Abstract
Airway epithelial cells are the initial site of infection with influenza viruses. The innate immune responses of airway epithelial cells to infection are important in limiting virus replication and spread. However, relatively little is known about the importance of this innate antiviral response to infection. Avian influenza viruses are a potential source of future pandemics; therefore, it is critical to examine the effectiveness of the host antiviral system to different influenza viruses. We used a human influenza (H3N2) and a low-pathogenic avian influenza (H11N9) to assess and compare the antiviral responses of Calu-3 cells. After infection, H3N2 replicated more effectively than the H11N9 in Calu-3 cells. This was not due to differential expression of sialic acid residues on Calu-3 cells, but was attributed to the interference of host antiviral responses by H3N2. H3N2 induced a delayed antiviral signaling and impaired type I and type III IFN induction compared with the H11N9. The gene encoding for nonstructural (NS) 1 protein was transfected into the bronchial epithelial cells (BECs), and the H3N2 NS1 induced a greater inhibition of antiviral responses compared with the H11N9 NS1. Although the low-pathogenic avian influenza virus was capable of infecting BECs, the human influenza virus replicated more effectively than avian influenza virus in BECs, and this was due to a differential ability of the two NS1 proteins to inhibit antiviral responses. This suggests that the subversion of human antiviral responses may be an important requirement for influenza viruses to adapt to the human host and cause disease.
Keywords: influenza, innate immune response, antiviral, IFN
Influenza A viruses are major respiratory pathogens that primarily infect human bronchial epithelial cells (BECs), and, as the initial barrier to the virus, the immune response elicited by these cells is important in determining the outcome of infection. Type I IFNs (IFN-α/β) and type III IFNs (IFN-λ1, IFN-λ2, and IFN-λ3) are critical components of the human innate immune system that provide immediate responses to limit viral infection and help initiate an appropriate adaptive immune system response to clear infection.
Influenza infection of airway epithelial cells is initiated by the binding of influenza hemaglutinin (HA) to sialic acid (SA) residues. Human influenza viruses have been found to preferentially bind to glycoproteins containing SA residues with a terminal α2,6Gal linkage (SAα2,6Gal), which are thought to predominate in the upper airway and proximal bronchial tree (1–3). In contrast, avian influenza viruses bind to SAα2,3Gal linkages mainly found in the lower airways (1, 2). These differences in binding preference and SA distribution in the respiratory system are thought to account for the reduced ability of avian influenza viruses to infect humans (2–4). Once bound, viruses are endocytosed and replicate in BECs. The cytoplasmic RNA helicase retinoic acid–inducible gene (RIG)–I recognizes the viral RNA and associates with tripartite motif protein 25. This enables RIG-I to associate with the adaptor protein, IFN-β promoter stimulator–1, on the mitochondria (5, 6), which leads to the subsequent phosphorylation of IFN regulatory factor (IRF) 3. Phosphorylated IRF-3 then translocates to the nucleus, where it initiates the expression of type I IFNs and chemokines, such as CXCL-10 (7–10). After secretion, type I IFNs bind to the type I IFN receptor 2 on the same and/or neighboring cells, and then signal via JAK1/STAT1 to induce the expression of over 300 IFN-stimulated genes (ISGs), including antiviral proteins, such as protein kinase R (PKR) and 2′-5′-Oligoadenylate synthetase (OAS), and positive regulators, RIG-I, Melanoma Differentiation-associated Protein 5 (MDA-5), and IRF7 (11–16). These ISGs are able to inhibit viral replication, amplify the antiviral responses, and also induce an antiviral state in neighboring airway epithelial cells (17, 18). Type III IFNs are newly discovered IFNs that, although they bind to different receptors, have similar mechanisms of inducing antiviral activity as type I IFNs (19). Viral infection, including influenza viruses and herpes simplex viruses, were shown to induce type III IFNs via RIG-I–IRF3 signaling pathway, and, once released, bind to IFN-λR1, and subsequently activate JAK1/STAT1 and initiate ISG expression (15, 16, 20).
In spite of these host immune responses, influenza viruses are known to replicate efficiently in host epithelial cells, and have developed virulence factors to subvert these responses. The influenza virus nonstructural (NS) 1 protein is a multifunctional protein that, when expressed, inhibits host mRNA processing and immune responses, particularly antiviral responses, that are activated against influenza (21–26). NS1 protein binds to tripartite motif protein 25–RIG-I complex, and disrupts downstream RIG-I signaling (21, 22). NS1 also binds to IRF3 and prevents the initiation of IFN responses (23).
