Abstract
IFN-γ expression increases during the inflammatory response after bleomycin injury in mice. IFN-γ deficiency attenuates lung inflammation and fibrosis. Because IFN-γ stimulates class II transactivator (CIITA) expression, which activates major histocompatibility class (MHC) II and represses collagen expression, it was hypothesized that CIITA mediates IFN-γ action after bleomycin injury. To test this hypothesis, two CIITA mouse lines, one carrying a mutation of the leucine-rich region of CIITA (CIITA C−/−) and one with a deletion extending into the GTP-binding domain (CIITA G−/−), were used. IFN-γ treatment of lung cells isolated from both strains of mice induced mutant CIITA expression, which did not activate MHC II transcription. Collagen expression was similar in both mutant mouse strains and comparable to C57BL/6 (wild-type) mice. When mice were exposed to intratracheal bleomycin, both strains of CIITA mutant mice retained body weight and altered inflammation at 14 days after bleomycin injury compared with bleomycin-treated wild-type mice. However, there was no difference in fibrosis as judged by histology, mRNA, and protein expression of lungs. Bronchoalveolar lavage cells from CIITA C−/− and C57BL/6 lungs were examined at 3, 7, and 14 days after bleomycin injury. CD4 mRNA expression in bronchoalveolar lavage cells was down-regulated, whereas IL-4 and IL-10 expression was up-regulated, in CIITA C−/− mice, indicating a diminished, skewed Th2 response. The expression of IFN-γ was the same in all mice tested. Combined, our data suggest that CIITA mutations altered the immune response without affecting fibrosis.
Keywords: collagen, CIITA, IFN-γ, fibrosis, bleomycin
Idiopathic pulmonary fibrosis is a progressive disease characterized by alveolar epithelial cell injury and hyperplasia, inflammatory cell accumulation, myofibroblast hyperplasia, deposition of extracellular matrix (ECM), and scar formation within the lung (1). The progression in human disease is not known because when patients seek medical treatment for symptoms the disease process is generally advanced, with little evident inflammation. Bleomycin-induced injury in the lungs is a well established model for studying the progression of pulmonary fibrosis (2).
Class II transactivator (CIITA/NLR1) is the first recognized member of the NLR family (3, 4). Mutations in CIITA cause the immunodeficiency bare lymphocyte syndrome (5), in which patients have no major histocompatibility class II (MHC II) proteins, resulting in increased susceptibility to infection. CIITA, referred to as a master regulator of MHC II, is the only member of the NLR family that functions in the nucleus as a transcription regulator. CIITA has several isoforms that are constitutively expressed in immune cells in addition to being induced by IFN-γ in multiple nonimmune cell types, including fibroblasts and smooth muscle cells, where it activates MHC II expression (6, 7). CIITA represses several genes in macrophages and dendritic cells such as IL-4 (8), IL-10 (9), E-cathepsin (10), MMP-9 (11), plexin (12), various thyroid-specific genes (13), and FasL (14). Most importantly, CIITA represses collagen transcription in mesenchymal cells through the N-terminal acidic domain and the proline/serine/threonine (PST) domain of CIITA (15, 16).
Mice with CIITA mutations have low levels of MHC II (17, 18). Our studies indicate that mice (herein referred to as CIITA C−/−) with a C-terminal deletion of the leucine-rich region (LRR) are hypomorphic mutants that express lowered amounts of a truncated CIITA protein, which loses its function as an activator of MHC II but maintains its function as a repressor of collagen transcription (6). A second mouse with a deletion in the GTP binding domain (herein referred to as CIITA G−/−) had reduced MHC II expression (17) and greater susceptibility to infection (19). Because IFN-γ contributes to bleomycin-induced lung inflammation and fibrosis (20–22) and CIITA is activated by IFN-γ, CIITA could be a mediator in bleomycin-induced disease. To examine the role of CIITA, CIITA mutant mice and C57BL/6 control mice were subjected to intratracheal bleomycin instillation. Combined, our data suggest that CIITA mutations altered the immune response to bleomycin injury without affecting fibrosis.
MATERIALS AND METHODS
Mice
Wild-type (WT) (C57BL/6) and CIITA hypomorphic mutant (C2tatm1Ccum, herein referred to as CIITA C−/−) mice (6, 18) were obtained from Jackson Laboratory (Bar Harbor, ME). The CIITA mutant mouse (17) (herein referred to as CIITA G−/−) was obtained from Dr. Jenny Ting (University of North Carolina). All mice were genotyped with published primers, and homozygous mice were used for experiments.
Plasmids
The col1a2-luciferase construct (pH20) (23) contains sequences from −221 to +54 bp of mouse col1a2 promoter fused to the luciferase reporter gene. The MHC II promoter fused to the luciferase reporter gene was a gift from Dr. Jenny Ting (12).
Bleomycin Experiments
Adult (8-wk-old) CIITA or C57BL/6 mice were anesthetized with isoflurane and treated with bleomycin (0.1 U/100 μL saline/20 g mouse) or saline (0.14 M NaCl) by intratracheal instillation as described previously (24). Animals were killed at 3 to 14 days after bleomycin instillation. Mice were weighed at time points indicated after instillation. Lungs were prepared as described previously (24). Approximately 10 to 20 sections were prepared from each block by serial sectioning. When appropriate, the right lung was placed in RNALater (Ambion, Foster City, CA) and processed for RNA.
Bronchoalveolar Lavage Cell Analysis
After anesthetizing mice, bronchoalveolar lavage (BAL) were collected using two 1-ml aliquots of PBS. Cells were centrifuged and resuspended in 1 ml PBS. Cells were pelleted and used for RNA isolation, and the BAL fluid (BALF) was used to analyze active TGF-β.
Active BALF TGF-β Analysis
In brief, 2 × 104 mink lung epithelial cells stably transfected with a plasminogen activator inhibitor-1 promoter–luciferase construct plated at a density of 2 × 104 per 96-well plate in complete DMEM until adherent (∼3 h) (25). BALF (100 μl) or hrTGF-β (50–1,000 pg/ml) standard was added to each well with media without serum in triplicate and incubated at 37°C for 20 hours. Cell lysates were read on an auto-injecting plate reader (BioTek, Winooski, VT) using 100 μL of luciferase assay substrate (Promega, Madison , WI). The average luciferase unit of BALF replicates were translated into active TGF-β concentrations using the average of standard triplicates and reported as picograms per milliliter.
