Abstract
Asthma is an inflammatory condition for which anti-inflammatory glucocorticoids are the standard of care. However, similar efficacy has not been shown for agents targeting inflammatory cells and pathways. This suggests a noninflammatory cell contributor (e.g., epithelium) to asthmatic inflammation. Herein, we sought to define the intrinsic and glucocorticoid-affected properties of asthmatic airway epithelium compared with normal epithelium. Human primary differentiated normal and asthmatic airway epithelia were cultured in glucocorticoid-free medium beginning at −48 hours. They were pulsed with dexamethasone (20 nM) or vehicle for 2 hours at −26, −2, +22, and +46 hours. Cultures were mechanically scrape-wounded at 0 hours and exposed continuously to bromodeoxyuridine (BrdU). Cytokine secretions were analyzed using cytometric bead assays. Wound regeneration/mitosis was analyzed by microscopy and flow cytometry. Quiescent normal (n = 3) and asthmatic (n = 6) epithelia showed similar minimal inflammatory cytokine secretion and mitotic indices. After wounding, asthmatic epithelia secreted more basolateral TGF-β1, IL-10, IL-13, and IL-1β (P < 0.05) and regenerated less efficiently than normal epithelia (+48 h wound area reduction = [mean ± SEM] 50.2 ± 7.5% versus 78.6 ± 7.7%; P = 0.02). Asthmatic epithelia showed 40% fewer BrdU+ cells at +48 hours (0.32 ± 0.05% versus 0.56 ± 0.07% of total cells; P = 0.03), and those cells were more dyssynchronously distributed along the cell cycle (52 ± 10, 25 ± 4, 23 ± 7% for G1/G0, S, and G2/M, respectively) than normal epithelia (71 ± 1, 12 ± 2, and 17 ± 2% for G1/G0, S, and G2/M, respectively). Dexamethasone pulses improved asthmatic epithelial inflammation and regeneration/mitosis. In summary, we show that inflammatory/fibrogenic cytokine secretions are correlated with dyssynchronous mitosis upon injury. Intermittent glucocorticoids simultaneously decreased epithelial cytokine secretions and resynchronized mitosis. These data, generated in an airway model lacking inflammatory cells, support the concept that epithelium contributes to asthmatic inflammation.
Keywords: asthma, epithelial cells, mitosis, inflammation, glucocorticoids
CLINICAL RELEVANCE.
These data, generated in an airway model lacking inflammatory cells, support the concept that asthmatic epithelium is intrinsically inflammatory, fibrogenic, and mitotically dyssynchronous. These results support our previously proposed model predicting that asthmatic inflammation is driven by intrinsic inflammatory, fibrogenic, and regenerative characteristics of epithelia that are rescued by glucocorticoids. If extended by further studies, antiinflammatory treatment of asthma with glucocorticoids may best be redirected to target pathological lung remodeling directly.
Asthma is a chronic inflammatory disease of the lower respiratory tract characterized by airway hyperresponsiveness and mucus obstruction (1). Pharmacologic analogs of cortisol (e.g., prednisone) have been used clinically since 1948 and remain the standard of care for the treatment of a variety of inflammatory diseases, including asthma (2). These glucocorticoids reduce the pathological inflammation that is central to asthma, and they are thought to control clinical asthma symptoms through their anti-inflammatory effects (3). For example, Martinez and coworkers report that inhaled fluticasone shows sustained (albeit reversible) improvement in the proportion of asthma episode-free days, a reflection of reduced inflammation when compared with a placebo over a 2-year study period (4). Many agents, such as recombinant IL-12 and IFN-γ, that specifically target inflammatory cells and their intercellular signaling pathways have not shown similar efficacy to glucocorticoids in human trials (reviewed in 5, 6). This argues against the idea that asthmatic inflammation is merely the result of interactions between external stimuli and classic inflammatory cells like eosinophils and T cells. Rather, it is likely to involve complex interactions among multiple cell types, including noninflammatory resident cells of the lung (i.e., airway epithelium, fibroblasts, and smooth muscle).
To clarify this inconsistency, we recently proposed a model placing airway epithelium at the center of a network of interacting inflammatory mediators (7). Due to its ability to simultaneously respond to airborne pathogens and environmental challenges and to interact with its tissue environments, airway epithelium is regarded as a key lung tissue in asthma (8–10). In addition, airway epithelium communication with lamina propria fibroblasts (11) and smooth muscle has been described (12). Our model predicts that asthmatic inflammation is driven by the intrinsic inflammatory, fibrogenic, and regenerative characteristics of epithelium that are rescued by glucocorticoids.
In the current paper we present data that support this proposed model. We used a well established in vitro system wherein human primary airway epithelial cells, lacking inflammatory cells, from normal and asthmatic individuals are differentiated at an air–liquid interface to morphologically mimic conducting airway epithelium (13, 14). Our experiments showed that when induced to regenerate, asthmatic epithelium is intrinsically inflammatory, fibrogenic, and mitotically dyssynchronous. Furthermore, intermittent glucocorticoid exposures simultaneously reduced asthmatic inflammation and resynchronized epithelial mitotic regeneration. Some of the results of these studies have been previously reported in the form of an abstract (15).
MATERIALS AND METHODS
Further details on several aspects of these experiments can be found in the online supplement.
