Abstract
Idiopathic pulmonary fibrosis (IPF) is a progressive fibrotic disease of the lung parenchyma, without curative treatment. Gremlin is a bone morphogenic protein (BMP) antagonist, its expression being increased in IPF lungs. It has been implicated in promoting myofibroblast accumulation, likely through inhibited fibroblast apoptosis and epithelial-to-mesenchymal transition. In the current study, we examined the effects of selective adenovirus-mediated overexpression of Gremlin in rat lungs. We show that transient Gremlin overexpression results in activation of alveolar epithelial cells with proliferation and apoptosis, as well as partly reversible lung fibrosis. We found myofibroblasts arranged in fibroblastic foci. Fibroblast proliferation occurred delayed as compared with epithelial changes. Fibrotic pathology significantly declined after Day 14, the reversal being associated with an increase of the epithelium-protective element, fibroblast growth factor (FGF)–10. Our data indicate that Gremlin-mediated BMP inhibition results in activation of epithelial cells and transient fibrosis, but also induction of epithelium-protective FGF10. A Gremlin–BMP–FGF10 loop may explain these results, and demonstrate that the interactions between different factors are quite complex in fibrotic lung disease. Increased Gremlin expression in human IPF tissue may be an expression of continuing epithelial injury, and Gremlin may be part of activated repair mechanisms.
Keywords: pulmonary fibrosis, gremlin, bone morphogenic protein, animal model, epithelial cell
CLINICAL RELEVANCE.
This research provides important mechanistic insights into the biological functions of the bone morphogenic protein (BMP) antagonist, Gremlin, in the lungs. Specifically, it shows that a balance of BMP/Gremlin is important for epithelial integrity and homeostasis. Too much Gremlin activity may contribute to the development of fibrotic tissue repair, and, as such, Gremlin may potentially become a novel molecular target in pulmonary fibrosis treatment.
Idiopathic pulmonary fibrosis (IPF) is a progressive, scar-forming, and disabling disease of the lung parenchyma, with poor prognosis similar to many malignant disorders. Despite continuous efforts, there is no successful therapy for IPF (1, 2). The histopathological correlate of IPF is usual interstitial pneumonia. It is characterized by spatiotemporal heterogeneity, with unaffected lung parenchyma adjacent to subepithelial myofibroblast accumulation in so-called fibroblastic foci and advanced scar tissue (honeycombing). The fibrotic pathology shows basal and subpleural predominance (3). The pathogenic concepts have significantly changed over the past 10 years, and now focus on dysregulated epithelial repair and apoptosis, as well as epithelial-to-mesenchymal transition (EMT), as very likely components of initiation and progression of the fibrotic process (4, 5).
Among the various factors and pathways that are likely involved in the fibrotic process are transforming growth factor (TGF)–β, platelet-derived growth factor, endothelin-1, vascular endothelial growth factor, and inhibitors of bone morphogenic proteins (BMPs) (6, 7). Gremlin belongs to the Cerberus/Dan family of binding proteins with antagonistic activity for BMP signaling (8). Elevated Gremlin levels have been found in IPF lung tissue and in association with experimental asbestosis in mice (9, 10). Gremlin is thought to be implicated in prevention of epithelial regeneration, but also in promotion of myofibroblast generation, likely through inhibition of BMP-induced fibroblast apoptosis and participation in EMT (9).
Fibroblast growth factors (FGFs) represent a family of mitogenic factors that signal through four transmembrane receptor tyrosine kinases, FGF receptors 1–4 (11). FGF7 and -10, and also FGF9 (through putative induction of FGF10), are involved in epithelial repair and regeneration (12–15). FGF10 has not only important functions in survival and proliferation of endogenous distal alveolar progenitor cells during lung development, but also attenuates bleomycin-induced pulmonary fibrosis in adult mice (16–18). The biological functions of FGF10 are transduced by binding to FGF receptor 2b, which is important for alveolar regeneration after lung injury (19). FGF10 has important protective effects for alveolar epithelial cells (AEC) in vitro and in vivo (14, 15, 20, 21).
Our study is the first to investigate overexpression of Gremlin in rat lungs using adenovirus-mediated gene transfer. We show that this results in alveolar epithelial activation (deviation from normal appearance) and partly reversible lung fibrosis. We found activated myofibroblasts in clusters of fibroblastic foci, as well as proliferating and apoptotic alveolar epithelial cells within fibrotic regions. Furthermore, these regions contained fibroblast proliferation. All changes declined significantly after Day 14; the reversal was associated with increased presence of epithelium-protective FGF10.
MATERIALS AND METHODS
Recombinant Adenovirus
The construction of the adenoviral vectors encoding mouse Gremlin (AdGRE) and green fluorescent protein (AdGFP), as well as of an empty control vector (AdDL70), have been described in detail elsewhere (22, 23).
Animal Experiments
All procedures were approved by the Animal Research Ethics Board of McMaster University (Hamilton, ON, Canada) and conducted in accordance with the guidelines of the Canadian Council of Animal Care and the Guide for the Care and Use of Laboratory Animals, NIH publication No. 86-23 (revised 1985). Animals were housed in specific-pathogen–free environment of the central animal facility at McMaster University at a 12 hour/12 hour light/dark cycle. Water and animal chow were provided ad libitum. Female Sprague-Dawley rats (225–250 g; Charles River, Wilmington, MA) received 1.5 × 108 pfu AdGRE or AdDL70 intratracheally under isoflurane anesthesia. Before sacrifice, quasistatic elastance was assessed by recording pressure–volume loops with a quantitative mechanical respirator (flexiVent; SCIREQ Scientific Respiratory Equipment, Montreal, PQ, Canada) under ketamine/xylazine anesthesia. The animals were killed by abdominal bleeding. Lung was removed en bloc and 5 ml sterile PBS was used to obtain bronchoalveolar lavage (BAL) fluid (BALF). Right and left lung were used for molecular biology and histology.
BAL
The BALF was centrifuged at 1,500 rpm for 10 minutes. The supernatant was stored at −80°C for further analysis. BAL cells were counted in a hemocytometer, centrifuged in a cytospin, and slides were stained for modified Wright-Giemsa (Sigma-Aldrich, Oakville, ON, Canada) according to the manufacturer's instruction. A total of 400 cells per animal were counted to obtain differential cell count.
Histological Evaluation
The left lung was inflated with 10% formalin at a pressure of 20 cm H20 and fixed for 24 hours. Lungs were paraffin embedded, cut at a thickness of 5 μm, and stained with hematoxylin and eosin and Picro-Sirius red (PSR). Relative collagen content was assessed with a semiquantitative scoring system on PSR-stained slides according to Ashcroft and colleagues as previously described (24, 25). Details regarding immunohistochemistry methodology and quantification can be found in the online supplement.