Avian influenza viruses are a potential source of future pandemics, as they have the capacity to undergo genetic mutation and reassortment with other strains of influenza from different hosts, and subsequently cause diseases in humans. The recent pandemic caused by the novel H1N1 influenza virus is a clear example of this phenomenon, and this virus is the result of triple reassortment between human, avian, and swine influenza viruses (27). Therefore, it is critically important to understand the mechanisms of pathogenesis of human and avian influenza viruses and the effectiveness of human antiviral responses to these viruses. In this study, we used a low-pathogenic avian influenza virus strain, H11N9, from a transhemispheric migratory bird. This strain is not known to cause disease in either avian or human hosts; therefore, we evaluated the ability of human H3N2 and avian H11N9 to infect Calu-3 BECs and primary BECs (pBECs), and investigated the antiviral responses to infection with these viruses.
MATERIALS AND METHODS
Viruses
Human influenza A/Wellington/43/2006 (H3N2) strain and a low-pathogenic avian strain, A/Sharp-tailed Sandpiper/Australia/6/2004, was obtained from the World Health Organization Collaborating Centre for Reference and Research on Influenza (Melbourne, VIC, Australia) (28). Influenza viruses were propagated and virus titer determined by plaque assays on Madin-Darby canine kidney cells (ATCC, Manassas, VA) (29).
Cell Culture and Viral Infection
Calu-3 and Madin-Darby canine kidney cells were maintained in minimum essential medium supplemented with 10% FBS and Dulbecco's modified Eagle's medium with 5% FBS, respectively. Human pBECs were obtained from healthy individuals by endobronchial brushing during fiberoptic bronchoscopy (30). Subjects had no history of smoking or lung disease, with normal lung function. All subjects gave written consent. pBECs were cultured as previously described (31). Virus infection was performed at multiplicity of infection of 5. Cells were treated with 100 μg/ml of Poly I:C (Sigma-Aldrich, NSW, Australia) as a positive control. For IFN-β and -λ1 pretreatments, Calu-3 cells were incubated with 1 ng/ml of IFN-β and -λ1 for 3 hours before infection.
Flow Cytometry
FITC-conjugated Sambucus niger agglutinin and Maackia amurensis lectins (Vector Laboratories, Qld, Australia) were used to identify SAα2,6Gal and SAα2,3Gal residues, respectively. Calu-3 cells were stained with 10 μg/ml of lectins, analyzed using flow cytometry (Becton and Dickinson), and results expressed as median fluorescence intensity.
RT-qPCR
RNA was extracted from infected Calu-3 cells using RNeasy Mini Kits (Qiagen, Vic, Australia) according to the manufacturer's instructions. RNA (1 μg) was reverse transcribed to cDNA and was used for RT-qPCR assays (Applied Biosystem, Vic, Australia). Ribosomal RNA (18S) was used as the reference gene. The cycle threshold (Ct) value obtained was normalized to that of the 18S gene, and expressed as fold induction over the medium control.
Immunoblotting and ELISA
Calu-3 cells were lysed in RIPA buffer, and proteins (10 μg) were resolved by SDS-PAGE and transferred onto nitrocellulose membranes for detection of IFN-β in supernatants, RIG-I and MDA-5 in the cell lysates. Glyceraldehyde 3-phosphate dehydrogenase was detected as a loading control. CXCL-10 and IFN-λ1 concentration was assessed using human CXCL-10 and IFN-λ1 ELISA kits (RnD, R&D Systems, NSW, Australia), according to the manufacturer's instructions.
Microarray Analysis
RNA was extracted from Calu-3 cells 24 hours after infection using RNeasy Mini Kits (Qiagen). RNA was amplified, hybridized onto beadchips, and scanned for gene expression analysis. Data sets were submitted to the Gene Expression Omnibus database (accession number, GSE19580; National Center for Biotechnology Information, Bethesda, MD).
Expression Plasmids and Transfection
Viral RNA was extracted from influenza viruses, reverse transcribed to cDNA, and used as templates to amplify NS genes by PCR. The gene was then cloned into an expression vector pcDNA3.3-TOPO (Invitrogen, Vic, Australia) and transfected into Calu-3 cells. The transfectant cells were stimulated with Poly I:C (100 μg/ml; Sigma-Aldrich) and NS1, IFN-β, IFN-λ1, CXCL-10 expression was measured.
Statistical Analysis
Data were expressed as mean (±SEM). Statistical analysis was performed using student's t test on virus replication. A one-way ANOVA was performed on mRNA relative fold change and a P value of less than 0.05 was considered significant. The study was approved by the University of Newcastle (Newcastle, NSW, Australia) Human Research Ethics Committee.