Cell Culture and Intermittent Glucocorticoid Exposures
Normal (n = 3) and asthmatic (n = 6) primary differentiated human airway (i.e., bronchial) epithelia grown in 12-well plates on collagen-coated Transwell membrane inserts at an air–liquid interface were obtained commercially (#AIR-606 and #AIR-606-Asthma; MatTek Corp., Ashland, MA). Donors underwent bronchoscopic brushing to provide epithelial cells. Descriptive donor information provided by MatTek Corporation for the individuals from whom cells were obtained is shown in Table 1.
TABLE 1.
DESCRIPTION OF HUMAN BRONCHIAL EPITHELIAL CELL DONORS*
| Donor Age (yr) | Gender | Race | Smoking | Medications | |
|---|---|---|---|---|---|
| Asthmatic | 7 | Female | Caucasian | No | Albuterol |
| 9 | Female | African American | No | Albuterol, fluticasone, salmeterol | |
| 27 | Female | African American | No | Unknown | |
| 43 | Female | African American | No | Oral and inhaled steroids | |
| 45 | Female | Caucasian | Yes | Albuterol, fluticasone, salmeterol | |
| 46 | Female | Caucasian | Yes | None | |
| Normal | 5 | Female | Caucasian | No | None |
| 13 | Male | Caucasian | No | None | |
| 33 | Female | Caucasian | No | None |
Provided by MatTek, Inc. (Ashland, MA).
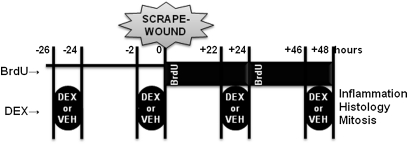
On arrival, the in vitro epithelia were washed with PBS, and the basal medium was replaced with proprietary defined medium supplied by the manufacturer. Cells were equilibrated at 37°C and 5% CO2 for 16 hours, followed by medium replacement with identical proprietary medium lacking glucocorticoids and epidermal growth factor (EGF). This condition was maintained for an additional 22 hours. Upon completion (i.e., −26 h on the experimental timeline shown in Figure 1), intermittent glucocorticoid exposures began. Dexamethasone (DEX) (20 nM) or PBS vehicle (VEH) was added to the apical and basolateral epithelial surfaces for 2 hours. At −24 hours, the medium was replaced with glucocorticoid- and EGF-free medium. This 2-hour DEX/VEH pulse was repeated every 24 hours (i.e., at −2, +22, and +46 h) until cell harvest at +48 hours.
Figure 1.
Experimental design for in vitro wounding of respiratory epithelia. Epithelia were pulsed for 2 hours every 24 hours with 20 nM dexamethasone (DEX) or vehicle (VEH) at the times shown. Mechanical scrape-wounding occurred at 0 hours with continuous apical and basolateral bromodeoxyuridine (BrdU) exposure until cell harvest at +48 hours for histological and mitotic analyses. Media samples were frozen before measurement of inflammatory cytokines.
Mechanical Injury Model
An in vitro epithelial injury model that allows for the study of epithelial repair processes in the lung was adapted for use in this study (16–18). Briefly, at 0 hours on the timeline in Figure 1, epithelia were scraped in two perpendicular lines with a p1000 pipette tip and placed in bromodeoxyuridine (BrdU)-containing (10 μM) medium. BrdU-containing medium was changed at +24 hours after the DEX/VEH pulse. Epithelia were incubated at 37°C and 5% CO2 until +48 hours. In some experiments, wounds were imaged daily (i.e., at 0, +24, and +48 h) using a 16× phase contrast objective lens, and wound area was measured in triplicate by a single operator blinded to the culture conditions using ImageJ Software (19). Contrast was enhanced equally in all images to improve wound visualization.
Analysis of Inflammatory Mediators and Cell Cycle Analysis
Inflammatory (i.e., IL-1β, IL-6, IL-10, and IL-13) and fibrogenic (i.e., transforming growth factor [TGF]-β1) cytokines were measured in apical and basolateral secretions at 0, +24, and +48 hours by flow cytometry. These cytokines were selected as an initial screening set for these experiments because of their prominent role in asthmatic inflammation and remodeling (5).
At +48 hours, cells were simultaneously labeled with the following according to the manufacturers' protocols: (1) a Carboxyfluorescein FLICA Apoptosis Poly-Caspase Detection Kit (Immunochemistry Technologies, LLC, Bloomington, MN) and (2) an APC BrdU Flow Kit containing 7-amino-actinomycin-D (BD Biosciences, San Jose, CA). Data were analyzed by means of the cell cycle analysis feature of FlowJo 7.6 (Tree Star, Inc., Ashland, OR).
Statistical Analysis
Statistical comparisons were performed in SPSS 17.0 software (SPSS Inc., Chicago, IL) using t test functions within time points. Results are reported as mean ± SEM unless otherwise noted.