Preparation of Lung Homogenate
One lobe of the right lung was homogenized in 3 ml of TRIzol (Invitrogen, Burlington, ON, Canada) for RNA isolation, and another lobe was homogenized in RIPA buffer (0.5 g tissue/ml RIPA) for protein isolation.
Experimental details regarding cell culture, RNA and protein isolation, Western blotting, coimmunoprecipitation experiments, and immunofluorescence staining of cells can be found in the online supplement.
Statistical Analysis
Data are presented as mean (±SEM). Two groups were compared with Student's t test and more than two groups with one-way ANOVA, followed by Neumann-Keuls multiple comparison test. P values less than 0.05 were considered significant. Statistical tests and graphs were done with GraphPad Prism 5.0 (GraphPad Inc., San Diego, CA).
RESULTS
Transgene Expression of AdGRE
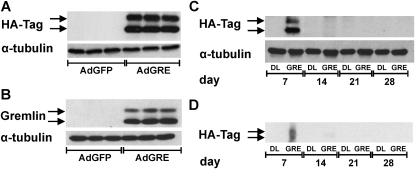
The Gremlin transgene expression induced by AdGRE was verified in vitro and in vivo. In A549 cells infected with AdGRE, Gremlin was detected by Western blot for hemagglutinin influenza virus epitope tag (HA-Tag) and Gremlin (Figures 1A and 1B). Furthermore, AdGRE-mediated Gremlin expression in rat lung was also detected in the lung tissue lysate (Figure 1C) and BALF (Figure 1D) at Day 7, but not at Days 14, 21, or 28 after intratracheal instillation of AdGRE, suggesting that AdGRE-mediated Gremlin expression lasts less than 2 weeks.
Figure 1.
Adenovirus encoding Gremlin (AdGRE) transgene expression. Transgene expression of adenovirus construct was verified in A549 cells by Western blot for (A) Hemagglutinin influenza virus epitope tag (HA-Tag) and (B) Gremlin (both show double band 20–25 kD, indicating that both detect transgenic Gremlin). The three bands per condition represent an n of 3 of a representative experiment. AdDL70, empty control adenovirus; AdGFP, adenovirus encoding green fluorescent protein. Transgene expression was found 7 days after intratracheal administration of AdGRE to rat lung in protein lysate of homogenized lung (C) and bronchoalveolar lavage (BAL) protein (D). α-Tubulin was used as loading control except in (D).
Time Course of Pulmonary Fibrosis in AdGRE Animals
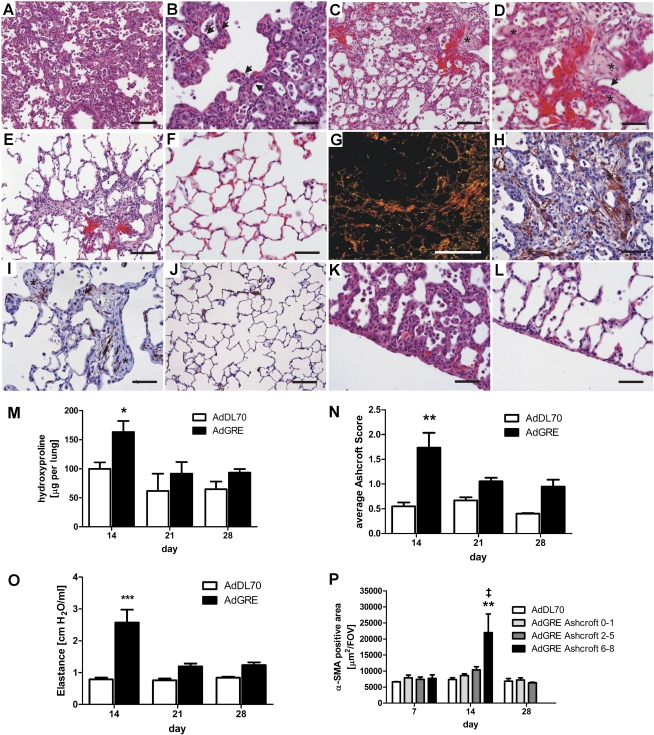
After administration of AdGRE to rat lungs, we observed alveolar septal thickening and activation of AEC, as well as inflammatory cell infiltration at Day 7 (Figures 2A and 2B). In AdDL70-treated animals, no inflammation or fibrosis was seen at any time point, similar to previously published data (Figure 2F) (26). At Day 14, multiple fibroblastic foci showed evidence of collagen deposition in PSR (Figure 2G) and accumulation of α–smooth muscle actin (SMA)+ myofibroblasts (Figure 2H), but also the presence of hyperplastic and activated AEC in the fibrotic regions (Figures 2C and 2D). We detected increased α-SMA positivity in severely fibrotic areas (Ashcroft 6–8) of AdGRE animals at this time point (Figure 2P). Lung collagen content, as measured by hydroxyproline levels (Figure 2M) and Ashcroft score (Figure 2N), were significantly increased at Day 14. This correlated with pathological lung physiology, as indicated by elevated lung elastance (Figure 2O). At Day 21, lung collagen yield was already declining (Figures 2M and 2N), α-SMA levels of the lung normalized (Figures 2I and 2P), and lung physiology improved (Figure 2O). Lung histology and immunohistochemistry showed focal accumulation of scar tissue and fibroblastic foci–like structures, with minimal, if any, α-SMA+ cells (Figures 2E and 2I). There were no further changes between Days 21 and 28 (Figures 2M–2P). The initial process extended toward the visceral pleura, and a moderate transient increase in pleura thickness, associated with cellular infiltration and activation of mesothelial cells, was found (Figures 2K and 2L).
Figure 2.