RESULT
Human Influenza H3N2 Replicates More Efficiently than Avian Influenza H11N9 Independently of Influenza Receptor Expression on Calu-3 Cells
We first examined the SA residue expression on Calu-3 cells, a cell line representing epithelial cells of the proximal lower respiratory tract. By staining the cells with lectin Sambucus niger agglutinin and Maackia amurensis lectin (MAL), Calu-3 cells showed a significantly higher SAα2,6Gal than SAα2,3Gal residues, which is similar to primary proximal airway epithelial cells (Figure 1A). Both H3N2 and H11N9 initially infected Calu-3 cells equally well, as evident with similar of HA content immediately after infection (Figure 1B), and replicated with similar kinetics. By 48 hours, however, H3N2 had replicated to a significantly higher titer compared with H11N9 (P = 0.009; Figure 1C). This indicates that, although there was only a low level of SAα2,3Gal residues, H11N9 was still able to infect Calu-3 cells and initially replicated as efficiently as H3N2. However, H3N2 appeared to be able to establish infection with greater replication at 48 hours.
Figure 1.
Sialic acid (SA) α2,6Gal and SAα2,3Gal residue levels, hemaglutinin (HA) level at 2 and 6 hours after infection, and H3N2 and H11N9 replication kinetics in Calu-3 cells. (A) SAα2,6Gal residue level is significantly higher than SAα2,3Gal residue on Calu-3 cells, which is similarly found in cells of upper respiratory tract. (B) After infection with H3N2 and H11N9 in Calu-3 cells, both viruses initially infected the cells equally well, as evident by similar HA level of H3N2 and H11N9 inside infected cells. (C) In addition, both viruses replicated to similar levels until 48 hours, when H3N2 replicated to a significantly higher titer than H11N9 (P = 0.009). Results were obtained from three independent experiments. SA residue levels were assessed by staining with fluorescein-labeled Sambucus niger agglutinin (SNA) and Maackia amurensis lectin (MAL), analyzed by flow cytometry, and are expressed as median fluorescence intensity. Results are representative of three independent experiments. Densitometry on the results from HA Western blot is expressed as PKR:glyceraldehyde 3-phosphate dehydrogenase (GAPDH) ratio, and is presented as fold induction from medium controls. Viral replication was measured by plaque assays, and the results are presented as SEM.
Induction of Antiviral Responses after H3N2 and H11N9 Infection
We then investigated the kinetics of the induction of antiviral responses to see if there were differences in the response of Calu-3 cells to infection by the two viruses. Poly I:C was used as a positive control of antiviral responses, and ultraviolet-inactivated virus was used as a negative control, which had no significant effect compared with the medium control (data not shown).
H3N2 led to an early induction of RIG-I (25-fold) by 6 hours, with a more modest increase in MDA-5 mRNA (fivefold) by 12 hours (Figures 2A and 2B). This was followed by a second late induction of both genes at 72 hours. H11N9 induced similar responses, although the later rise in RIG-I from 24 to 72 hours was more modest and gradual. H3N2 induced Toll-like receptor–3 mRNA at 6 hours, with a second rise from 48 to 72 hours, which occurred to a greater extent than for H11N9 (Figure 2C). Both viruses induced PKR and IFN-β mRNA from 48 to 72 hours (Figures 2D and 2E), with H11N9 inducing an earlier IFN-β response.
Figure 2.
Induction of antiviral response genes to influenza infection in Calu-3 cells. The relative induction of mRNA was measured by RT-qPCR, normalized to the housekeeping gene, 18S ribosomal RNA, and expressed as fold change from media-treated controls. H3N2 and H11N9 induced an early up-regulation of (A) retinoic acid–inducible gene (RIG)–I mRNA at 6 hours, whereas (B) MDA-5 mRNA was not increased until 48 hours. (C) Toll-like receptor (TLR)–3 mRNA expression was increased at 12–72 hours. Both viruses expressed (D) PKR and (E) IFN-β mRNA from 48 to 72 hours. H3N2 induced earlier and greater RIG-I and TLR3 responses, whereas H11N9 induced an earlier IFN-β response.
The protein expression of RIG-I and MDA-5, as well as IFN-β, IFN-λ1, and CXCL-10, was also assessed after infection. In contrast to observations with mRNA, H3N2 induced delayed RIG-I protein expression until 48 hours, and this induction was significantly lower compared with H11N9 (Figure 3A). Similarly, MDA-5 protein was only detected at 48 hours after H11N9 infection (Figure 3B), but not at earlier time points by both viruses H3N2 and H11N9 (data not shown). As inductions were mostly observed at 48 hours after infection, IFN-β, IFN-λ1, CXCL-10, and PKR protein induction was measured at 48 hours. H3N2 induced minimal IFN-β, IFN-λ1 (P = 0.005), CXCL-10 (P = 0.002), and PKR protein production (P = 0.05) at this time point compared with medium control, and induction of these antiviral proteins by H3N2 were significantly reduced compared with induction by H11N9 (Figures 4A–4D). Furthermore, infection in pBECs also showed a similar IFN induction pattern. H11N9 infection induced significantly higher levels of IFN-β and CXCL-10 protein compared with H3N2 infection (IFN-β, P = 0.002; CXCL-10, P = 0.011; Figure 5), further confirming the difference in antiviral responses between the two influenza infections.