RESULTS
Injured Asthmatic Epithelium Is Inflammatory and Fibrogenic
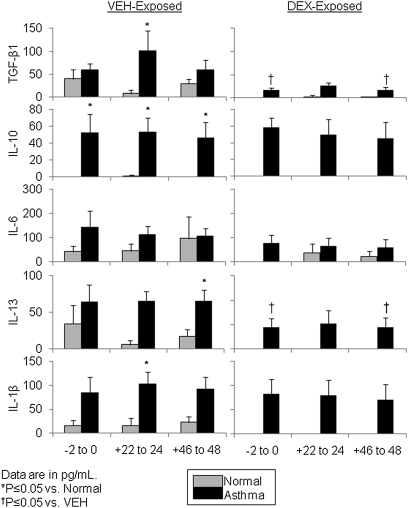
Flow cytometric bead assays were used to quantify a select screening group of inflammatory (i.e., IL-1β, IL-6, IL-10, and IL-13) and fibrogenic (i.e., TGF-β1) cytokines in apical and basolateral secretions from asthmatic and normal epithelia at 0, +24, and +48 hours after wounding. Normal and asthmatic epithelia at time 0 hours (i.e., before wounding) exhibited statistically similar levels of the cytokines investigated except for higher basolateral secretion of IL-10. However, asthmatic epithelia basolaterally secreted significantly higher levels of four of the five cytokines during wound healing (Figure 2). In particular, there was a significant between-group (i.e., asthma > normal) difference for secretion of TGF-β1, IL-10, IL-13, and IL-1β (all P < 0.05) for at least one time point during wound healing (Figure 2). IL-6 was the only cytokine for which basolateral secretion was not significantly different between asthmatic and normal epithelia. With DEX pulses, asthmatic TGF-β1 and IL-13 basolateral secretion were significantly reduced. Although generally higher than in basolateral secretions, cytokine levels in apical secretions were not different between wounded untreated asthmatic and normal epithelia (data not shown).
Figure 2.
Asthmatic epithelial basolateral secretions are relatively inflammatory after wounding. Levels of specific cytokines (TGF-β1, IL-10, IL-6, IL-13, and IL-1β) measured by cytometric bead assay are shown for basolateral epithelial secretions from asthmatic and normal epithelia at 0, +24, and +48 hours. Asthmatic epithelia had significantly higher basolateral secretion of TGF-β1, IL-10, IL-13, and IL-1β at one or more time points. Dexamethasone (DEX) pulses decreased secretion of TGF-β1 and IL-13 in asthmatic epithelia. Data are shown as mean ± SEM in pg/mL.
Asthmatic Epithelial Cell Mitosis Is Slow and Dyssynchronous
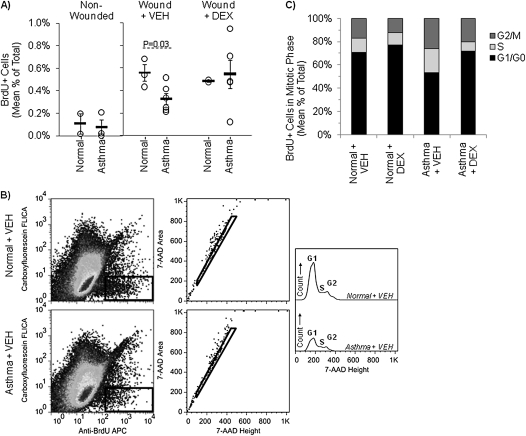
Epithelia were harvested into single-cell suspensions at +48 hours for flow cytometry. Nonwounded asthmatic and normal epithelia showed similar minimal background levels of BrdU+ cells, an indicator of mitosis (Figure 3A). Due to the lack of mitotic cells, no cell cycle analysis was performed on samples from this condition.
Figure 3.
Mitosis is diminished in asthmatic epithelium and increased by pulse DEX. Flow cytometry was used to measure the presence of BrdU in single cell suspensions of normal and asthmatic human airway epithelia at +48 hours. (A) The quantity of BrdU positivity is shown as the percent of total counted cells in non-wounded, wounded, and wounded DEX-pulsed epithelial cultures. Asthmatic epithelial wounds (n = 6) showed approximately 40% fewer mitotically active cells than normal epithelial wounds (n = 3), but intermittent DEX exposures abrogated this difference. (B) Shown are gating and cell cycle analyses for one representative wounded normal and one representative wounded asthmatic epithelial culture. All events measured by the cytometer were gated on the BrdU+FLICA− population (left panels). The selected cells were further gated by 7-AAD height and area to remove cell doublets (middle panels). Finally, 7-AAD height was used to identify the cell cycle distribution of the gated cell population (right panel). (C) Compared with wounded normal epithelia, wounded asthmatic epithelia at +48 hours showed a more even distribution of BrdU+ cells among the cell cycle phases (i.e., G1/G0, S, and G2/M) consistent with mitotic dyssynchrony. Intermittent exposures of the asthmatic epithelia to DEX improved cell cycle synchrony as shown by normalization of the percentage of mitotic cells in each cell cycle phase.
Wounded asthmatic epithelia showed 40% fewer BrdU+ cells than wounded normal epithelia (mean ± SEM: 0.32 ± 0.05% versus 0.56 ± 0.07% of total cells; P = 0.03). Exposure of normal cells to pulses of DEX did not significantly alter the quantity of BrdU+ cells in normal epithelia, whereas the quantity of asthmatic epithelial mitosis approximated normal levels with DEX pulses (0.55 ± 0.13%; P = 0.19 versus wounded untreated asthmatic cells) (Figure 3A).