Collagen Extraction and Sircol Assay for Collagenous Protein
Lungs were minced, homogenized, and subjected to neutral salt extraction in RIPA buffer (50 mM Tris [pH 7.5], 150 mM NaCl, 1% NP-40, 0.5% deoxycholate, and protease inhibitors) for 24 hours with agitation by rotation at 4°C. Homogenates were pelleted by centrifugation, extracted in 0.5 N acetic acid, and stirred for 24 hours at 4°C. An equivalent amount of supernatant, relevant to the mass of tissue used in the extraction, or rat-tail collagen (standard) was precipitated and quantified using Sircol reagent (Biocolor, Carrickfergus, UK) per the manufacturer's protocol.
Statistical Analysis
Data are presented as mean values ± SE. Statistical analyses of parametric group data were performed by ANOVA with post hoc comparisons by the method of Scheffé. A probability value of P < 0.05 was considered significant. Nonparametric group data (disease scores, histology) were performed using the Mann-Whitney test.
RESULTS
Collagen Gene Expression Is Repressed by IFN-γ WT and CIITA-Deficient Cells
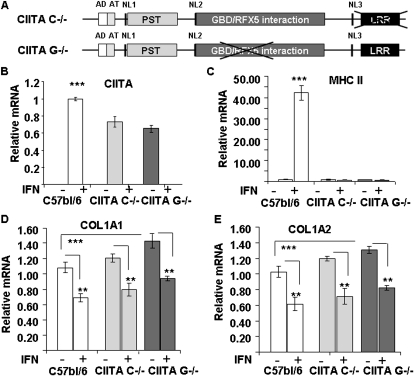
Our earlier results (15, 26) suggest that CIITA is critical for IFN-γ–induced repression of collagen transcription by human lung fibroblasts, yet the CIITA C−/− deficient mice with a deletion of the 13th and 14th exons coding for the C-terminal LRR domain (18) (Figure 1A) produced a truncated CIITA protein that maintains its ability to repress collagen (6). A second mouse line (kindly supplied by Dr. Jenny Ting), called CIITA G−/−, with a mutation in the CIITA gene spanning the GTP-binding domain, was examined because it was reported that these mice show no detectable CIITA mRNA (17). To test whether these mice are true knockouts, lung fibroblasts were isolated from homozygote CIITA G−/− and compared with lung fibroblasts from WT and homozygote CIITA C−/− mice. Experiments were performed using cells between the third and sixth passages. When cells reached confluence, they were treated with and without IFN-γ and analyzed for CIITA, MHC II, and collagen gene expression using real-time PCR. Real-time PCR was able to detect IFN-γ–induced CIITA mRNA in lung fibroblasts with no significant difference between the two CIITA-deficient fibroblasts using previously described primers (6) between the 5th and 6th exons upstream of the GDP binding domain deletion (Figure 1A). The genotype of these animals indicated that they were homozygous for the GTP domain deletion. There was significantly (25%) more CIITA steady-state mRNA expressed by WT fibroblasts than either of the CIITA mutant cell lines (Figure 1B).
Figure 1.
Expression of class II transactivator (CIITA), major histocompatibility class (MHC II), and collagen type I mRNA in wild-type (WT) and CIITA hypomorphic mice. (A) Diagram of the CIITA mutation in CIITA C−/− (top) and CIITA G−/− mouse DNA (bottom). The CIITA repression domains of collagen are acidic domain (AD) and proline/serine/threonine (PST) domain (light gray). The leucine-rich region (LRR) (black) is deleted in the CIITA C−/− mouse, and the GDP binding domain (dark gray) is deleted in the CIITA G−/− mouse. (B–E) Lung fibroblast cells isolated from WT C57BL/6 (white bars), CIITA C−/− (light gray bars), or CIITA G−/− (dark gray bars) were treated with or without IFN-γ as indicated. The mRNA levels of CIITA (B), HLA-DRα (MHC II) (C), col1a1 (D), and col1a2 (E) were determined by real-time PCR. Data are presented as relative mRNA levels compared with WT samples performed in triplicate (mean ± SD). Statistical analysis by ANOVA with post hoc comparisons by Scheffé. **P < 0.01; ***P < 0.001. A representative experiment out of three repeats is shown. Cell isolation and tranfection methods as described in online supplement.
IFN-γ activated MHC II expression in WT lung fibroblasts but did not activate MHC II expression in CIITA C−/− or CIITA G−/− lung fibroblasts (Figure 1C). On the other hand, IFN-γ reduced collagen type I expression by approximately 40% in all lung fibroblasts (Figures 1D and 1E). There was significantly more collagen mRNA expression in the CIITA G−/− deficient cells than in the C57BL/6 cells.
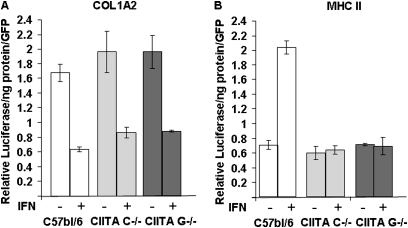
IFN-γ Represses the Collagen Promoter but Does Not Activate the MHC II Promoter in Lung Fibroblasts Isolated from CIITA C−/− and CIITA G−/− Mice
A CIITA molecule with a GTP-binding domain deletion was hypothesized to repress collagen promoter activity but not to activate the MHC II promoter, similar to the CIITA molecule with the C-terminal deletion in the LRR, because the repression domains are in the N-terminal portion of the molecule (6, 15). To test this hypothesis, lung fibroblasts from C57BL/6, CIITA C−/−, and CIITA G−/− cells were transfected with COL1A2 or MHC II promoter luciferase constructs and treated with and without IFN-γ. IFN-γ repressed collagen promoter activity but failed to activate MHC II promoter activity in CIITA C−/− and CIITA G−/− cells (Figure 2). There was no difference between the two CIITA mutant cell lines, suggesting that both lines expressed mutant CIITA that could repress collagen without activation of MHC II.
Figure 2.