Figure 3.
RIG-I and MDA-5 protein production in response to influenza infection in Calu-3 cells. The protein induction of RIG-I and MDA-5 was detected 48 hours after infection by Western blot. (A) H3N2 only induced the significant production of RIG-I at 48 hours, whereas RIG-I was detected at 6, 24, and 48 hours after H11N9 infection, and the induction was significantly up-regulated compared with H3N2 at 48 hours. (B) Similarly, H11N9, but not H3N2 infection, resulted in a detectable MDA-5 expression at 48 hours. Results are representative of three independent experiments, expressed as protein:GAPDH ratio, and presented as fold induction from media-treated controls.§ and §§Significant reduction from medium control;*, **, and ***significant induction from medium control.
Figure 4.
IFN-β, IFN-λ, CXCL-10, and PKR protein production in response to influenza infection in Calu-3 cells. The protein induction of IFN-β and PKR was measured by Western blot; IFN-λ1 and CXCL-10 protein was measured by ELISA. (A) IFN-β and (B) IFN-λ production was not induced by H3N2 infection, but was significantly up-regulated by H11N9 infection compared with media-treated controls. (C) CXCL-10 production was significantly induced after H11N9, but not H3N2 infection. (D) PKR protein was induced by both viruses, and induction was also higher in H11N9 infection compared with that in H3N2 (P = 0.055). Results are representative of three independent experiments, and densitometry of the results from IFN-β Western blot is expressed as fold induction from media-treated controls. PKR densitometry was expressed as PKR:GAPDH ratio, and is presented as fold induction from media-treated controls. *, **, and ***Significant induction from medium control.
Figure 5.
IFN-β and CXCL-10 protein production in response to influenza infection in primary bronchial epithelial cells (pBECs). The protein induction of IFN-β and CXCL-10 was measured by Western blot at 48 hours after infection. (A) IFN-β and (B) CXCL-10 protein induction was significantly higher in H11N9 infection compared with that in H3N2 infection at 48 hours. Results are representative of three independent experiments, and densitometry of the results from IFN-β Western blot is expressed as fold induction from media-treated controls. * and **Significant induction from medium control.
IFN-β and IFN-λ1 Have Major Roles in Limiting Influenza Viral Replication in Calu-3 Cells
To determine if the minimal induction of type I and type III IFNs after H3N2 infection led to the increase in replication compared with H11N9, cells were pretreated with IFN-β or IFN-λ1 alone, or in combination, before infection, and viral replication was measured at 48 hours. Pretreatment with IFN-β or IFN-λ1 alone at 1 ng/ml significantly decreased the viral replication of H3N2 and H11N9 by threefold, and the combination of both IFNs further reduced the replication of both viruses (Figure 6).
Figure 6.
The antiviral effect of IFN-β and IFN-λ1 against influenza infection in Calu-3 cells. IFN-β and IFN-λ1 (1 ng/ml) was used to prime Calu-3 cells before influenza infection, and viral replication was analyzed by plaque assay at 48 hours after infection. Both H3N2 and H11N9 had a three- to fourfold decrease in viral replication when Calu-3 cells were pretreated with IFN-β and IFN-λ1 alone, and replication was further reduced when IFN-β and IFN-λ1 were used in combination. Results are representative of three independent experiments.
H3N2 Virus Infection Leads to Widespread Reductions in Antiviral Responses in Calu-3 Cells
To investigate if the difference in antiviral responses by H3N2 and H11N9 was widespread across different antiviral signaling pathways, gene expression in infected cells was examined at 24 hours by microarray analysis.
Infection with H3N2 and H11N9 resulted in the altered expression of 13,388 genes after normalization to media-treated controls. ANOVA with the more stringent Benjamini-Hochberg correction identified 2,155 genes that were differentially expressed (P ≤ 0.005) (see Figure E1 in the online supplement). Of these, 330 genes were altered by twofold or greater (either up- or down-regulated), with 41 genes differentially expressed in response to H3N2 infection, 300 in response to H11N9, and 35 altered in response to both H3N2 and H11N9 infection. Gene ontology analysis revealed that H3N2 infection only induced the ISG OAS2, whereas, in contrast, H11N9 infection up-regulated many immune and virus responses genes, including IFN-β, IFN-λ1, and numerous ISGs.