To evaluate normal and asthmatic epithelial mitosis during wound repair, flow cytometric cell cycle analysis for DNA content (i.e., 7-amino-actinomycin-D) was performed by gating on BrdU+ cells with no detectable caspase activation (i.e., apoptosis) (Figure 3B). Caspase+ (i.e., apoptotic) cells were rare in all conditions. Cells in active mitosis were presumed to be regenerating the scrape wound because of the extremely low rate of background mitosis in nonwounded cultures. Normal epithelial mitosis was fairly synchronous (e.g., > 70% of cells in G1/G0) in the absence and presence of pulse DEX (Figure 3C). Conversely, mitotically active asthmatic epithelial cells exhibited a dyssynchronous distribution among the cell cycle phases (53 ± 5, 21 ± 3, and 26 ± 4% for G1/G0, S, and G2/M, respectively) compared with normal epithelia (71 ± 1, 12 ± 2, and 17 ± 2% for G1/G0, S, and G2/M, respectively). DEX-pulsed asthmatic cells showed similarly synchronous mitotic activity to normal cells.
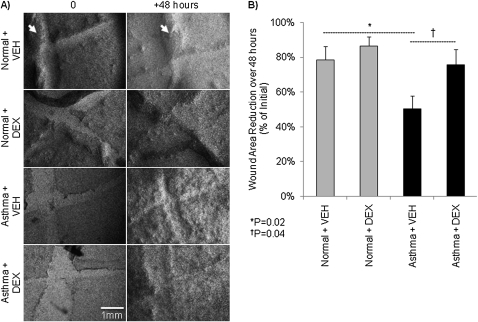
Normal and Asthmatic Epithelia Exhibit Differential Wound Healing
Wounded normal and asthmatic epithelia were imaged by bright field microscopy daily at 0, +24, and +48 hours using a 16× phase-contrast objective lens. Normal epithelial wounds showed visible healing regardless of DEX pulse (Figure 4A). Alternatively, the asthmatic scars appeared relatively thin and were still visible at +48 hours regardless of DEX pulses. This was evaluated quantitatively by measurement of the wound area. Normal wound area decreased from 0 to +48 hours by 78.6 ± 7.7% with vehicle alone and by 86.8 ± 5.4% with DEX pulses. However, in the absence of DEX, asthmatic epithelial wound area decreased by significantly less (50.2 ± 7.5%; P = 0.02) than normal epithelia. With DEX pulses, asthmatic wound narrowing improved significantly (75.7 ± 9%; P = 0.04) (Figure 4B).
Figure 4.
Regeneration of asthmatic human airway epithelia is impaired. (A) Contrast-enhanced bright-field microscopy (16×) images of scrape-wounded primary differentiated human airway epithelia at wounding (i.e., 0 h) and +48 h from representative normal and asthmatic donors. Normal epithelial wounds showed complete healing in all conditions (i.e., 20 nM DEX or VEH) by +48 hours. Similarly cultured asthmatic epithelia showed thinly repaired wounds at +48 hours regardless of DEX exposure. The wounds are pale appearing X-shaped regions. Thick-appearing dark areas (arrows) are heaped up areas of epithelium resulting from the scraping process. (B) Percent wound area reduction over 48 hours according to culture condition and DEX exposure. Wound area was measured in triplicate by a single operator blinded to the culture conditions using ImageJ Software (19).
DISCUSSION
We studied cultures of human primary differentiated asthmatic and normal airway epithelia cultured at an air–liquid interface. As in a recent study (20), we found that confluent, quiescent normal, and asthmatic epithelial cultures were similar, with minimal secretion of cytokines and mitotic activity as evidenced by BrdU labeling. However, upon mechanical wounding, asthmatic and normal epithelia exhibited different responses. The asthmatic epithelial cultures showed increased basolateral secretion of inflammatory/fibrogenic cytokines (as exemplified by TGF-β1, IL-10, IL-13, and IL-1β) and showed slow, poorly synchronized mitosis relative to normal controls. This was associated with the poor wound repair observed for asthmatic epithelia. Those markers of inflammation and dyssynchronous regeneration were attenuated by intermittent glucocorticoid pulses. These results support our proposed model that predicts asthmatic inflammation is driven by intrinsic inflammatory, fibrogenic, and regenerative characteristics of airway epithelium that are rescued by glucocorticoids (7).
Cytokine (i.e., TGF-β1, IL-10, IL-13, and IL-1β) secretion in our experiments in response to epithelial injury is important given accumulating evidence for airway epithelium–induced inflammatory cell recruitment (21, 22) and the proliferation of fibroblasts (23–25) and smooth muscle (12). In particular, basolateral secretion of TGF-β1, which was increased in asthmatic epithelia in our experiments, is one of the key mediators of fibroblast and smooth muscle proliferation (26) and is a central component of our previously published airway epithelial stress response gene/protein network (27). Furthermore, IL-1β activates many inflammatory genes in asthma (28), and IL-13 is a critical mediator of the classical Th2 asthmatic inflammation (29, 30). Conversely, IL-10 is a potent immunoregulatory and antiinflammatory cytokine that suppresses eosinophils (31), decreases airway hyperresponsiveness (32, 33), and is increased during acute viral exacerbations of asthma (34). The elevated basolateral secretion of IL-10 from asthmatic epithelium at all time points suggests a constitutive epithelial counter-regulation of inflammation in vitro. This runs counter to reports of decreased IL-10 in BAL fluid from individuals with asthma (35, 36). However, this difference may be accounted for by BAL fluid cytokines reflecting both apical epithelial and inflammatory cell secretions.