Transient pulmonary fibrosis after AdGRE. AdGRE induces transient pulmonary fibrosis in the rodent lung. At Day 7 (A and B), hyperplastic, injured epithelial cells (arrows in [B]) are visible within thickened alveolar walls together with cellular infiltration. At Day 14 (C and D), multiple fibroblastic foci (FF) are found (asterisks in [C and D]). At Day 21 (E), residual scar tissue is detected. No cellular infiltration or matrix deposition was seen in lungs of AdDL70 animals at Day 14 (F). Picro-Sirius red (PSR)–stained section shows collagen deposition at Day 14 (G). At Day 14, activated, α–smooth muscle actin (SMA)+ myofibroblasts are found within FF (asterisks in [H]), whereas FF are almost α-SMA free at Day 21 (asterisk in [I]). Normal α-SMA content in AdDL70 animals ([J] Day 14). α-SMA staining is seen sparely, mainly in vascular structures and isolated cells, and in alveolar walls. Changes and injury extend to the pleura at Day 7 (K), and residual damage is still seen at Day 21 (L). Transiently increased collagen content at Day 14 is confirmed by increased lung hydroxyproline (M) and elevated average Ashcroft score (N). Lung elastance is also increased at Day 14 (O). Quantification of α-SMA+ area revealed increased α-SMA+ area in Ashcroft 6–8 areas at Day 14(P). Please note that there were no Ashcroft 6–8 areas at Day 28 in the AdGRE group. Each column represents means (±SEM) of four to six animals per group. *P < 0.05, **P < 0.01, ***P < 0.0001 versus AdDL70; ‡P < 0.01 versus AdGRE Ashcroft 0–1. (A–F and K–L) hematoxylin and eosin (H&E) staining; (G) PSR; (H–J) α-SMA immunohistochemistry (IHC). Original magnification: (G) 25×; (A, C, E, and J) 200×; (B, D, F, H, I, K, and L) 400×. Scale bar, 200 μm (G), 100 μm (A, C, E, and J), 50 μm (B, D, F, H, I, K, and L).
BALF Differential Cell Count
We detected elevated total cell counts in BALF of AdGRE animals as compared with AdDL70 animals (Table 1). The majority of cells were macrophages/monocytes, but AdGRE-treated animals also showed a relevant elevation of neutrophil counts at Day 7 (Table 1).
TABLE 1.
BRONCHOALVEOLAR LAVAGE FLUID DIFFERENTIAL CELL COUNTS
| Day 7 AdGRE | AdDL70 | Day 14 AdGRE | AdDL70 | Day 21 AdGRE | AdDL70 | Day 28 AdGRE | AdDL70 | |
|---|---|---|---|---|---|---|---|---|
| Total cells | 141.43 ± 24.25 | 9.71 ± 0.82 | 46.0 ± 8.04 | 7.60 ± 1.88 | 25.75 ± 5.88 | 5.38 ± 0.69 | 17.20 ± 2.944 | 8.50 ± 1.04 |
| Macrophages | 85.86 ± 14.59 | 9.10 ± 0.77 | 40.34 ± 6.64 | 7.33 ± 1.72 | 22.30 ± 5.09 | 5.09 ± 0.63 | 16.07 ± 2.73 | 8.01 ± 1.02 |
| Neutrophils | 49.64 ± 9.64 | 0.16 ± 0.08 | 0.19 ± 0.11 | 0.12 ± 0.10 | 0.11 ± 0.07 | 0.002 ± 0.0006 | 0.016 ± 0.006 | 0.003 ± 0.001 |
| Lymphocytes | 5.49 ± 0.98 | 0.43 ± 0.075 | 4.19 ± 1.55 | 0.15 ± 0.074 | 1.75 ± 0.60 | 0.23 ± 0.067 | 0.71 ± 0.16 | 0.41 ± 0.044 |
Defintition of abbreviations: AdGRE, adenoviral vectors encoding mouse Gremlin; AdDL70, empty control vector.
Values presented are n × 104 cells/ml, and represent mean ± SEM values.
BMP Signaling after Gremlin Overexpression
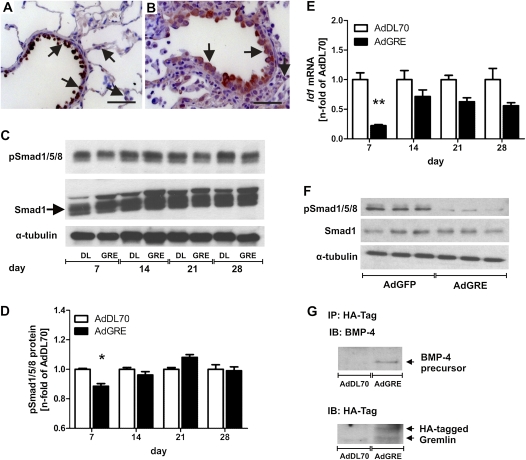
Smad1/5/8 phosphorylation was transiently reduced in the lungs of AdGRE rats by Day 7 as compared with AdDL70 animals. It was back to normal levels at Day 14, and the reduction appears to have been mainly localized to epithelial cells (Figures 3A–3D). In addition, the BMP-dependent gene, Id1, was down-regulated in the lung after AdGRE, predominantly at Day 7 (Figure 3E). The inhibitory effect of overexpressing Gremlin on BMP-specific Smad phosphorylation was also verified in a simple lung epithelial cell A549 culture 72 hours after AdGRE (Figure 3F). In addition, an endogenous 50-kD BMP-4 precursor protein was coimmunoprecipitated with HA-tagged Gremlin in lung protein lysate of AdGRE animals (Figure 3G), suggesting that overexpressed Gremlin may interact with BMP-4 and inhibit BMP-4 activity. Coimmunoprecipitation experiments with BMP-7 did not reveal an intracellular interaction between BMP-7 or its precursor molecule and Gremlin (data not shown).
Figure 3.
Bone morphogenic protein (BMP) signaling and GRE–BMP-4 interaction. Reduced pSmad1/5/8 level in AdGRE animals at Day 7 (B and C) as compared with AdDL70 animals (A and C) by IHC and Western blot of lung protein lysate. The total Smad1 double band is indicated by an arrow in (C). pSmad1+ epithelial cells are outlined by arrows in (A and B). Please note the reduced overall staining intensity in the AdGRE animal (B) as compared with AdDL70 (A). (D) Semiquantitative densitometry of pSmad1/5/8 protein normalized to α-tubulin, expressed as n fold of pSmad1/5/8 protein versus AdDL70. (E) Reduced Id1 mRNA expression in lung homogenate of AdGRE animals, indicating reduced BMP-4 signaling, expressed as n fold mRNA versus AdDL70. (F) Reduced pSmad1/5/8 protein level in total protein lysate of A549 72 hours after AdGRE administration as compared with AdGFP (control). (G) Co-immunoprecipitation (IP) HA-tagged Gremlin and BMP-4 showing that an approximately 50-kD BMP-4 precursor protein interacts with Gremlin in AdGRE rat lungs at Day 7. α-Tubulin was used as loading control in (C and F). (A and B) Original magnification, 400×; scale bar, 20 μm. (D and E) Each bar represents the mean (±SEM) of three to six animals per group. *P < 0.05, **P < 0.01 versus AdDL70. IB, immunoblot.