H3N2 NS1 Is More Effective in Suppressing IFN Responses in Calu-3 Cells Compared with H11N9 NS1
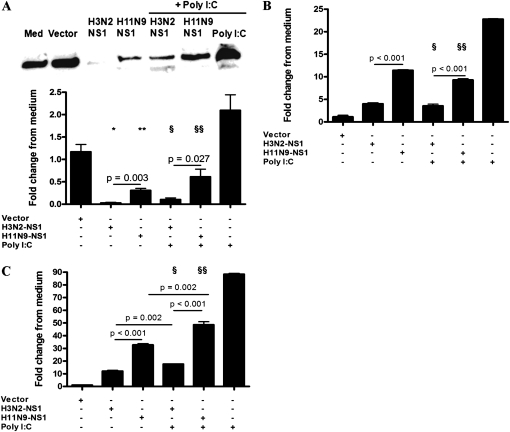
The influenza NS1 protein is known to inhibit antiviral responses in Calu-3 cells; therefore, we determined if the NS1 of H3N2 was more effective in suppressing immune responses compared with that of H11N9. The NS1 genes of H3N2 and H11N9 were transfected into Calu-3 cells, which were then stimulated with Poly I:C. NS1 of both H3N2 and H11N9 were expressed to similar levels in Calu-3 cells (Figure E2), and significantly down-regulated IFN-β, IFN-λ1, and CXCL-10 protein production compared with media-treated controls (Figures 7A–7C). IFN-β, IFN-λ1, and CXCL-10 protein production in response to Poly I:C was inhibited by NS1 of both viruses, but the suppression of these antiviral proteins was significantly greater by the NS1 protein of H3N2 compared with that of H11N9 virus. Vector alone did not induce these proteins above media-treated controls. This clearly demonstrates that human Calu-3 cells respond poorly to H3N2, due to the enhanced NS1-induced suppression of antiviral responses, which was less effective in H11N9 NS1.
Figure 7.
Induction of IFN-β, IFN-λ1, and CXCL-10 protein production after nonstructural (NS) 1 transfection and Poly I:C stimulation in Calu-3 cells. The protein induction of IFN-β was measured by Western blot, and IFN-λ1, and CXCL-10 protein was measured by ELISA. (A) H3N2-NS1 and H11N9-NS1 transfection significantly down-regulated IFN-β production after Poly I:C stimulation, with a significantly greater effect of H3N2-NS1. (B) IFN-λ1 and (C) CXCL-10 production was also significantly reduced by H3N2-NS1 compared with H11N9-NS1. IFN-β blots are representative of experiments performed in three independent experiments; densitometry on the results from the IFN-β Western blot was performed, and is presented as fold induction from medium controls. * and **Significant reduction from medium control; § and §§significant reduction compared with induction by Poly I:C.
DISCUSSION
The innate antiviral response to influenza by the airway epithelial cells is the first line of defense against infection, and influences the subsequent adaptive immune response and the effective clearance of the virus. In this study, we have demonstrated that human influenza H3N2 replicated more efficiently than avian H11N9 influenza virus in human Calu-3 cells. The increase in replication of H3N2 was not due to the greater expression of SAα2,6Gal residues on the Calu-3 cells, as similar levels of H3N2 and H11N9 titer were observed shortly after infection, demonstrating a similar ability for both viruses to enter into airway epithelial cells. Instead, differences in replication were attributed to the ability of H3N2 to impair host cellular innate immune responses, with reduced induction of RIG-I and downstream release of IFN-β, IFN-λ1, PKR, and CXCL-10 compared with H11N9. We have also demonstrated that this impaired antiviral response was induced by the NS1 protein of H3N2, which more effectively inhibited these antiviral responses compared with that of H11N9.
Calu-3 cells are representative of human proximal airway epithelial cells, and predominantly express SAα2,6Gal residues. These residues are the binding site for human influenza virus on epithelial cells, whereas avian influenza viruses appear to bind more readily to SAα2,3Gal, the level of which was found to be less abundant as SAα2,6Gal residue in the proximal airway epithelial cells (2–4). Despite this observation, we demonstrate that both H3N2 and H11N9 viruses were able to readily infect these human airway epithelial cells to a similar extent based on HA level and similar initial replication titer. This suggests that either only a low level of SAα2,3Gal is sufficient to allow viral entry to establish infection, or that other receptors may also play a role in influenza infection, such as caveolae and other lipid-linked glycoproteins (32, 33). A study has shown that a SAα2,6Gal-deficient mice could in fact allow influenza virus to replicate to a similar extent as in the wild-type mice, supporting the theory that other factors are in play in the susceptibility to influenza infection (34).