In addition to inflammation, epithelial regeneration is of particular importance in asthma due to the fact that many typical asthma triggers, including tobacco smoke and viruses, are known to induce apoptotic injury in airway epithelium (reviewed in 37). Epithelial stress/injury, independent of inflammation, has been observed in moderate and severe childhood asthma (38). In fact, airway epithelial injury, in the forms of physical damage to the columnar cell layer and apoptosis (39, 40), is a hallmark of asthma (41). Puchelle and colleagues have shown that regeneration of normal human airway epithelium in response to injury includes three stages: cell spreading/migration, proliferation, and differentiation (42, 43). Using a similar in vitro mechanical injury model to the one used in our study, Wadsworth and colleagues showed that normal human differentiated airway epithelial wounds closed over the initial 16 to 24 hours. This primarily reflected cell migration mediated by autocrine EGF secretion that subsequently led to mitosis of epithelial cells within the wound (18). Our results for normal epithelium wound repair were temporally similar to those described by Wadsworth and colleagues (18). However, the thinly repaired wounds in asthmatic cultures observed in our study are consistent with effective migration without effective regeneration. This needs to be defined in future studies.
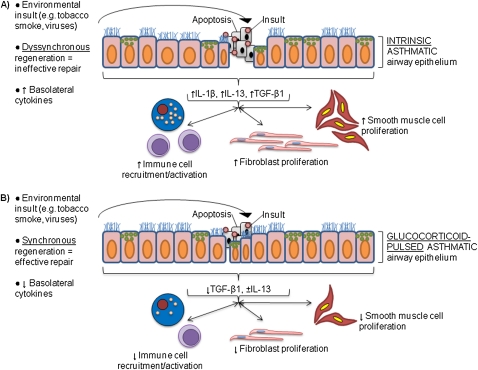
The simultaneous resolution of inflammation and resynchronization of epithelial mitotic regeneration on exposure to intermittent glucocorticoids after in vitro injury addresses an important inconsistency in asthma. That is, despite well demonstrated antiinflammatory efficacy, inhaled glucocorticoids have not been shown to improve long-term pathologic airway remodeling. The classic model is that asthmatic inflammation leads to long-term pathological lung function decline (i.e., remodeling). However, this is not supported by several trials that have shown inhaled glucocorticoids improve lung function in the short-term, but lung function regresses toward the placebo group over the course of several years (4, 44, 45). Recently, this was confirmed by a large trial comparing inhaled budesonide with placebo in children and adults with recent-onset, mild persistent asthma. In this study, the initial pre- and postbronchodilator differences in FEV1 between the treatment and placebo groups disappeared by the fourth year of the study (46). Furthermore, asthmatic bronchial biopsy reticular layer thickness does not decrease with inhaled glucocorticoid treatment unless given at relatively high doses (47). These patients were only studied for 2 years, so any sustained long-term effect of relatively high-dose glucocorticoids remains unclear. Therefore, our data address this inconsistency in asthma by supporting our proposed alternative model of glucocorticoid efficacy in asthma (Figure 5). In our model, direct antiinflammatory effects of intermittent glucocorticoid dosing are accompanied by simultaneous resynchronization of epithelial mitosis, thereby reducing pathological lung remodeling in asthma.
Figure 5.
Proposed model for glucocorticoid efficacy in asthma. Tobacco smoke and viruses are among many agents known to induce apoptosis in airway epithelium (reviewed in Ref. 37), prompting regenerative processes. (A) Untreated asthmatic airway epithelium is characterized by dyssynchronous regeneration that ineffectively repairs apoptotic regions of epithelium. The concomitant basolateral inflammatory cytokine secretion (e.g., increased IL-1β and TGF-β1, variable IL-10) would lead to pathological immune cell recruitment/activation as well as fibroblast and smooth muscle cell proliferation. (B) In our proposed model for glucocorticoid efficacy in asthma, intermittent glucocorticoid dosing simultaneously mediates antiinflammation in injured asthmatic epithelium and increases the ability of asthmatic epithelium to synchronize its mitosis. This leads to more effective regeneration of injured regions.
Pulsatile secretion of endogenous adrenal glucocorticoids (i.e., cortisol in humans) can reset an organism's internal and peripheral circadian clocks (48), and this has been shown to occur in the bronchiolar epithelium, where it is mediated by Clara cells (49). The result of this is synchronous progression of a tissue's cells through normal regeneration/mitosis. The intermittent glucocorticoid exposure scheme used in our experiments was a gross reflection of a circadian peak in circulating endogenous glucocorticoid levels. Although crude by comparison to in vivo glucocorticoid circadian fluctuations, a 2-hour-pulse glucocorticoid exposure is sufficient to induce precursors to the inhibition of mitosis, including cyclin-dependent kinase inhibitor p57kip2 (50) and clock gene Per1 (51).