TGF-β Expression and Signaling in AdGRE Animals
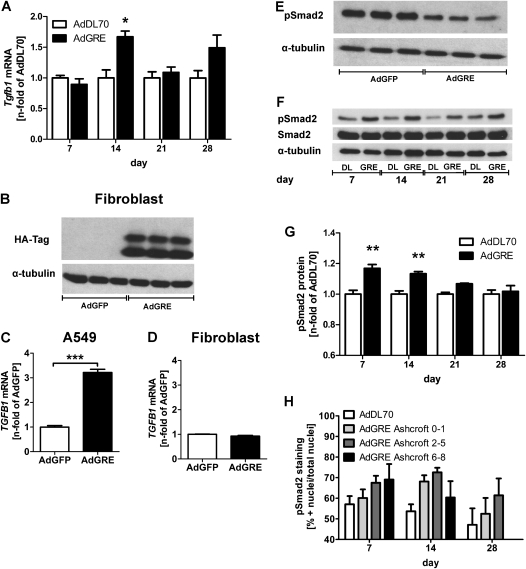
Consistent with the pathological changes shown in Figure 2, there was a transient induction of Tgfb1 mRNA expression 14 days after AdGRE, but there was no significant elevation at later time points (Figure 4A). To determine whether Gremlin has a regulatory role in TGFB1 expression, the level of TGFB1 expression at the mRNA level was then analyzed in cultured human lung cells. Interestingly, AdGRE-infected lung epithelial cells (A549) had a threefold increase in TGFB1 mRNA at 72 hours, whereas AdGRE-infected primary human lung fibroblasts did not have such change after Gremlin overexpression (Figures 4C and 4D), although the levels of transgenic protein produced by both cell types were similar (Figures 1A and 4B). Furthermore, phosphorylated Smad (pSmad) 2 content of whole-cell lysate of A549 cells was reduced after AdGRE transfection (Figure 4E). In contrast, TGF-β–specific Smad2 phosphorylation in lung protein lysate was transiently elevated at Days 7 and 14, but declined at Days 21 and 28 after AdGRE administration (Figures 4F and 4G). Using colocalization algorithms and quantitative immunofluorescence, we detected a nonsignificant trend toward increasing percentage of pSmad2+ nuclei, with rising degree of fibrosis at Day 7, but also a gradual decline after Day 14, especially in highly fibrotic regions (Ashcroft 6–8), in AdGRE animals (Figure 4H).
Figure 4.
Transforming growth factor (Tgfb1) mRNA expression and signaling, (A) Tgfb1 mRNA expression in lung homogenate of AdGRE-treated rats as compared with AdDL70 animals. Each column represents the mean (±SEM) of four to six animals per group. *P < 0.05 versus AdDL70. (B) Representative detection of transgenic (HA-tagged) Gremlin in stimulated primary lung fibroblasts (n = 3 per group). α-Tubulin was used as loading control. (C and D) TGFB1 mRNA expression in A549 cells (C) and primary human lung fibroblasts (D) after 72 hours of AdGRE versus AdGFP. ***P < 0.0001 versus AdGFP. (E) Representative Western blot showing reduced pSmad2 protein levels in AdGRE transfected A549 cells. α-Tubulin as loading control. (F) Representative Western blot of pSmad2, Smad2, and α-tubulin (loading control) in protein lysate of AdGRE lungs versus AdDL70. (G) Densitometry of pSmad2 in AdGRE versus AdDL70. Each bar represents the mean (±SEM) of n fold of AdDL70, two to five animals per group. **P < 0.01 versus AdDL70. (H) Percentage of pSmad2+ nuclei versus total nuclei in AdDL70 lungs and AdGRE lungs in areas of high interstitial damage (Ashcroft 6–8), moderate interstitial damage (2–5), and no changes (0–1). Each bar represents the mean (±SEM) of three to six animals per group.
Apoptosis and Proliferation
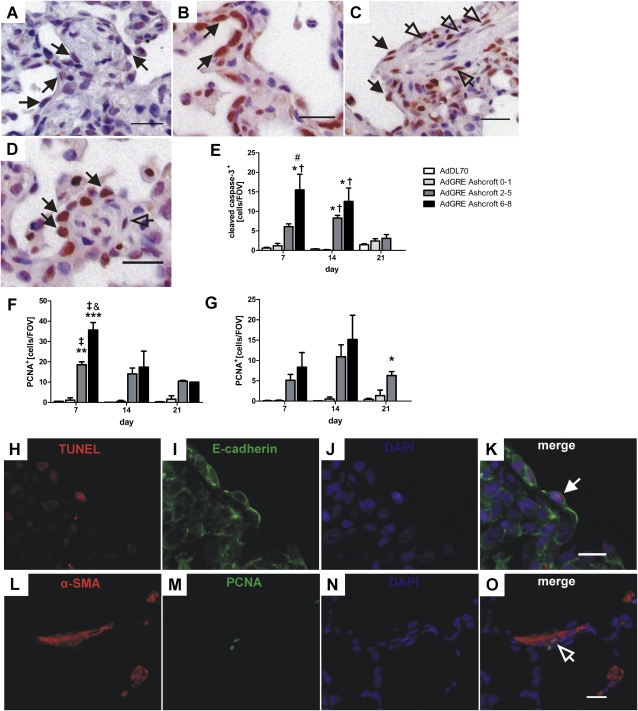
We found relevant levels of apoptotic (cleaved caspase-3+ or terminal deoxynucleotidyl transferase dUTP nick end labeling [TUNEL]+/E-cadherin+) epithelial cells with cuboid AEC type 2 (AEC2) or hyperplastic appearance at Days 7 and 14 after AdGRE administration (Figures 5A, 5E, and 5H–5K). Using further double immunostaining, we have demonstrated that apoptotic AECs were both AEC type 1 (AEC1) (TUNEL+/T1α+) and AEC2 (TUNEL+/Surfactant protein-C) (see Figure E1 in the online supplement). These apoptotic epithelial cells were mainly found around fibroblastic foci (Figure 5A), and their numbers increased gradually with rising degree of fibrosis (Figure 5E). The level of epithelial apoptosis was almost back to normal at Day 21 after AdGRE exposure (Figure 5E).
Figure 5.