Postendocytosis events clearly play a key role in determining the success of infection. After endocytosis into Calu-3 cells, cytosolic RNA helicase RIG-I recognizes influenza viral RNA and signals through IRF3 to induce the expression and production of type I IFNs. The secreted IFNs then bind to the type I IFN receptor 2 on the same and/or neighboring cells, and amplify the antiviral responses by inducing ISG expression via JAK1/STAT1 pathways. Type III IFNs are also induced through similar pathways (15, 16, 20), and via a distinct receptor, IFN-λR1 (19), they signal through the same JAK1/STAT1 pathway to induce ISG responses (15, 16, 35).
H3N2 induced a more robust expression of RIG-I mRNA compared with H11N9, and late induction was also observed for MDA-5 as well as Toll-like receptor–3, again with a greater induction by H3N2 infection. Paradoxically, at the protein level, RIG-I and MDA-5 were more potently suppressed by H3N2 infection, and this correlated with the higher level of H3N2 replication. The disparity between mRNA and protein induction could be explained by the NS1 protein that inhibits the host mRNA processing, thereby minimizing the IFN responses in the infected cells (36–38).
Type I and type III IFNs are critically important in establishing the antiviral state in the infected microenvironment (39, 40). Whereas priming of Calu-3 cells with either IFN-β or IFN-λ1 alone reduced viral replication by twofold, concurrent administration of both types of IFN further limited viral infection by fivefold. Despite the antiviral effect of IFNs, H3N2 still replicated to a greater extent than H11N9, suggesting that one type of IFN alone may not be sufficient to counteract the suppressive effect of H3N2 NS1 protein. The NS1 protein of H5N1 has been shown to be resistant to IFN pretreatment in vitro via unidentified pathways (41), and it is possible that H3N2 NS1 also has similar ability to a certain extent. The mechanisms by which NS1 confers resistance to antiviral cytokines are still unclear, but it is known that a glutamic acid at position 92 is required for this activity.
We also observed residual levels of IFN-β in the absence of infection. The release of IFN-β may be constitutive, and has also been hypothesized to play a major role in epithelial cell priming for a more robust antiviral response to infection (12, 42). In our case, Calu-3 cells and pBECs appeared to be unable to sustain this constitutive release in H3N2 infection, which was at least partly due to the suppressive activity of its NS1 protein.
Human H3N2 influenza virus was able to inhibit the host IFN response and replicate more efficiently in Calu-3 cells than the H11N9 virus. We have demonstrated that the NS1 protein of human influenza more effectively inhibited IFN responses than the avian strain, and this is at least partly responsible for the enhanced replication of H3N2. This further demonstrates that the enhanced replication of H3N2 may result from the activity of the NS1 protein in suppression of antiviral responses. Collectively, these results indicate that there are differences in the level of immune suppression by the NS1 protein of different strains of influenza, and this appears to be an important factor determining whether influenza viruses have high or low pathogenic properties in human airway cells.
The potent antiviral suppression by human influenza NS1 protein may be due to the selection pressure that drives the evolution of the virus for better adaptation in human populations. In addition, influenza viruses that develop an NS1 protein that is effective in human cells are more likely to lead to sustained infection in humans, and this may be an important factor in the zoonotic transmission of avian influenza to humans. Nevertheless, it remains unclear which set of residues differentiates the NS1 of high- or low-pathogenic influenza viruses, although a change in one residue (92E) has been implicated in increased disease severity with H5N1 NS1 in pigs (41). Our study demonstrates that differences in NS1 at the genetic level may potentially serve as a marker for influenza pathogenicity, and are an important determinate for replication in human Calu-3 cells.
We cannot rule out that other influenza virulence factors, such as PB1-F2 protein, may also be involved in the antiviral suppression. By targeting inner mitochondrial membrane, PB1-F2 is able to cause mitochondrial membrane permeabilization, leading to apoptosis of the infected cells, and thereby increasing the virulence of the virus (43–45). However, we did not observe a significant level of apoptosis at 48 hours after infection (both early apoptosis and late apoptosis/necrosis), which could be due the nature of immortalized cell line.
In summary, we have demonstrated that a human influenza virus that has had a long period of adaption in man replicates to a higher level in human airway cells than a low-pathogenic avian virus in Calu-3 cells. This is not determined by the ability of the virus to enter the host cell, but rather the ability of the virus to inhibit the postendocytotic host innate immune response. Both type I and type III IFNs can limit influenza replication. We have shown that infection with H3N2 impairs these IFN responses through the action of its NS1 protein, whereas the NS1 protein from H11N9 virus is far less effective at inhibiting these responses. This study demonstrates that the ability of an influenza virus to inhibit the early innate immune response in human Calu-3 cells is an important factor in promoting its ability to infect humans. Therefore, factors that impair this early response could enhance infection, whereas factors that enhance the antiviral response may have quite profound effects on limiting infection. Both of these avenues should be further explored to look for reasons why individuals may be susceptible to infection, and to explore this as a potential therapeutic pathway.