IFN-γ represses a collagen promoter activity but does not activate MHC II promoter activity in CIITA hypomorphic mice. (A) Collagen promoter (col1a2) or (B) an MHC II promoter (DRA300) was transfected in triplicate, along with a GFP plasmid, into lung fibroblast cells isolated from WT C57BL/6 (white bars), CIITA C−/− (light gray bars), or CIITA G−/− (dark gray bars), followed by treatment with IFN-γ for 24 hours. Luciferase activities were normalized for protein concentration and GFP fluorescence. A representative experiment out of three repeats is shown. Cell isolation and transfection methods as described in online supplement.
CIITA Hypomorphic Mice Have Attenuated Weight Loss after Bleomycin Exposure
To understand the role of CIITA during inflammation and fibrosis, mice were challenged with intratracheal instillation of bleomycin. Because IFN-γ deficiency attenuates bleomycin-induced lung inflammation (22, 27) and induces CIITA expression in lung fibroblast cells, experiments were performed to directly evaluate whether CIITA mediates pulmonary inflammatory response to bleomycin using both lines of CIITA hypomorphic mice and C57BL/6 control mice. Four experiments were conducted with four or five mice per group. Mice were weighed at instillation and several times before harvest at 14 days. The control mice lost significantly (P < 0.01) more weight than either of the CIITA hypomorphic lines of mice 14 days after bleomycin treatment (Table 1). At this dosage of bleomycin, there were no differences in mortality among groups.
TABLE 1.
WEIGHT AND SURVIVAL RATE BEFORE AND 14 DAYS AFTER BLEOMYCIN TREATMENT
| Mouse strain | Exposure | n | Pre-exposure Weight (g) | Post-exposure Weight (g) | Survival |
|---|---|---|---|---|---|
| C57bl/6 | Bleomycin | 15 | 20.7 ± 1.0 | 16.2 ± 0.9 ** | 17/19 |
| Saline | 9 | 22.5 ± 1.2 | 24.5 ± 1.1 | 11/11 | |
| CIITA C-/- | Bleomycin | 15 | 22.6 ± 1.1 | 20.3 ± 1.1 | 15/18 |
| Saline | 14 | 23.4 ± 0.8 | 24.6 ± 0.9 | 11/11 | |
| CIITA G-/- | Bleomycin | 12 | 20.5 ± 0.6 | 20.0 ± 0.7 | 12/14 |
| Saline | 10 | 21.5 ± 0.8 | 22.1 ± 0.9 | 10/10 |
Results are presented as mean ± SE. Weight data were analyzed by one-way ANOVA with post comparisons by the method of Scheffé **P > 0.01.
CIITA Hypomorphic Mice Have Attenuated Lung Disease at 14 Days after Bleomycin Injury
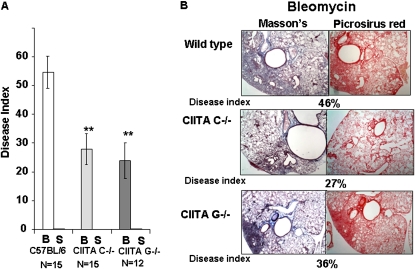
Lungs were examined for the pathologic response to bleomycin after 14 days. Sections of lungs were stained with Masson's trichrome stain or with picrosirius red, which stains all collagenous proteins (Figure 3B). The disease index, an estimate of the proportion of the lung that is diseased, was used to describe the extent of the disease process (Figure 3A) (28). This process was performed by two investigators on Masson's stained sections. The disease index takes into consideration the percentage of lung area involved with mononuclear cell infiltration, interstitial thickening, distortion of lung architecture, and abnormal ECM. The CIITA mutant mice had significantly fewer patches of interstitial thickening and alveolar inflammation with less architectural distortion, typically involving an average of 23 to 27% of lung area. This was significantly less (P = 0.002) than the more widespread inflammatory changes that were present in C57BL/6 lungs (54% of lung area) (Figure 3A). The saline-treated mice showed no discernable inflammation or distortion of lung architecture (data not shown). This supports that hypothesis that CIITA deficiency attenuates bleomycin response.
Figure 3.
CIITA hypomorphic mice have less disease but equivalent fibrosis compared with WT mice. (A) Histological sections from the right caudal, left central, and left hilar regions of lung were coded using a quantitative scale (0–100%) of lung area involved with abnormal cellular infiltration, interstitial thickening, or architectural distortion of native lung architecture. The average disease index (± SE) was graphed for bleomycin (B) and saline (S) lungs from C57BL/6 (white bars), CIITA C−/− (light gray bars), and CIITA G−/− (dark gray bars). N = the number of lungs analyzed. Data were analyzed by nonparametric group data using Mann-Whitney test. (B) Representative histological sections of bleomycin-treated lungs stained with Masson's trichrome (left panels) and picrosirus red (right panels). Masson's trichrome = blue, ECM; red, cells. Picrosirus = red, all types of collagen.
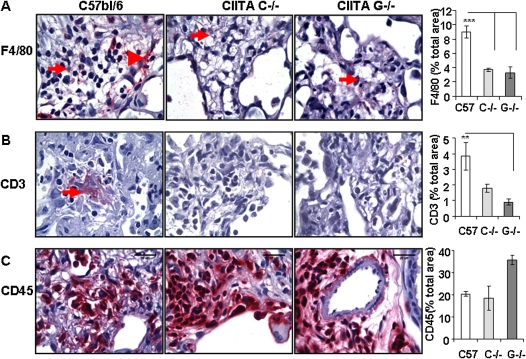
CIITA Mutant Mice Have Fewer Macrophages and T Cells in Lungs than Control Mice
Lungs were immunostained with antibodies specific for activated macrophage (F4/80), T cell (CD3), and leukocyte common antigen (CD45) cell surface markers and counterstained with hematoxylin. Pictures were taken of all tissue on the slide, and the red stain and total area of lung on each slide was quantified using Image-Pro Plus software (MediaCybermetrics, Bethesda, MD). F4/80+ macrophages were clearly detected in the bleomycin-treated lungs (Figure 4A). There were strongly stained macrophages in fibrotic areas of WT lung (Figure 4A, arrowhead) and within the interstitial regions that were missing in the CIITA mutant lungs. The CIITA mutant bleomycin-treated lungs had significantly less staining of F4/80+ macrophages, which correlated with the disease scores. There were few detectable CD3-stained T cells in the CIITA mutant bleomycin-treated lungs, although there was visible staining (3–6%) in the C57BL/6 bleomycin-treated lungs (Figure 4B). On the other hand, there was strong CD45 staining of all lungs, especially surrounding blood vessels and capillaries (Figure 4C). CD45+ cells were present in saline-treated lungs, and recruitment of CD45+ cells increased after bleomycin injury in all strains of mice, with no significant differences between WT and CIITA mutant mice. Recruitment of CD45+ cells was equivalent, although there were reductions in the T-cell and macrophage subsets analyzed.