Apoptosis and proliferation in AdGRE. (A) IHC for cleaved caspase-3, demonstrating representative apoptotic epithelial cells (black arrows) in AdGRE rat lung at Day 14. (B–D) IHC for proliferating cell nuclear antigen (PCNA), demonstrating representative proliferating epithelial cells (black arrows) at Day 7 (B) and 14 (C and D) and representative proliferating fibroblasts (arrows) at Day 14 (C and D). Counterstaining with Mayer's hematoxylin. Original magnification, 400× (A–D); scale bar, 20 μm (A–D). (E–G) Quantification of cleaved caspase-3+ epithelial cells (E), PCNA+ epithelial cells (F), and PCNA+ fibroblasts (G), according to degree of interstitial damage in AdGRE-treated animals as compared with AdDL70 rats. Each bar represents the mean (±SEM) of two to three animals per group. *P < 0.05, **P < 0.01, ***P < 0.0001, all versus AdDL70; †P < 0.05, ‡P < 0.01, both versus AdGRE Ashcroft 0–1; #P < 0.05, and P < 0.01, both versus AdGRE Ashcroft 2–5. (H–K) Double immunofluorescence for E-cadherin (green) and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL; red), indicating a representative apoptotic epithelial cell (white arrow) in AdGRE rat lung at Day 7. (L–O) Double immunofluorescence for α-SMA (red) and PCNA (green) to demonstrate a proliferating myofibroblast (arrow). Nuclear counterstaining with 4′-6′-diamidino-2-phenylindole (DAPI (H–O). Original magnification, 400× (H–O); scale bar, 10 μm (K and O).
Hyperplastic epithelial cells were also frequently positive for the proliferation marker, proliferating cell nuclear antigen (PCNA) (Figures 5B–5D). Proliferation, as quantified by counting the number of PCNA+ cuboid (AEC2) or hyperplastic epithelial cells, was highest at Day 7, and decreased afterward (Figure 5F). Additional double immunostaining revealed that proliferating AECs were positive for AEC1 (PCNA+/T1α+) or AEC2 (PCNA+/SP-C+) markers (Figure E1). The amount of proliferating epithelial cells correlated with the fibrotic score, and highest levels were present in severely fibrotic areas (Ashcroft 6–8) (Figure 5F). Proliferating cells were found overlying thickened alveolar walls, as well as around fibroblastic foci (Figures 5B–5D).
Proliferating (PCNA+) fibroblasts occurred delayed as compared with injured epithelium, and the number of PCNA+ fibroblasts peaked at Day 14, and was also directly related to the degree of surrounding fibrosis (Figure 5G). PCNA+ fibroblasts were mostly found in and around fibroblastic foci, and were mainly α-SMA+ myofibroblasts, as demonstrated by double immunofluorescence for PCNA and α-SMA (Figures 5C, 5D, and 5L–5O).
Expression of FGFs in AdGRE
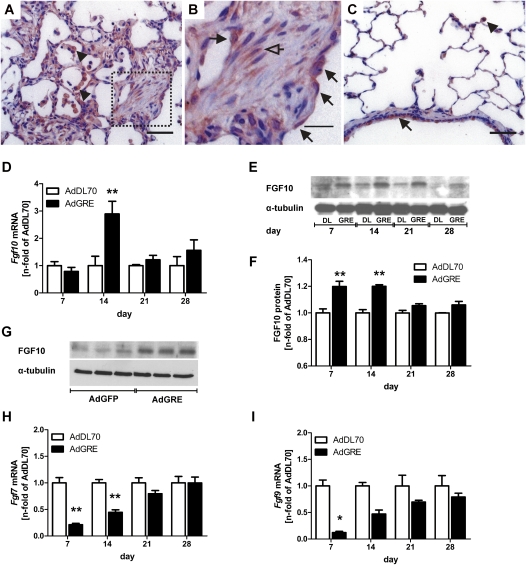
FGF10 was elevated by Day 14 (mRNA) or Days 7 and 14 (Western blot), and the expression was localized to epithelial cells in fibrotic regions, predominantly around fibroblastic foci, and some fibroblasts (Figures 6A–6F). In AdDL70-treated lungs, FGF10 expression was almost exclusively detected in airway and alveolar epithelial cells and alveolar macrophages (Figure 6C). We were able to increase existing FGF10 expression in A549 by transfection with AdGRE (Figure 6G). In contrast to FGF10, FGF-7, and -9 were both found transiently down-regulated at Days 7 and 14 after AdGRE administration (Figures 6H and 6I).
Figure 6.
Expression of fibroblast growth factor (FGF)–7, FGF9 and FGF10 in AdGRE. A–C: Representative FGF10 IHC in AdGRE animal at Day 14 (A and B) compared with AdDL70 Day 14 (C). (B) Boxed area from (A) enlarged. Expression is found in epithelial cells (black arrows), fibroblasts (arrows) and alveolar macrophages (arrowheads) in AdGRE lungs, and in epithelial cells (black arrows) and alveolar macrophages (arrowheads) in AdDL70 lungs. (D) Fgf10 mRNA expression in lung homogenate of AdGRE-treated rats as compared with AdDL70 animals. Each column represents the mean (±SEM) of four to six animals per group. **P < 0.01 versus AdDL70. (E) Representative Western blot of FGF10 protein expression in AdGRE- and AdDL70-treated rats. α-Tubulin was used to ensure equal loading. (F) Densitometric quantification of FGF10 protein content in AdGRE versus AdDL70 rats. Each bar represents the mean (±SEM) of n fold of AdDL70, two to five animals per group. **P < 0.01 versus AdDL70. (G) Representative Western blot of FGF10 protein expression in A549 cell transfected with AdGRE or AdGFP. α-tubulin was used to ensure equal loading. (H and I) Fgf7 (H) and Fgf9 (I) mRNA expression in lung homogenate of AdGRE-treated rats compared with AdDL70 animals. Each column represents the mean (±SEM) of four to six animals per group. *P < 0.05, **P < 0.01 versus AdDL70.
DISCUSSION
IPF is a devastating disease of the lung parenchyma, with a complex underlying pathobiology and no efficacious therapy (1, 2). Various pathways with possible therapeutic relevance are being investigated, and recent publications suggest that the BMP antagonist, Gremlin, could be an interesting and novel target (7). Gremlin belongs to the Cerberus/Dan family of binding proteins with antagonistic activity for BMP signaling (8). Human IPF lungs exhibit elevated Gremlin levels, as do mouse lungs with experimental asbestosis. Gremlin has been implicated in promoting myofibroblast differentiation, likely through inhibition of BMP-induced fibroblast apoptosis and participation in EMT (9). The detailed physiological and pathophysiological roles of Gremlin are still unclear. Our study is the first to investigate the selective overexpression of Gremlin in adult rat lungs using adenovirus-mediated gene transfer. We show that transient Gremlin overexpression results in alveolar epithelial injury, followed by modest lung tissue fibrosis that is partly reversible. We found accumulating α-SMA+ myofibroblasts arranged in fibroblastic foci, as well as apoptotic and proliferating AECs in fibrotic regions. Fibroblast proliferation occurred slightly delayed in the fibrotic areas. All these changes were declining after Day 14. This reversal was associated with elevated production of the epithelium-protective factor, FGF10, in fibrotic areas.