This study is the first step to validate our proposed model for airway epithelial–derived inflammation in asthma (6), and we acknowledge several limitations. First, the use of commercially available epithelia limited the clinical information available for each donor. Second, despite our attempts to age-, gender-, and race-match the normal donors to the donors with asthma, the limited sample availability prevented this. Third, this limited availability resulted in heterogeneity among the donors with asthma with regard to their smoking status and treatment regimens at the time of tissue availability. In fact, one adult donor with asthma was taking oral and inhaled glucocorticoids, suggesting particularly severe asthma. Epithelial cells from this donor showed comparatively less resynchronization with pulse DEX exposures, raising the possibility of a glucocorticoid-resistant phenotype. Finally, our data only show correlations between inflammation and dyssynchronous mitosis in response to acute injury and therefore do not directly address the long-term remodeling consequences implicated by our model. Measurement of inflammatory cytokines at time points interval to the 24-hour time points studied in these experiments as well as chronic and repeated wounding experiments need to be performed to address this.
In summary, these data, generated in an airway model lacking inflammatory cells, support the concept that asthmatic epithelium is intrinsically inflammatory, fibrogenic, and mitotically dyssynchronous. These results support our previously proposed model predicting that asthmatic inflammation is driven by the intrinsic inflammatory, fibrogenic, and regenerative characteristics of epithelium that are rescued by glucocorticoids (7). If extended by further studies, antiinflammatory treatment of asthma with glucocorticoids may best be redirected to target pathological lung remodeling directly.
Supplementary Material
Supported by grants K23RR020069 from the National Institutes of Health, Bethesda, MD (R.J.F.) and by institutional grants from Children's National Medical Center, Washington, DC (R.J.F.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2010-0029OC on August 12, 2010
Author Disclosure: E.H. has received consultancy/lecture fees from QI2 ($1,001–$5,000), Astra Zenica ($1,001–$5,000), Sanofie-Aventis ($1,001–$5,000), and Grifols ($1,001–$5,000); has served on the board for Siemens Medical Systems (less than $1,000); holds stock ownership with VIDA Diagnostics (more than $100,001); and has received a sponsored grant from NIH (more than $100,001). R.F. has received a sponsored grant from NIH (more than $100,001). S.I. has received a sponsored grant from NIH (more than $100,001). P.D. has received support from NIH ($75,000). None of the other authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Busse W, Banks-Schlegel S, Noel P, Ortega H, Taggart V, Elias J. Future research directions in asthma: an NHLBI Working Group report. Am J Respir Crit Care Med 2004;170:683–690. [DOI] [PubMed] [Google Scholar]
- 2.Larj M, Bleecker E. Therapeutic responses in asthma and COPD: corticosteroids. Chest 2004;126:138S–149S. [DOI] [PubMed] [Google Scholar]
- 3.Expert Panel Report 3 (EPR-3). Guidelines for the diagnosis and management of asthma: summary report 2007. J Allergy Clin Immunol 2007;120:S94–S138. [DOI] [PubMed] [Google Scholar]
- 4.Guilbert TW, Morgan WJ, Zeiger RS, Mauger DT, Boehmer SJ, Szefler SJ, Bacharier LB, Lemanske RF Jr, Strunk RC, Allen DB, et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med 2006;354:1985–1997. [DOI] [PubMed] [Google Scholar]
- 5.Barnes PJ. The cytokine network in asthma and chronic obstructive pulmonary disease. J Clin Invest 2008;118:3546–3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lemanske RF Jr. Asthma therapies revisited: what have we learned? Proc Am Thorac Soc 2009;6:312–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freishtat RJ, Nagaraju K, Jusko W, Hoffman EP. Glucocorticoid efficacy in asthma: is improved tissue remodeling upstream of anti-inflammation. J Investig Med 2010;58:19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holgate ST, Lackie PM, Davies DE, Roche WR, Walls AF. The bronchial epithelium as a key regulator of airway inflammation and remodelling in asthma. Clin Exp Allergy 1999;29:90–95. [DOI] [PubMed] [Google Scholar]
- 9.Kicic A, Sutanto EN, Stevens PT, Knight DA, Stick SM. Intrinsic biochemical and functional differences in bronchial epithelial cells of children with asthma. Am J Respir Crit Care Med 2006;174:1110–1118. [DOI] [PubMed] [Google Scholar]
- 10.Davies DE, Holgate ST. Asthma: the importance of epithelial mesenchymal communication in pathogenesis. Inflammation and the airway epithelium in asthma. Int J Biochem Cell Biol 2002;34:1520–1526. [DOI] [PubMed] [Google Scholar]
- 11.Davies DE, Wicks J, Powell RM, Puddicombe SM, Holgate ST. Airway remodeling in asthma: new insights. J Allergy Clin Immunol 2003;111:215–225, quiz 226. [DOI] [PubMed] [Google Scholar]
- 12.Malavia NK, Raub CB, Mahon SB, Brenner M, Panettieri RA Jr, George SC. Airway epithelium stimulates smooth muscle proliferation. Am J Respir Cell Mol Biol 2009;41:297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu R, Zhao YH, Chang MM. Growth and differentiation of conducting airway epithelial cells in culture. Eur Respir J 1997;10:2398–2403. [DOI] [PubMed] [Google Scholar]
- 14.Fulcher ML, Gabriel S, Burns KA, Yankaskas JR, Randell SH. Well-differentiated human airway epithelial cell cultures. In: Picot J, editor. Human cell culture protocols. Totowa, NJ: Humana Press Inc.; 2004. pp. 183–206. [DOI] [PubMed]
- 15.Freishtat RJ, Watson AM, Benton AS, Iqbal SF, Pillai DK, Rose MC, Hoffman EP. Asthmatic bronchial epithelium is intrinsically inflammogenic, mitotically dyssynchronous, and is rescued by glucocorticoids. Am J Respir Crit Care Med 2010;181:A2491. [Google Scholar]
- 16.Geiser T, Ishigaki M, van Leer C, Matthay MA, Broaddus VCH. (2)O(2) inhibits alveolar epithelial wound repair in vitro by induction of apoptosis. Am J Physiol Lung Cell Mol Physiol 2004;287:L448–L453. [DOI] [PubMed] [Google Scholar]
- 17.Geiser T, Jarreau PH, Atabai K, Matthay MA. Interleukin-1beta augments in vitro alveolar epithelial repair. Am J Physiol Lung Cell Mol Physiol 2000;279:L1184–L1190. [DOI] [PubMed] [Google Scholar]
- 18.Wadsworth SJ, Nijmeh HS, Hall IP. Glucocorticoids increase repair potential in a novel in vitro human airway epithelial wounding model. J Clin Immunol 2006;26:376–387. [DOI] [PubMed] [Google Scholar]
- 19.Rasband WS. ImageJ. Bethesda, MD: US National Institutes of Health; 1997–2009.