Figure 4.
CIITA hypomorphic mice have fewer T cells and macrophages in bleomycin-treated lungs compared with WT mice. Representative histological sections of bleomycin-treated lungs stained with antibody to (A) F4/80 (1:50), (B) CD3 (1:100), and (C) CD45 (1:250). Red staining material is antibody stain. Slides were counterstained with hematoxylin. Nine pictures covering the entire slide were analyzed for red and total lung area using Image-pro plus. The average percent red ± SE from three or four animals was graphed (right panels). Statistical analysis by ANOVA with post hoc comparisons by Scheffé. **P < 0.05.
CIITA Mutant Mice Have Equivalent Fibrosis and Collagen to Control Mice at 14 Days after Bleomycin Injury
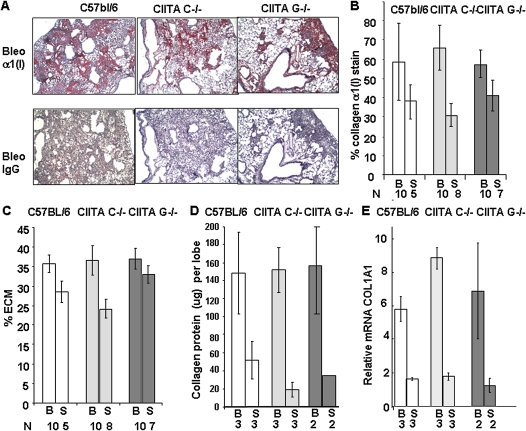
To detect alterations in ECM, higher-magnification images of lungs stained with Masson's trichrome were analyzed for blue ECM staining using Image-Pro Plus. Bleomycin-treated lungs contained more ECM than untreated lungs; however, there were no significant differences in the amount of ECM between CIITA mutant and control animals (Figure 5C). Sections stained for collagen with picrosirius red, which measures all collagenous proteins, also showed no difference in the amount of collagen between bleomycin-treated C57BL/6, CIITA C−/−, or CIITA G−/− lungs (data not shown). Adjacent sections were incubated with collagen α1(I) antibody and counterstained with hematoxylin (Figure 5A). Analysis of the slides indicated that there was 56 to 63% collagen type I in the bleomycin-treated lungs from all three strains of mice (Figure 5B). Saline-treated lungs contained 37 to 41% collagen type I. These data suggest that bleomycin-induced fibrosis in was similar at 14 days in all strains of mice.
Figure 5.
Fibrosis is not altered in CIITA hypomorphic mice. (A) Representative histological sections of bleomycin (Bleo)-treated lungs stained with antibody to collagen α1(I) (1:200) and counterstained with hematoxylin. Red staining is collagen. (B) A graph of percent collagen by antibody staining in bleomycin- (B) and saline-treated (S) lungs at 14 days after bleomycin injury. Six pictures of each lung were analyzed. N = number of lungs. (C) A graph of percent ECM from Masson's stained slides in bleomycin- (B) and saline-treated (S) lungs at 14 days after bleomycin injury. Six pictures of each lung were analyzed. N = the number of lungs analyzed. (B and C) C57BL/6 (white bars); CIITA C−/− (light gray bars); CIITA G−/− (dark gray bars). (D) Proteins were acid extracted from lungs 14 days after bleomycin (B) or saline (S) instillation as described in Materials and Methods. Collagen was measured by Sircol assay, which measures all collagenous proteins. (E) RNA was extracted from lungs 14 days after bleomycin (B) or saline (S) treatment, and COL1A1 mRNA was measured and normalized to 18S RNA. Each sample was measured in triplicate. In D E, data were analyzed by ANOVA with post hoc comparisons by the method of Scheffé.
To analyze whether newly synthesized uncrosslinked collagen content was similar in CIITA mutant mice compared with control mice, proteins from lungs were extracted from bleomycin- and saline-treated mice at 14 days after bleomycin injury. Collagen content in the left lobe was measured by the Sircol method, which is specific for all collagenous proteins. Collagen increased upon bleomycin treatment. However, there were no differences in the amount of acid-extracted collagen among CIITA mutant and control mice (Figure 5D). To determine whether collagen mRNA increased, mRNA was extracted from the right lobe of lung tissue. The mRNA for collagen increased at 14 days after bleomycin treatment. There was no difference in collagen mRNA produced among the two CIITA mouse strains compared with WT mice (Figure 5E). This supports the morphological data that no attenuation of fibrosis occurs as a result of CIITA mutations.
On the other hand, MHC II expression was not detected by qRT-PCR (CT values ranged from 35 to 40) in CIITA mutant mice, whereas MHC II expression was detected in C57BL/6 mice (CT values in the 20s), suggesting that the CIITA mutations were loss-of-function mutations for MHC II transcription activation but not for collagen repression.