Various growth factors and mediators are involved in progressive pulmonary fibrosis, among them TGF-β—especially through the Smad2/3 signaling pathway, platelet-derived growth factor, and several chemokines (6, 27). Using adenovirus-mediated gene transfer, our group has identified two mediators, the selective overexpression of which results in progressive lung fibrosis: TGF-β and IL-1β (28, 29). In contrast, a larger number of factors does not lead to a persistent fibrotic phenotype using the same approach (e.g., TNF-α, connective tissue growth factor, or bleomycin) (26, 30, 31). Similar to these experiments, overexpression of the BMP antagonist Gremlin results in transient lung fibrosis, as shown by an increase in lung collagen content and enhanced lung stiffness at Day 14. Gremlin inhibits the BMP-4–Smad1/5/8 signaling pathway, which has important functions during lung development. Specifically, BMP-4 decreases epithelial proliferation and promotes distal differentiation of epithelial cells (32, 33). In line with this observation, we found activated, hyperplastic alveolar epithelium at Days 7 and 14 after AdGRE. Relevant numbers of these cells were positive for PCNA or cleaved caspase-3, indicating active proliferation and apoptosis. BMP signaling was decreased in AdGRE rat lungs at Day 7, correlating with the time of highest proliferation and apoptosis, but we also detected a trend toward a transient induction of pSmad1/5/8 at Day 21. This might be related to fibrosis resolution and epithelial repair found after Day 14 in this model. In addition, we also demonstrate a physical intracellular interaction between overexpressed Gremlin and a BMP-4 precursor molecule, but not BMP-7, similar to what has been described during lung development (22, 34). These data point toward activation of the epithelium with subsequent apoptosis and proliferation after Gremlin-induced BMP inhibition. It is interesting that there were more proliferating than apoptotic epithelial cells. These results are consistent with those of previous reports indicating that Gremlin can promote proliferation of epithelial tumor cells (35). In addition, we found an increase in inflammatory cells in BALF, with a significant elevation of neutrophil numbers, possibly related to release of neutrophil chemoattractants from apoptotic epithelial cells (36, 37).
In addition to effects on epithelial cells, Gremlin has been suggested to inhibit BMP-related mesenchymal cell apoptosis and thereby promote fibroblast proliferation (9). In our study, epithelial effects precede myofibroblast proliferation and their accumulation in fibroblastic foci. These findings peaked by Day 14 before decreasing and returning to near normal. This delay between epithelial and mesenchymal responses may be due to reduced BMP-4 secretion from epithelial cells, with subsequent effects on fibroblast proliferation. It could also be explained by epithelial cell–derived TGF-β1 due to direct or indirect effects of Gremlin. Our in vitro and in vivo data show elevated TGF-β1 production in AdGRE-treated A549 cells, and transiently enhanced TGF-β signaling in lung tissue of AdGRE rats. The earlier increase in Smad2 phosphorylation as compared with TGF-β1 gene expression could be related to the transgenic overexpression at Day 7, and we have shown similar discrepancies before (25). This indicates a potential role for Gremlin in epithelial–mesenchymal crosstalk during lung injury, wound repair, and fibrosis.
Epithelial cells are believed to be a source of myofibroblasts in fibrotic lung disease via EMT (5). Koli and colleagues (9) have shown that overexpression of Gremlin in A549 cells can promote TGF-β–induced EMT through accelerated down-regulation of the epithelial adhesion molecule, E-cadherin.
We have reported before that experimental models of reversible fibrosis, such as bleomycin injury in Balb/c mice or overexpression of connective tissue growth factor and TGFb3, are associated with a lack of persistent up-regulation of endogenous TGFb1 and tissue inhibitor of metalloproteinases-1 (26, 38–40). The only transient increase in TGF-β and tissue inhibitor of metalloproteinases-1 was also seen in the model described here, and provides possible explanations for the reversible nature of Gremlin-induced lung fibrosis. In addition, TGF-β signaling was inhibited in our in vitro experiments, despite elevated TGF-β production, indicating that Gremlin overexpression could also activate TGF-β antagonistic mediators. However, the time course of enhanced TGF-β signaling might not be the only explanation for the limitation of the fibrotic process after Day 14. The phenotypic changes of AEC and the appearance of myofibroblasts suggest involvement of epithelium-protective factors, such as the FGF family. We investigated expression levels of FGF7, -9, and -10. FGF9 may not be directly involved in epithelial regeneration like FGF7 and -10, but it can positively regulate FGF10 expression (12, 13). After AdGRE, Fgf7 and -9 mRNA was decreased from Day 7 to Day 14, and normalized thereafter. The reduced expression of these factors might be a reflection of epithelial activation and subsequent fibrotic injury. In addition, the close correlation between transiently increased TGF-β signaling and decreased Fgf7 points toward antagonistic effects of TGF-β on FGF7 (41). In contrast to low Fgf7 gene expression, we found selective up-regulation of FGF10 after AdGRE, which likely contributes to the maintenance of structural integrity in this model. FGF10 exerts strong protective effects on alveolar epithelium, and has a pivotal role in maintaining epithelial progenitor cell proliferation during development (14–16). Furthermore, epithelium-specific overexpression of FGF10 attenuates pulmonary fibrosis induced by bleomycin, likely through stabilization of AEC2 (18). This seems to be mediated, at least in part, through reduction of TGF-β expression and/or activity (18). FGF10 up-regulation might also be involved in the decreased Smad2 phosphorylation of A549 under Gremlin overexpression. We believe that enhanced expression of FGF10 in this disease model is induced through Gremlin-mediated inhibition of BMP signaling, similarly to a Gremlin–BMP–FGF feedback loop that has been characterized in limb bud growth (42). Although reduction in Fgf10 levels is likely related to fibrogenesis, Fgf10 expression normalizes too late to primarily account for resolution of fibrosis or epithelial restoration. Whereas some contribution of Fgf10 is likely, FGF10 is, due to its early increase, a more promising candidate to explain the relatively fast epithelial regeneration and partial resolution of fibrosis in the AdGRE model.