- 20.Parker J, Sarlang S, Thavagnanam S, Williamson G, O'Donoghue D, Villenave R, Power U, Shields M, Heaney L, Skibinski G. A 3-D well-differentiated model of pediatric bronchial epithelium demonstrates unstimulated morphological differences between asthmatic and nonasthmatic cells. Pediatr Res 2010;67:17–22. [DOI] [PubMed] [Google Scholar]
- 21.Cheng D-s, Han W, Chen SM, Sherrill TP, Chont M, Park G-Y, Sheller JR, Polosukhin VV, Christman JW, Yull FE, et al. Airway epithelium controls lung inflammation and injury through the NF-{kappa}B pathway. J Immunol 2007;178:6504–6513. [DOI] [PubMed] [Google Scholar]
- 22.Hammad H, Lambrecht BN. Dendritic cells and epithelial cells: linking innate and adaptive immunity in asthma. Nat Rev Immunol 2008;8:193–204. [DOI] [PubMed] [Google Scholar]
- 23.Perng D-W, Wu Y-C, Chang K-T, Wu M-T, Chiou Y-C, Su K-C, Perng R-P, Lee Y-C. Leukotriene C4 induces TGF-{beta}1 production in airway epithelium via p38 kinase pathway. Am J Respir Cell Mol Biol 2006;34:101–107. [DOI] [PubMed] [Google Scholar]
- 24.Hostettler KE, Roth M, Burgess JK, Gencay MM, Gambazzi F, Black JL, Tamm M, Borger P. Airway epithelium-derived transforming growth factor-beta is a regulator of fibroblast proliferation in both fibrotic and normal subjects. Clin Exp Allergy 2008;38:1309–1317. [DOI] [PubMed] [Google Scholar]
- 25.Royce SG, Tan L, Koek AA, Tang MLK. Effect of extracellular matrix composition on airway epithelial cell and fibroblast structure: implications for airway remodeling in asthma. Ann Allergy Asthma Immunol 2009;102:238–246. [DOI] [PubMed] [Google Scholar]
- 26.Makinde T, Murphy RF, Agrawal DK. The regulatory role of TGF-[beta] in airway remodeling in asthma. Immunol Cell Biol 2007;85:348–356. [DOI] [PubMed] [Google Scholar]
- 27.Freishtat RJ, Benton AS, Watson AM, Wang Z, Rose MC, Hoffman EP. Delineation of a gene network underlying the pulmonary response to oxidative stress in asthma. J Investig Med 2009;57:756–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenwasser LJ. Biologic activities of IL-1 and its role in human disease. J Allergy Clin Immunol 1998;102:344–350. [DOI] [PubMed] [Google Scholar]
- 29.Walter DM, McIntire JJ, Berry G, McKenzie ANJ, Donaldson DD, DeKruyff RH, Umetsu DT. Critical role for IL-13 in the development of allergen-induced airway hyperreactivity. J Immunol 2001;167:4668–4675. [DOI] [PubMed] [Google Scholar]
- 30.Wills-Karp M. The gene encoding interleukin-13: a susceptibility locus for asthma and related traits. Respir Res 2000;1:19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takanaski S, Nonaka R, Xing Z, O'Byrne P, Dolovich J, Jordana M. Interleukin 10 inhibits lipopolysaccharide-induced survival and cytokine production by human peripheral blood eosinophils. J Exp Med 1994;180:711–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Makela MJ, Kanehiro A, Borish L, Dakhama A, Loader J, Joetham A, Xing Z, Jordana M, Larsen GL, Gelfand EW. IL-10 is necessary for the expression of airway hyperresponsiveness but not pulmonary inflammation after allergic sensitization. Proc Natl Acad Sci USA 2000;97:6007–6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Justice JP, Shibata Y, Sur S, Mustafa J, Fan M, Van Scott MR. IL-10 gene knockout attenuates allergen-induced airway hyperresponsiveness in C57BL/6 mice. Am J Physiol Lung Cell Mol Physiol 2001;280:L363–L368. [DOI] [PubMed] [Google Scholar]
- 34.Grissell TV, Powell H, Shafren DR, Boyle MJ, Hensley MJ, Jones PD, Whitehead BF, Gibson PG. Interleukin-10 gene expression in acute virus-induced asthma. Am J Respir Crit Care Med 2005;172:433–439. [DOI] [PubMed] [Google Scholar]
- 35.Borish L, Aarons A, Rumbyrt J, Cvietusa P, Negri J, Wenzel S. Interleukin-10 regulation in normal subjects and patients with asthma. J Allergy Clin Immunol 1996;97:1288–1296. [DOI] [PubMed] [Google Scholar]