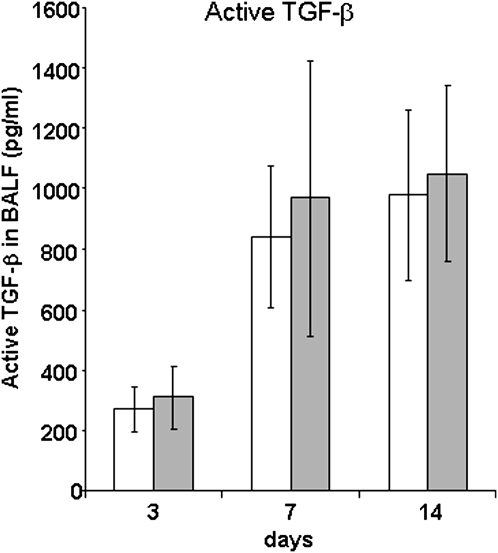
CIITA Mutant Mice Have Equivalent Amounts of Active TGF-β in BALF at all Time Points
TGF-β, which can be measured in BALF, plays an important role in fibrosis (29) and collagen transcription in vivo (24). Because IFN-γ down-regulates TGF-β during fibrosis (30), which could be mediated by CIITA, active TGF-β1 concentrations in BALF from CIITA lungs were compared with WT mice at 3, 7, and 14 days after treatment with saline or bleomycin. There were marked, yet equivalent, increases in BALF TGF-β1 in bleomycin-treated mice (Figure 6A) at 7 and 14 days after bleomycin injury, in keeping with the unaltered fibrosis of these CIITA mutant strains of mice. RNA extracted from lungs after BAL cell removal was measured for TGF-β content at each time point and in saline-treated lungs. The steady-state level of TGF-β was similar in all stains of mice (data not shown).
Figure 6.
CIITA hypomorphic mouse has equivalent amounts of active BALF TGF-β and lung TGF-β expression to WT mice. BALF was collected from WT (white bars) or CIITA C−/− (gray bars) mice at 3, 7, and 15 days and added to mink lung epithelial cells as described in Materials and Methods. Luciferase activity was measured in triplicate and plotted against a standard curve to generate TGF-β concentrations (pg/ml). Triplicate concentrations of TGF-β for WT (white bars) or CIITA C−/− mice (dark gray bars) were combined for each time point (n = 5).
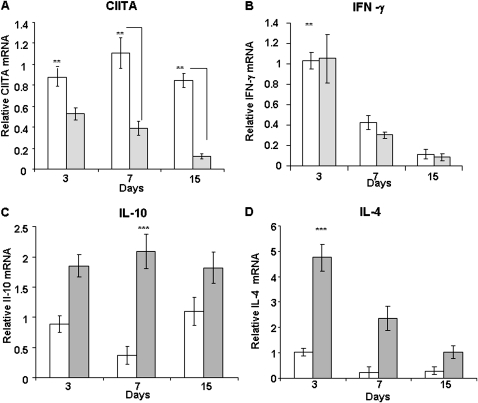
Expression of CIITA in BAL Cells Is High in Control Mice, whereas the Mutant CIITA Decreases with Time after Bleomycin Treatment
Inflammatory cells, primarily macrophages, lymphocytes, and neutrophils (25), accumulate in BALF after bleomycin treatment. Because CIITA is constitutively expressed in most inflammatory cells, BAL cells were collected and counted at 3, 7, and 15 days after bleomycin treatment at time of death. Because CIITA G−/− mice were indistinguishable from CIITA C−/− mice, for this time study we focused on the commercially available CIITA C−/− and C57BL/6 control mice (five animals per group). There were equivalent numbers of BAL cells in the fluid at 3 and 7 days. However, at 15 days after bleomycin treatment, there were more cells recovered from the C57BL/6 (40.8 × 106 ± 11.3 cells) compared with the CIITA C−/− (18.1 × 106 ± 9.9 cells) mice. In the normal C57BL/6 mice, there was high expression of CIITA at each time point (Figure 7A). Consistent with our previous findings in fibroblasts, a truncated form of CIITA mRNA was expressed by BAL cells from CIITA C−/− mice. This form of CIITA mRNA was present at approximately 40% of normal levels in 3-day BAL cells and was further decreased with time after bleomycin treatment.
Figure 7.
CIITA hypomorphic mouse BAL cells express higher levels of IL-4 and IL-10 cytokines than in WT mice. BAL cells collected from WT (white bars) or CIITA C−/− (gray bars) mice at 3, 7, and 15 days as described in Materials and Methods. RNA was extracted from BAL cells and analyzed by QRT-PCR in triplicate for (A) CIITA, (B) IFN-γ, (C) IL-10, and (D) IL-4 mRNA levels. One representative experiment out of three is shown. WT (white bars) or CIITA C−/− (gray bars); n = 5 animals per group. Data were analyzed by ANOVA with post hoc comparisons by the method of Scheffé.
In addition to constitutively expressed CIITA, in cells such as B-cells, CIITA expression is also up-regulated by IFN-γ. Therefore, IFN-γ expression was measured in BAL cells. There was equivalent expression of IFN-γ in control and CIITA mutant BAL cells that decreased with time after bleomycin injury (Figure 7B). The time course of IFN-γ expression was similar to that of the expression of mutant CIITA, but WT CIITA expression was higher throughout the time course.
IL-4 and IL-10 Were Highly Expressed by CIITA Mutant BAL Cells, but their Temporal Expression Differed
CIITA may play a significant role in the transcription of cytokines, such as IL-10 and IL-4, expressed by Th2 cells (9, 31). Because CIITA has been demonstrated to repress these cytokines (32), steady-state mRNA expression levels of cytokines were evaluated in BAL cells at different times after bleomycin treatment. IL-10 expression in CIITA C−/− BAL cells was significantly elevated above WT at every time point and did not decrease with time, similar to the expression of WT CIITA mRNA (Figure 7C). IL-4 expression in CIITA mutant BAL cells was significantly higher than WT cells at 3 days and decreased with time after bleomycin treatment (Figure 7D), similar to IFN-γ mRNA expression. Overall, IL-4 and IL-10 expression was higher in CIITA mutant BAL cells compared with WT cells.
Because it has been reported that CIITA mutant mice have fewer CD4 T cells in circulation (18) and because bleomycin injury primarily recruits CD4 cells (27), CD4 expression in BAL cells and remaining lung tissue was measured (Figure E1, see the online supplement.). There was significantly less CD4 mRNA expression at every time point in BAL cells or lung tissue in all treatments by CIITA C−/− mice compared with C57BL/6 control mice.