In conclusion, our data indicate that Gremlin-mediated BMP inhibition is involved in activation of epithelial cells, with proliferation dominating over apoptosis and EMT. Adenovirus-mediated overexpression of Gremlin induces transient lung fibrosis, with myofibroblast and collagen accumulation in the lung, which is likely related to epithelial injury, but also local fibroblast proliferation. The selective up-regulation of FGF10, together with epithelial proliferation in response to Gremlin-mediated BMP inhibition, suggests that Gremlin may be part of the physiological repair process of the lung epithelium: inhibition of epithelial BMP signaling leads to proliferation, but also apoptosis. TGF-β production from epithelial cells after BMP inhibition or epithelial apoptosis leads to myofibroblast accumulation to support injury repair. Inhibition of BMP signaling results in up-regulation of FGF10 to limit epithelial injury and promote regeneration. The previously described elevation of Gremlin in IPF tissue may be an expression of continuing epithelial injury, and Gremlin may be a reflection of the ongoing repair mechanisms.
Supplementary Material
Acknowledgments
The authors acknowledge the expert technical help of Fuqin Duan, Xueya Feng, Mary Jo Smith, Mary Bruni, Jennifer Wattie, and Jane Ann Smith. Laszlo Farkas has a research fellowship from the German Research Foundation, and Martin Kolb holds a Canadian Institutes for Health Research New Investigator Award.
This work was supported by the Canadian Institutes for Health Research (“Pathogenic Mechanisms in Persistent Pulmonary Fibrosis”), German Research Foundation grant FA841/2-1 (L.F.), and National Institutes of Health/National Heart, Lung, and Blood Institute grant HL68597 (W.S.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2010-0070OC on January 7, 2011
Author Disclosure: J.G. received consultancy fees from GlaxoSmithKline for $5,001–$10,000 and a sponsored grant for $50,001–$100,000, and sponsored grants from the National Institutes of Health (NIH) and the Canadian Institutes for Health Research for more than $100,001 each. M.K. received consultancy fees from GlaxoSmithKline for $5,001–$10,000 and a sponsored grant for more than $100,001. W.S. received a sponsored grant from NIH for more than $100,001. None of the other authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med 2000;161:646–664. [DOI] [PubMed] [Google Scholar]
- 2.Kim R, Meyer KC. Review: therapies for interstitial lung disease: past, present and future. Ther Adv Respir Dis 2008;2:319–338. [DOI] [PubMed] [Google Scholar]
- 3.Visscher DW, Myers JL. Histologic spectrum of idiopathic interstitial pneumonias. Proc Am Thorac Soc 2006;3:322–329. [DOI] [PubMed] [Google Scholar]
- 4.Selman M, Pardo A. Role of epithelial cells in idiopathic pulmonary fibrosis: from innocent targets to serial killers. Proc Am Thorac Soc 2006;3:364–372. [DOI] [PubMed] [Google Scholar]
- 5.Willis BC, duBois RM, Borok Z. Epithelial origin of myofibroblasts during fibrosis in the lung. Proc Am Thorac Soc 2006;3:377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ask K, Martin GE, Kolb M, Gauldie J. Targeting genes for treatment in idiopathic pulmonary fibrosis: challenges and opportunities, promises and pitfalls. Proc Am Thorac Soc 2006;3:389–393. [DOI] [PubMed] [Google Scholar]
- 7.Selman M, Pardo A, Kaminski N. Idiopathic pulmonary fibrosis: aberrant recapitulation of developmental programs? PLoS Med 2008;5:e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Topol LZ, Marx M, Laugier D, Bogdanova NN, Boubnov NV, Clausen PA, Calothy G, Blair DG. Identification of drm, a novel gene whose expression is suppressed in transformed cells and which can inhibit growth of normal but not transformed cells in culture. Mol Cell Biol 1997;17:4801–4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koli K, Myllarniemi M, Vuorinen K, Salmenkivi K, Ryynanen MJ, Kinnula VL, Keski-Oja J. Bone morphogenetic protein–4 inhibitor Gremlin is overexpressed in idiopathic pulmonary fibrosis. Am J Pathol 2006;169:61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Myllarniemi M, Lindholm P, Ryynanen MJ, Kliment CR, Salmenkivi K, Keski-Oja J, Kinnula VL, Oury TD, Koli K. Gremlin-mediated decrease in bone morphogenetic protein signaling promotes pulmonary fibrosis. Am J Respir Crit Care Med 2008;177:321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braun S, auf dem Keller U, Steiling H, Werner S. Fibroblast growth factors in epithelial repair and cytoprotection. Philos Trans R Soc Lond B Biol Sci 2004;359:753–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colvin JS, White AC, Pratt SJ, Ornitz DM. Lung hypoplasia and neonatal death in Fgf9-null mice identify this gene as an essential regulator of lung mesenchyme. Development 2001;128:2095–2106. [DOI] [PubMed] [Google Scholar]
- 13.Ware LB, Matthay MA. Keratinocyte and hepatocyte growth factors in the lung: roles in lung development, inflammation, and repair. Am J Physiol Lung Cell Mol Physiol 2002;282:L924–L940. [DOI] [PubMed] [Google Scholar]
- 14.Upadhyay D, Bundesmann M, Panduri V, Correa-Meyer E, Kamp DW. Fibroblast growth factor–10 attenuates h2o2-induced alveolar epithelial cell DNA damage: role of MAPK activation and DNA repair. Am J Respir Cell Mol Biol 2004;31:107–113. [DOI] [PubMed] [Google Scholar]
- 15.Upadhyay D, Panduri V, Kamp DW. Fibroblast growth factor-10 prevents asbestos-induced alveolar epithelial cell apoptosis by a mitogen-activated protein kinase–dependent mechanism. Am J Respir Cell Mol Biol 2005;32:232–238. [DOI] [PubMed] [Google Scholar]
- 16.Ramasamy SK, Mailleux AA, Gupte VV, Mata F, Sala FG, Veltmaat JM, Del Moral PM, De Langhe S, Parsa S, Kelly LK, et al. FGF10 dosage is critical for the amplification of epithelial cell progenitors and for the formation of multiple mesenchymal lineages during lung development. Dev Biol 2007;307:237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sekine K, Ohuchi H, Fujiwara M, Yamasaki M, Yoshizawa T, Sato T, Yagishita N, Matsui D, Koga Y, Itoh N, et al. FGF10 is essential for limb and lung formation. Nat Genet 1999;21:138–141. [DOI] [PubMed] [Google Scholar]