Supplementary Material
Acknowledgments
The authors thank Ms. Kristy Parsons and Ms. Melina Tooze for technical assistance, and Dr. Katherine Baines for assistance in microarray analysis.
This work was supported by National Health and Medical Research Council grants 510762 and 401314.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2010-0157OC on August 12, 2010
Author Disclosure: P.A.W. has served on the board for Astra Zeneca ($1,001–$5,000), has received lecture fees from Astra Zeneca ($1,001–$5,000) and GlaxoSmithKline ($1,001–$5,000), and has received industry-sponsored grants from GlaxoSmithKline ($10,001–$50,000); none of the other authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Ito T, et al. Receptor specificity of influenza A viruses correlates with the agglutination of erythrocytes from different animal species. Virology 1997;227:493–499. [DOI] [PubMed] [Google Scholar]
- 2.Ito T, et al. Differences in sialic acid–galactose linkages in the chicken egg amnion and allantois influence human influenza virus receptor specificity and variant selection. J Virol 1997;71:3357–3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryan-Poirier K, et al. Changes in H3 influenza A virus receptor specificity during replication in humans. Virus Res 1998;56:169–176. [DOI] [PubMed] [Google Scholar]
- 4.Rogers GN, Paulson JC. Receptor determinants of human and animal influenza virus isolates: differences in receptor specificity of the H3 hemagglutinin based on species of origin. Virology 1983;127:361–373. [DOI] [PubMed] [Google Scholar]
- 5.Kawai T, et al. IPS-1, an adaptor triggering RIG-I– and Mda5-mediated type I interferon induction. Nat Immunol 2005;6:981–988. [DOI] [PubMed] [Google Scholar]
- 6.Seth RB, et al. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell 2005;122:669–682. [DOI] [PubMed] [Google Scholar]
- 7.Le Goffic R, et al. Cutting edge: influenza A virus activates TLR3-dependent inflammatory and RIG-I–dependent antiviral responses in human lung epithelial cells. J Immunol 2007;178:3368–3372. [DOI] [PubMed] [Google Scholar]
- 8.Loo YM, et al., Distinct RIG-I and MDA5 signaling by RNA viruses in innate immunity. J Virol 2008;82:335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsukura S, et al. Role of RIG-I, MDA-5, and PKR on the expression of inflammatory chemokines induced by synthetic dsRNA in airway epithelial cells. Int Arch Allergy Immunol 2007;143:80–83. [DOI] [PubMed] [Google Scholar]
- 10.Pichlmair A, et al. RIG-I–mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science 2006;314:997–1001. [DOI] [PubMed] [Google Scholar]
- 11.Marie I, Durbin JE, Levy DE. Differential viral induction of distinct interferon-alpha genes by positive feedback through interferon regulatory factor–7. EMBO J 1998;17:6660–6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sato M, et al. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity 2000;13:539–548. [DOI] [PubMed] [Google Scholar]
- 13.Jouanguy E, et al. Human primary immunodeficiencies of type I interferons. Biochimie 2007;89:878–883. [DOI] [PubMed] [Google Scholar]
- 14.Marcello T, et al. Interferons alpha and lambda inhibit hepatitis C virus replication with distinct signal transduction and gene regulation kinetics. Gastroenterology 2006;131:1887–1898. [DOI] [PubMed] [Google Scholar]
- 15.Kotenko SV, et al. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat Immunol 2003;4:69–77. [DOI] [PubMed] [Google Scholar]
- 16.Sheppard P, et al. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat Immunol 2003;4:63–68. [DOI] [PubMed] [Google Scholar]
- 17.de Veer MJ, et al. Functional classification of interferon-stimulated genes identified using microarrays. J Leukoc Biol 2001;69:912–920. [PubMed] [Google Scholar]
- 18.Haller O, Kochs G, Weber F. The interferon response circuit: induction and suppression by pathogenic viruses. Virology 2006;344:119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Onoguchi K, et al. Viral infections activate types I and III interferon genes through a common mechanism. J Biol Chem 2007;282:7576–7581. [DOI] [PubMed] [Google Scholar]