DISCUSSION
Our earlier publications (6, 15, 26, 33, 34) demonstrate that IFN-γ–induced CIITA in fibroblasts activates MHC II and represses collagen transcription. Collagen repression is primarily through the N-terminal portion of the molecule in an acidic domain and in the PST domain (15, 16, 33). The PST domain interacts with co-repressor molecules on the collagen promoter in a phosphorylation-dependent manner and suppresses CIITA activity (33, 35). A CIITA C−/− hypomorphic mouse containing a deletion of the C-terminal LRR domain of CIITA maintains the ability to repress collagen gene expression but has a loss-of-function for activating MHC II expression (6). Our results demonstrate that the CIITA G−/− mouse containing the targeted deletion of the GTP-binding domain in CIITA (17) has a very similar phenotype to the CIITA C−/− mouse in a bleomycin-induced pulmonary fibrosis model. Although the original research (17) claims that there is no mutant CIITA mRNA, the mutant CIITA mRNA is expressed by cultured lung fibroblasts (Figure 1), BAL cells (Figure 7), and lung tissue extracted from these mice (data not shown). A small amount of mutant CIITA protein with predicted size is detectable from IFN-γ–treated fibroblast cell extracts (data not shown), which may be sufficient to repress collagen transcription during inflammation and IFN-γ stimulation. Cultured lung fibroblast cells with mutant CIITA maintain the ability to repress collagen but lose the ability to activate MHC II, as judged by mRNA expression (Figure 1), protein (data not shown), and transcription assays (Figures 2). This suggests that the CIITA mutant mice after injury have altered inflammatory responses due to the loss of MHC II with little change in fibrogenesis.
IFN-γ null mice have attenuated bleomycin disease, although there is controversy as to whether IFN-γ deficiency alters fibrosis (22, 27). Because CIITA is induced in a variety of cell types, including lung fibroblasts upon stimulation by IFN-γ, we hypothesized that CIITA may mediate changes in the lungs during bleomycin injury. In support of this hypothesis, both strains of CIITA mutant mice had lower weight loss, fewer deaths, and lower disease scores on histological examination of bleomycin-diseased lungs than C57BL/6 control mice, similar to IFN-γ–deficient mice. There were fewer T cells and macrophages present in bleomycin-diseased CIITA mutant lungs. Attenuation of disease may be due to altered inflammatory cell response as a result of incapacitated CIITA.
Inflammatory cells, primarily monocyte/macrophages along with lymphocytes and neutrophils, accumulate after bleomycin injury (25). This process is not altered by CIITA mutations because CD45-stained cells are equivalent (Figure 4C). Monocytes differentiate into proinflammatory macrophages (M1) through a classical pathway involving IFN-γ stimulation (36). A subset of macrophages becomes MHC II producing APC cells between 3 to 5 days after bleomycin treatment when IFN-γ is produced (37). Antiinflammatory macrophages (M2) are alternatively activated by IL-4 (and IL-13), whereas IL-10 deactivates macrophages at later stages. The CIITA mutant mice had no detectable MHC II mRNA in bleomycin-treated lungs. BAL cells produced higher amounts of IL-4 and IL-10 after bleomycin treatment (9, 31). Most importantly, there are fewer F4/80+ macrophages in bleomycin-treated lungs in the CIITA mutant mice even though recruitment of CD45+ blood cells is equivalent. The damaged WT lungs have cells with high levels of F4/80, which are missing in the CIITA damaged hypomorphic lungs (Figure 4A). Other researchers have demonstrated that there are F4/80 high-expressing macrophages that produce higher levels of MHC II and F4/80 low-expressing macrophages that express IL-4 (38). The CIITA hypomorphic mice may have a less activated subpopulation of macrophages in bleomycin-treated lungs.
IFN-γ is a cytokine that is produced primarily by CD4+ T-helper Th1 cells during bleomycin injury along with natural killer and a few B cells (22). Investigators have postulated that polarized T cells regulate organ fibrosis, with the CD4 Th2 cells being considered profibrotic (39, 40). CIITA deficiency increases IL-10 (9) and IL-4 (31), lowers CD4 T-cell population (18), and drives Th1 to Th2 differentiation (41). Our results confirm that CD4 expression in BAL cells and in lung are lower (data are provided in the online supplement), with fewer CD3+ T cells (Figure 4B) in the CIITA mutant fibrotic lung than in control lungs.
Most importantly, although CIITA hypomorphic diseased lungs have lower disease scores, fewer inflammatory BAL cells, and decreased accumulation of macrophages and T cells in lungs, there was equivalent fibrosis, collagen deposition, collagen protein, and collagen mRNA compared with WT lungs at 15 days (Figure 5). There is significant up-regulation of IL-10 and IL-4 levels in CIITA hypomorphic mice after bleomycin instillation up to 14 days, with no significant change in overall fibrosis (Figures 5 and 7). This is intriguing because, although it is generally agreed that IL-10 and IL-4 promote an antiinflammatory agenda during bleomycin-induced pulmonary fibrosis, controversy remains regarding their roles in the fibrogenic process (42, 43). The discrepancy could be attributed to different genetic background of animals used, the time window of observation (14 versus 28 d), and the dosages of bleomycin (and hence the magnitude of the initial inflammatory response). IL-4 by itself fails to stimulate collagen production in lung fibroblast cells, suggesting that IL-4 probably affects fibrogenesis in an indirect manner, likely through stimulating TGF-β from alveolar epithelial cells (44). Because TGF-β levels did not vary significantly in BALF or the lungs (Figure 6), it is reasonable to postulate that up-regulated IL-4 levels are insufficient to cause additional TGF-β release. Finally, pulmonary fibrosis progresses in a milieu of cytokines and growth factors.
In summary, although CIITA mutant mice had altered immune responses to bleomycin injury, there was no change in fibrosis in these mice, suggesting that their altered responses are not coupled to lung fibrosis. Alternatively, because these mice do produce small quantities of IFN-γ–induced mutant CIITA with the collagen repression domain, we do not preclude the possibility that the mutant form of CIITA actively represses collagen in vivo just as well as the WT CIITA. These data suggest that there may be forms of CIITA that repress collagen synthesis without altering the immune response. It is of great interest to determine if the repression domain of CIITA will alter the fibrotic process without increasing the immunological profile in idiopathic pulmonary fibrosis.
Supplementary Material
Acknowledgments
of the authors thank Maureen Guiney and Yedan Li For technical assistance, Dr. Jenny Ting for providing mice, and Dr. Matthew Layne for suggestions and critical reading of this manuscript. Dr. Xu is now at the Atherosclerosis Research Center, Department of Pathophysiology, Nanjing Medical University, Nanjing, Jiangsu 210029, China.