- 36.Message SD, Laza-Stanca V, Mallia P, Parker HL, Zhu J, Kebadze T, Contoli M, Sanderson G, Kon OM, Papi A, et al. Rhinovirus-induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL-10 production. Proc Natl Acad Sci USA 2008;105:13562–13567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tesfaigzi Y. Roles of apoptosis in airway epithelia. Am J Respir Cell Mol Biol 2006;34:537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fedorov IA, Wilson SJ, Davies DE, Holgate ST. Epithelial stress and structural remodelling in childhood asthma. Thorax 2005;60:389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen L, Xueping E, Tarsi J, Ramkumar T, Horiuchi TK, Cochran R, DeMartino S, Schechtman KB, Hussain I, Holtzman MJ, et al and the NSARP. Epithelial cell proliferation contributes to airway remodeling in severe asthma. Am J Respir Crit Care Med 2007;176:138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bucchieri F, Puddicombe SM, Lordan JL, Richter A, Buchanan D, Wilson SJ, Ward J, Zummo G, Howarth PH, Djukanovic R, et al. Asthmatic bronchial epithelium is more susceptible to oxidant-induced apoptosis. Am J Respir Cell Mol Biol 2002;27:179–185. [DOI] [PubMed] [Google Scholar]
- 41.Holgate ST. The airway epithelium is central to the pathogenesis of asthma. Allergol Int 2008;57:1–10. [DOI] [PubMed] [Google Scholar]
- 42.Zahm JM, Kaplan H, Herard AL, Doriot F, Pierrot D, Somelette P, Puchelle E. Cell migration and proliferation during the in vitro wound repair of the respiratory epithelium. Cell Motil Cytoskeleton 1997;37:33–43. [DOI] [PubMed] [Google Scholar]
- 43.Puchelle E, Zahm JM, Tournier JM, Coraux C. Airway epithelial repair, regeneration, and remodeling after injury in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2006;3:726–733. [DOI] [PubMed] [Google Scholar]
- 44.The Childhood Asthma Management Program Research Group. Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med 2000;343:1054–1063. [DOI] [PubMed] [Google Scholar]
- 45.Murray CS, Woodcock A, Langley SJ, Morris J, Custovic A. Secondary prevention of asthma by the use of inhaled fluticasone propionate in Wheezy INfants (IFWIN): double-blind, randomised, controlled study. Lancet 2006;368:754–762. [DOI] [PubMed] [Google Scholar]
- 46.Busse WW, Pedersen S, Pauwels RA, Tan WC, Chen YZ, Lamm CJ, O'Byrne PM. The Inhaled Steroid Treatment As Regular Therapy in Early Asthma (START) study 5-year follow-up: effectiveness of early intervention with budesonide in mild persistent asthma. J Allergy Clin Immunol 2008;121:1167–1174. [DOI] [PubMed] [Google Scholar]
- 47.Sont JK, Willems LN, Bel EH, van Krieken JH, Vandenbroucke JP, Sterk PJ. Clinical control and histopathologic outcome of asthma when using airway hyperresponsiveness as an additional guide to long-term treatment. The AMPUL Study Group. Am J Respir Crit Care Med 1999;159:1043–1051. [DOI] [PubMed] [Google Scholar]
- 48.Knutsson U, Dahlgren J, Marcus C, Rosberg S, Bronnegard M, Stierna P, Albertsson-Wikland K. Circadian cortisol rhythms in healthy boys and girls: relationship with age, growth, body composition, and pubertal development. J Clin Endocrinol Metab 1997;82:536–540. [DOI] [PubMed] [Google Scholar]
- 49.Gibbs JE, Beesley S, Plumb J, Singh D, Farrow S, Ray DW, Loudon AS. Circadian timing in the lung; a specific role for bronchiolar epithelial cells. Endocrinology 2009;150:268–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Puddicombe SM, Torres-Lozano C, Richter A, Bucchieri F, Lordan JL, Howarth PH, Vrugt B, Albers R, Djukanovic R, Holgate ST, et al. Increased expression of p21waf cyclin-dependent kinase inhibitor in asthmatic bronchial epithelium. Am J Respir Cell Mol Biol 2003;28:61–68. [DOI] [PubMed] [Google Scholar]
- 51.Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schutz G, Schibler U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 2000;289:2344–2347. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.