- 20.Ank N, et al. Lambda interferon (IFN-lambda), a type III IFN, is induced by viruses and IFNs and displays potent antiviral activity against select virus infections in vivo. J Virol 2006;80:4501–4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gack MU, et al. Influenza A virus NS1 targets the ubiquitin ligase TRIM25 to evade recognition by the host viral RNA sensor RIG-I. Cell Host Microbe 2009;5:439–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mibayashi M, et al. Inhibition of retinoic acid–inducible gene I–mediated induction of beta interferon by the NS1 protein of influenza A virus. J Virol 2007;81:514–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Talon J, et al. Activation of interferon regulatory factor 3 is inhibited by the influenza A virus NS1 protein. J Virol 2000;74:7989–7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bergmann M, et al. Influenza virus NS1 protein counteracts PKR-mediated inhibition of replication. J Virol 2000;74:6203–6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li S, et al. Binding of the influenza A virus NS1 protein to PKR mediates the inhibition of its activation by either PACT or double-stranded RNA. Virology 2006;349:13–21. [DOI] [PubMed] [Google Scholar]
- 26.Min JY, Krug RM. The primary function of RNA binding by the influenza A virus NS1 protein in infected cells: inhibiting the 2′-5′ oligo (A) synthetase/RNase L pathway. Proc Natl Acad Sci USA 2006;103:7100–7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dawood FS, et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med 2009;360:2605–2615. [DOI] [PubMed] [Google Scholar]
- 28.Hurt AC, et al. Isolation of avian influenza viruses from two different transhemispheric migratory shorebird species in Australia. Arch Virol 2006;151:2301–2309. [DOI] [PubMed] [Google Scholar]
- 29.Huprikar J, Rabinowitz S. A simplified plaque assay for influenza viruses in Madin-Darby kidney (MDCK) cells. J Virol Methods 1980;1:117–120. [DOI] [PubMed] [Google Scholar]
- 30.Hurd SZ. Workshop summary and guidelines: investigative use of bronchoscopy. J Allergy Clin Immunol 1991;88:808–814. [DOI] [PubMed] [Google Scholar]
- 31.Wark PA, et al. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med 2005;201:937–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nunes-Correia I, et al. Caveolae as an additional route for influenza virus endocytosis in MDCK cells. Cell Mol Biol Lett 2004;9:47–60. [PubMed] [Google Scholar]
- 33.Rapoport EM, et al. Search for additional influenza virus to cell interactions. Glycoconj J 2006;23:115–125. [DOI] [PubMed] [Google Scholar]
- 34.Glaser L, et al. Effective replication of human influenza viruses in mice lacking a major alpha2,6 sialyltransferase. Virus Res 2007;126:9–18. [DOI] [PubMed] [Google Scholar]
- 35.Doyle SE, et al. Interleukin-29 uses a type 1 interferon–like program to promote antiviral responses in human hepatocytes. Hepatology 2006;44:896–906. [DOI] [PubMed] [Google Scholar]
- 36.Chen Z, Li Y, Krug RM. Influenza A virus NS1 protein targets poly(A)-binding protein II of the cellular 3′-end processing machinery. EMBO J 1999;18:2273–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nemeroff ME, et al. Influenza virus NS1 protein interacts with the cellular 30 kDa subunit of CPSF and inhibits 3′ end formation of cellular pre-mRNAs. Mol Cell 1998;1:991–1000. [DOI] [PubMed] [Google Scholar]
- 38.Noah DL, Twu KY, Krug RM. Cellular antiviral responses against influenza A virus are countered at the posttranscriptional level by the viral NS1A protein via its binding to a cellular protein required for the 3′ end processing of cellular pre-mRNAS. Virology 2003;307:386–395. [DOI] [PubMed] [Google Scholar]
- 39.Levy DE, Darnell JE Jr. Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol 2002;3:651–662. [DOI] [PubMed] [Google Scholar]
- 40.Levy DE, Garcia-Sastre A. The virus battles: IFN induction of the antiviral state and mechanisms of viral evasion. Cytokine Growth Factor Rev 2001;12:143–156. [DOI] [PubMed] [Google Scholar]
- 41.Seo SH, Hoffmann E, Webster RG. Lethal H5N1 influenza viruses escape host anti-viral cytokine responses. Nat Med 2002;8:950–954. [DOI] [PubMed] [Google Scholar]
- 42.Taniguchi T, Takaoka A. A weak signal for strong responses: interferon-alpha/beta revisited. Nat Rev Mol Cell Biol 2001;2:378–386. [DOI] [PubMed] [Google Scholar]
- 43.Conenello GM, et al. A single mutation in the PB1–F2 of H5N1 (HK/97) and 1918 influenza A viruses contributes to increased virulence. PLoS Pathog 2007;3:1414–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zamarin D, et al. Influenza virus PB1-F2 protein induces cell death through mitochondrial ANT3 and VDAC1. PLoS Pathog 2005;1:e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zamarin D, Ortigoza MB, Palese P. Influenza A virus PB1-F2 protein contributes to viral pathogenesis in mice. J Virol 2006;80:7976–7983. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.