This work was supported by NIH grant R01 HL68094 (B.D.S.) and American Heart Association postdoctoral training grant 0525981T (Y.X.).
Originally Published in Press as DOI: 10.1165/rcmb.2009-0416OC on August 12, 2010
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Moore BB, Hogaboam CM. Murine models of pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 2008;294:L152–L160. [DOI] [PubMed] [Google Scholar]
- 2.Lotze MT, Zeh HJ, Rubartelli A, Sparvero LJ, Amoscato AA, Washburn NR, Devera ME, Liang X, Tor M, Billiar T. The grateful dead: damage-associated molecular pattern molecules and reduction/oxidation regulate immunity. Immunol Rev 2007;220:60–81. [DOI] [PubMed] [Google Scholar]
- 3.Wilmanski JM, Petnicki-Ocwieja T, Kobayashi KS. NLR proteins: integral members of innate immunity and mediators of inflammatory diseases. J Leukoc Biol 2008;83:13–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harton JA, Linhoff MW, Zhang J, Ting JP. Cutting edge: CATERPILLER: a large family of mammalian genes containing CARD, pyrin, nucleotide-binding, and leucine-rich repeat domains. J Immunol 2002;169:4088–4093. [DOI] [PubMed] [Google Scholar]
- 5.Reith W, Mach B. The bare lymphocyte syndrome and the regulation of MHC expression. Annu Rev Immunol 2001;19:331–373. [DOI] [PubMed] [Google Scholar]
- 6.Xu Y, McDonald J, Perloff E, Buttice G, Schreiber BM, Smith BD. Collagen and major histocompatibility class II expression in mesenchymal cells from CIITA hypomorphic mice. Mol Immunol 2007;44:1720–1732. [DOI] [PubMed] [Google Scholar]
- 7.Buttice G, Miller J, Wang L, Smith BD. Interferon-gamma induces major histocompatibility class II transactivator (CIITA), which mediates collagen repression and major histocompatibility class II activation by human aortic smooth muscle cells. Circ Res 2006;98:472–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou X, Jiang Y, Lu L, Ding Q, Jiao Z, Zhou Y, Xin L, Chou KY. MHC class II transactivator represses human IL-4 gene transcription by interruption of promoter binding with CBP/p300, STAT6 and NFAT1 via histone hypoacetylation. Immunology 2007;122:476–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yee CS, Yao Y, Xu Q, McCarthy B, Sun-Lin D, Tone M, Waldmann H, Chang CH. Enhanced production of IL-10 by dendritic cells deficient in CIITA. J Immunol 2005;174:1222–1229. [DOI] [PubMed] [Google Scholar]
- 10.Yee CS, Yao Y, Li P, Klemsz MJ, Blum JS, Chang CH. Cathepsin E: a novel target for regulation by class II transactivator. J Immunol 2004;172:5528–5534. [DOI] [PubMed] [Google Scholar]
- 11.Nozell S, Ma Z, Wilson C, Shah R, Benveniste EN. Class II major histocompatibility complex transactivator (CIITA) inhibits matrix metalloproteinase-9 gene expression. J Biol Chem 2004;279:38577–38589. [DOI] [PubMed] [Google Scholar]
- 12.Wong AW, Brickey WJ, Taxman DJ, van Deventer HW, Reed W, Gao JX, Zheng P, Liu Y, Li P, Blum JS, et al. CIITA-regulated plexin-A1 affects T-cell-dendritic cell interactions. Nat Immunol 2003;4:891–898. [DOI] [PubMed] [Google Scholar]
- 13.Mori-Aoki A, Pietrarelli M, Nakazato M, Caturegli P, Kohn LD, Suzuki K. Class II transactivator suppresses transcription of thyroid-specific genes. Biochem Biophys Res Commun 2000;278:58–62. [DOI] [PubMed] [Google Scholar]
- 14.Chang CH, Gourley TS, Sisk TJ. Function and regulation of class II transactivator in the immune system. Immunol Res 2002;25:131–142. [DOI] [PubMed] [Google Scholar]
- 15.Xu Y, Wang L, Buttice G, Sengupta PK, Smith BD. Major histocompatibility class II transactivator (CIITA) mediates repression of collagen (COL1A2) transcription by interferon gamma (IFN-gamma). J Biol Chem 2004;279:41319–41332. [DOI] [PubMed] [Google Scholar]
- 16.Zhu XS, Ting JP. A 36-amino-acid region of CIITA is an effective inhibitor of CBP: novel mechanism of gamma interferon-mediated suppression of collagen alpha(2)(I) and other promoters. Mol Cell Biol 2001;21:7078–7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Itoh-Lindstrom Y, Piskurich JF, Felix NJ, Wang Y, Brickey WJ, Platt JL, Koller BH, Ting JP. Reduced IL-4-, lipopolysaccharide-, and IFN-gamma-induced MHC class II expression in mice lacking class II transactivator due to targeted deletion of the GTP-binding domain. J Immunol 1999;163:2425–2431. [PubMed] [Google Scholar]
- 18.Chang CH, Guerder S, Hong SC, van Ewijk W, Flavell RA. Mice lacking the MHC class II transactivator (CIITA) show tissue-specific impairment of MHC class II expression. Immunity 1996;4:167–178. [DOI] [PubMed] [Google Scholar]
- 19.Repique CJ, Li A, Brickey WJ, Ting JP, Collins FM, Morris SL. Susceptibility of mice deficient in the MHC class II transactivator to infection with Mycobacterium tuberculosis. Scand J Immunol 2003;58:15–22. [DOI] [PubMed] [Google Scholar]
- 20.Giri SN, Hyde DM, Marafino BJ Jr. Ameliorating effect of murine interferon gamma on bleomycin-induced lung collagen fibrosis in mice. Biochem Med Metab Biol 1986;36:194–197. [DOI] [PubMed] [Google Scholar]
- 21.Hyde DM, Henderson TS, Giri SN, Tyler NK, Stovall MY. Effect of murine gamma interferon on the cellular responses to bleomycin in mice. Exp Lung Res 1988;14:687–704. [DOI] [PubMed] [Google Scholar]