- 18.Gupte VV, Ramasamy SK, Reddy R, Lee J, Weinreb PH, Violette SM, Guenther A, Warburton D, Driscoll B, Minoo P, et al. Overexpression of fibroblast growth factor–10 during both inflammatory and fibrotic phases attenuates bleomycin-induced pulmonary fibrosis in mice. Am J Respir Crit Care Med 2009;180:424–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hokuto I, Perl AK, Whitsett JA. FGF signaling is required for pulmonary homeostasis following hyperoxia. Am J Physiol Lung Cell Mol Physiol 2004;286:L580–L587. [DOI] [PubMed] [Google Scholar]
- 20.Jimenez PA, Rampy MA. Keratinocyte growth factor–2 accelerates wound healing in incisional wounds. J Surg Res 1999;81:238–242. [DOI] [PubMed] [Google Scholar]
- 21.Igarashi M, Finch PW, Aaronson SA. Characterization of recombinant human fibroblast growth factor (FGF)–10 reveals functional similarities with keratinocyte growth factor (FGF-7). J Biol Chem 1998;273:13230–13235. [DOI] [PubMed] [Google Scholar]
- 22.Shi W, Zhao J, Anderson KD, Warburton D. Gremlin negatively modulates BMP-4 induction of embryonic mouse lung branching morphogenesis. Am J Physiol Lung Cell Mol Physiol 2001;280:L1030–L1039. [DOI] [PubMed] [Google Scholar]
- 23.Bett AJ, Haddara W, Prevec L, Graham FL. An efficient and flexible system for construction of adenovirus vectors with insertions or deletions in early regions 1 and 3. Proc Natl Acad Sci USA 1994;91:8802–8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ashcroft T, Simpson JM, Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol 1988;41:467–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farkas L, Farkas D, Ask K, Moller A, Gauldie J, Margetts P, Inman M, Kolb M. VEGF ameliorates pulmonary hypertension through inhibition of endothelial apoptosis in experimental lung fibrosis in rats. J Clin Invest 2009;119:1298–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonniaud P, Margetts PJ, Kolb M, Haberberger T, Kelly M, Robertson J, Gauldie J. Adenoviral gene transfer of connective tissue growth factor in the lung induces transient fibrosis. Am J Respir Crit Care Med 2003;168:770–778. [DOI] [PubMed] [Google Scholar]
- 27.Kelly M, Kolb M, Bonniaud P, Gauldie J. Re-evaluation of fibrogenic cytokines in lung fibrosis. Curr Pharm Des 2003;9:39–49. [DOI] [PubMed] [Google Scholar]
- 28.Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor–beta1 induces prolonged severe fibrosis in rat lung. J Clin Invest 1997;100:768–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kolb M, Margetts PJ, Anthony DC, Pitossi F, Gauldie J. Transient expression of IL-1beta induces acute lung injury and chronic repair leading to pulmonary fibrosis. J Clin Invest 2001;107:1529–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sime PJ, Marr RA, Gauldie D, Xing Z, Hewlett BR, Graham FL, Gauldie J. Transfer of tumor necrosis factor–alpha to rat lung induces severe pulmonary inflammation and patchy interstitial fibrogenesis with induction of transforming growth factor–beta1 and myofibroblasts. Am J Pathol 1998;153:825–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borzone G, Moreno R, Urrea R, Meneses M, Oyarzun M, Lisboa C. Bleomycin-induced chronic lung damage does not resemble human idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2001;163:1648–1653. [DOI] [PubMed] [Google Scholar]
- 32.Bellusci S, Henderson R, Winnier G, Oikawa T, Hogan BL. Evidence from normal expression and targeted misexpression that bone morphogenetic protein (BMP-4) plays a role in mouse embryonic lung morphogenesis. Development 1996;122:1693–1702. [DOI] [PubMed] [Google Scholar]
- 33.Weaver M, Yingling JM, Dunn NR, Bellusci S, Hogan BL. BMP signaling regulates proximal–distal differentiation of endoderm in mouse lung development. Development 1999;126:4005–4015. [DOI] [PubMed] [Google Scholar]
- 34.Sun J, Zhuang FF, Mullersman JE, Chen H, Robertson EJ, Warburton D, Liu YH, Shi W. BMP4 activation and secretion are negatively regulated by an intracellular Gremlin–BMP4 interaction. J Biol Chem 2006;281:29349–29356. [DOI] [PubMed] [Google Scholar]
- 35.Sneddon JB, Zhen HH, Montgomery K, van de Rijn M, Tward AD, West R, Gladstone H, Chang HY, Morganroth GS, Oro AE, et al. Bone morphogenetic protein antagonist Gremlin 1 is widely expressed by cancer-associated stromal cells and can promote tumor cell proliferation. Proc Natl Acad Sci USA 2006;103:14842–14847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lawson WE, Polosukhin VV, Stathopoulos GT, Zoia O, Han W, Lane KB, Li B, Donnelly EF, Holburn GE, Lewis KG, et al. Increased and prolonged pulmonary fibrosis in surfactant protein C–deficient mice following intratracheal bleomycin. Am J Pathol 2005;167:1267–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sato E, Koyama S, Masubuchi T, Takamizawa A, Kubo K, Nagai S, Izumi T. Bleomycin stimulates lung epithelial cells to release neutrophil and monocyte chemotactic activities. Am J Physiol Lung Cell Mol Physiol 1999;276:L941–L950. [DOI] [PubMed] [Google Scholar]
- 38.Ask K, Bonniaud P, Maass K, Eickelberg O, Margetts PJ, Warburton D, Groffen J, Gauldie J, Kolb M. Progressive pulmonary fibrosis is mediated by TGF-β isoform 1 but not TGF-β3. Int J Biochem Cell Biol 2008;40:484–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kolb M, Bonniaud P, Galt T, Sime PJ, Kelly MM, Margetts PJ, Gauldie J. Differences in the fibrogenic response after transfer of active transforming growth factor-β1 gene to lungs of “fibrosis-prone” and “fibrosis-resistant” mouse strains. Am J Respir Cell Mol Biol 2002;27:141–150. [DOI] [PubMed] [Google Scholar]
- 40.Gauldie J, Bonniaud P, Sime P, Ask K, Kolb M. TGF-β, Smad3 and the process of progressive fibrosis. Biochem Soc Trans 2007;35:661–664. [DOI] [PubMed] [Google Scholar]
- 41.Zhang F, Nielsen LD, Lucas JJ, Mason RJ. Transforming growth factor-β antagonizes alveolar type II cell proliferation induced by keratinocyte growth factor. Am J Respir Cell Mol Biol 2004;31:679–686. [DOI] [PubMed] [Google Scholar]
- 42.Verheyden JM, Sun X. An FGF/Gremlin inhibitory feedback loop triggers termination of limb bud outgrowth. Nature 2008;454:638–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.