- 22.Chen ES, Greenlee BM, Wills-Karp M, Moller DR. Attenuation of lung inflammation and fibrosis in interferon-gamma-deficient mice after intratracheal bleomycin. Am J Respir Cell Mol Biol 2001;24:545–555. [DOI] [PubMed] [Google Scholar]
- 23.Goldberg H, Helaakoski T, Garrett LA, Karsenty G, Pellegrino A, Lozano G, Maity S, de Crombrugghe B. Tissue-specific expression of the mouse alpha 2(I) collagen promoter: studies in transgenic mice and in tissue culture cells. J Biol Chem 1992;267:19622–19630. [PubMed] [Google Scholar]
- 24.Agarwal AR, Goldstein RH, Lucey E, Ngo HQ, Smith BD. Cell-specific expression of the alpha 1 (I) collagen promoter-CAT transgene in skin and lung: a response to TGF-beta subcutaneous injection and bleomycin endotracheal instillation. J Cell Biochem 1996;63:135–148. [DOI] [PubMed] [Google Scholar]
- 25.Schissel SL, Dunsmore SE, Liu X, Shine RW, Perrella MA, Layne MD. Aortic carboxypeptidase-like protein is expressed in fibrotic human lung and its absence protects against bleomycin-induced lung fibrosis. Am J Pathol 2009;174:818–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sengupta P, Xu Y, Wang L, Widom R, Smith BD. Collagen alpha1(I) Gene (COL1A1) is repressed by RFX family. J Biol Chem 2005;280:21004–21014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Segel MJ, Izbicki G, Cohen PY, Or R, Christensen TG, Wallach-Dayan SB, Breuer R. Role of interferon-gamma in the evolution of murine bleomycin lung fibrosis. Am J Physiol Lung Cell Mol Physiol 2003;285:L1255–L1262. [DOI] [PubMed] [Google Scholar]
- 28.Snider GL, Celli BR, Goldstein RH, O'Brien JJ, Lucey EC. Chronic interstitial pulmonary fibrosis produced in hamsters by endotracheal bleomycin: lung volumes, volume-pressure relations, carbon monoxide uptake, and arterial blood gas studied. Am Rev Respir Dis 1978;117:289–297. [DOI] [PubMed] [Google Scholar]
- 29.Giri SN, Hyde DM, Hollinger MA. Effect of antibody to transforming growth factor beta on bleomycin induced accumulation of lung collagen in mice. Thorax 1993;48:959–966 (see comments). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gurujeyalakshmi G, Giri SN. Molecular mechanisms of antifibrotic effect of interferon gamma in bleomycin-mouse model of lung fibrosis: downregulation of TGF-beta and procollagen I and III gene expression. Exp Lung Res 1995;21:791–808. [DOI] [PubMed] [Google Scholar]
- 31.Sisk TJ, Gourley T, Roys S, Chang CH. MHC class II transactivator inhibits IL-4 gene transcription by competing with NF-AT to bind the coactivator CREB binding protein (CBP)/p300. J Immunol 2000;165:2511–2517. [DOI] [PubMed] [Google Scholar]
- 32.Patel DR, Li W, Park JS, Sofi MH, Gourley TS, Hangoc G, Kaplan MH, Chang CH. Constitutive expression of CIITA directs CD4 T cells to produce Th2 cytokines in the thymus. Cell Immunol 2005;233:30–40. [DOI] [PubMed] [Google Scholar]
- 33.Xu Y, Harton JA, Smith BD. CIITA mediates interferon-gamma repression of collagen transcription through phosphorylation-dependent interactions with co-repressor molecules. J Biol Chem 2008;283:1243–1256. [DOI] [PubMed] [Google Scholar]
- 34.Xu Y, Farmer SR, Smith BD. Peroxisome proliferator-activated receptor gamma interacts with CIITA/RFX5 complex to repress type I collagen gene expression. J Biol Chem 2007;282:26046–26056. [DOI] [PubMed] [Google Scholar]
- 35.Xu Y, Sengupta PK, Seto E, Smith BD. RFX family proteins differentially interact with HDACs to repress collagen alpha 2(I) gene (COL1A2) expression. J Biol Chem 2006;281:9260–9270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gordon S. Alternative activation of macrophages. Nat Rev Immunol 2003;3:23–35. [DOI] [PubMed] [Google Scholar]
- 37.Yang HZ, Cui B, Liu HZ, Chen ZR, Yan HM, Hua F, Hu ZW. Targeting TLR2 attenuates pulmonary inflammation and fibrosis by reversion of suppressive immune microenvironment. J Immunol 2009;182:692–702. [DOI] [PubMed] [Google Scholar]
- 38.Bassaganya-Riera J, Misyak S, Guri AJ, Hontecillas R. PPAR gamma is highly expressed in F4/80(hi) adipose tissue macrophages and dampens adipose-tissue inflammation. Cell Immunol 2009;258:138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shimizu K, Shichiri M, Libby P, Lee RT, Mitchell RN. Th2-predominant inflammation and blockade of IFN-gamma signaling induce aneurysms in allografted aortas. J Clin Invest 2004;114:300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol 2004;4:583–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel DR, Kaplan MH, Chang CH. Altered Th1 cell differentiation programming by CIITA deficiency. J Immunol 2004;173:5501–5508. [DOI] [PubMed] [Google Scholar]
- 42.Izbicki G, Or R, Christensen TG, Segel MJ, Fine A, Goldstein RH, Breuer R. Bleomycin-induced lung fibrosis in IL-4-overexpressing and knockout mice. Am J Physiol Lung Cell Mol Physiol 2002;283:L1110–L1116. [DOI] [PubMed] [Google Scholar]
- 43.Huaux F, Liu T, McGarry B, Ullenbruch M, Phan SH. Dual roles of IL-4 in lung injury and fibrosis. J Immunol 2003;170:2083–2092. [DOI] [PubMed] [Google Scholar]
- 44.Wen FQ, Kohyama T, Liu X, Zhu YK, Wang H, Kim HJ, Kobayashi T, Abe S, Spurzem JR, Rennard SI. Interleukin-4- and interleukin-13-enhanced transforming growth factor-beta2 production in cultured human bronchial epithelial cells is attenuated by interferon-gamma. Am J Respir Cell Mol Biol 2002;26:484–490. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.