Abstract
The zinc finger transcription factor, Ikaros, is a central regulator of hematopoiesis. It is required for the development of the earliest B cell progenitors and at later stages for VDJ recombination and B cell receptor expression. Mature B cells rely on Ikaros to set the activation threshold for various stimuli, and to choose the correct antibody isotype during class switch recombination. Thus, Ikaros contributes to nearly every level of B cell differentiation and function.
Keywords: Ikaros, B cells development, B cell activation, Class switch recombination
INTRODUCTION
The Ikaros (Ikzf1) zinc finger transcription factor is a critical regulator of hematopoiesis. Originally identified as a protein binding regulatory regions of the lymphocyte specific genes dntt (terminal deoxynucleotidyl transferase)[1] and cd3d[2], Ikaros was soon shown to be required for fetal T cell development[3], as well as important steps in adult thymic development, such as the pre-T cell receptor (TCR) checkpoint and CD4 vs CD8 T cell differentiation[4-6]. Far from being T cell specific, Ikaros is expressed in virtually all hematopoietic cells in mice[7,8] and humans[9-13], and even in the murine neuro-endocrine system[14]. In mice, Ikaros has been shown to be crucial for hematopoietic stem cell function and renewal, and promotes the differentiation of conventional and plasmacytoid dendritic cells, natural killer cells, neutrophils and erythrocytes[15-21]. Furthermore, Ikaros is a critical tumor suppressor because mice bearing mutations in the Ikzf1 gene develop T lymphomas with near complete penetrance[22-24], and Ikzf1 mutations are found in multiple types of human B and T cell lymphomas and leukemias[25-27]. In addition to these key roles in T cells and non-lymphoid lineages, Ikaros is a critical regulator of B cell lymphopoiesis and function, and this is discussed in detail below. Importantly, unless otherwise stated, experiments referred to in this review were performed in the murine system.
MECHANISMS OF IKAROS-MEDIATED TRANSCRIPTIONAL REGULATION
Ikaros acts as both a transcriptional activator and repressor, depending in large part on the co-factors with which it interacts (Table 1). Mechanisms of Ikaros-mediated repression fit into three broad categories: chromatin modification, co-repressor recruitment, and competition. First, Ikaros colocalizes with pericentromeric heterochromatin in lymphocytes[28], and interacts with components of histone deacetylase (HDAC) complexes, including Sin3A and Sin3B (Sin3 complex), the chromatin remodeling Mi-2b ATPase (NuRD complex), and HDAC-1 and HDAC2 (both Sin3 and NuRD complexes)[29-32]. Thus, Ikaros could contribute to transcriptional repression by recruiting genes to heterochromatin[33], and/or by recruiting chromatin-modifying complexes to specific genes to enforce repressive chromatin. Second, in vitro assays have shown that Ikaros can recruit the C terminal binding protein (CtBP) and CtBP interacting protein (CtIP) co-repressors, which may in turn repress transcription by interacting directly with the basal transcriptional machinery (TATA binding protein and transcription factor IIB)[32,34,35]. Finally, Ikaros competes with positive factors to repress transcription of genes such Igll1 [Lambda5; competes with early B cell factor (EBF)][36], dntt (terminal deoxynucleotidyl transferase; competes with Ets)[37], and Hes1 (competes with the RBP-Jk/Notch complex)[38].
Table 1.
Ikaros co-factors
| Protein/complex | Citation | System |
| NuRD complex (Mi2b, HDACs) | [31] | Over-expression in murine T cells |
| [30] | Over-expression in a murine DP thymocyte cell line | |
| [41] | Endogenous proteins in a murine erythro-leukemia cell line | |
| [30] | Over-expression in non-lymphoid cell lines | |
| Sin3 complex (Sin3a/b, HDACs) | [34] | Over-expression in non-lymphoid cell lines and in murine T cells |
| [32] | Over-expression in non-lymphoid cell lines | |
| CtBP | [34] | Over-expression in non-lymphoid cell lines and in murine T cells |
| [32] | Over-expression in non-lymphoid cell lines | |
| CtIP | [35] | Over-expression in non-lymphoid cell lines and in murine T cells |
| SWI/SNF complex (BRG1, BAFs) | [31] | Over-expression in murine T cells |
| [41] | Endogenous proteins in a murine erythro-leukemia cell line | |
| pTEFb (cdk9) | [43] | Endogenous proteins in murine yolk sac erythroid cells |
CtBP: C terminal binding protein; CtIP: CtBP interacting protein; pTEFb: Positive transcriptional elongation factor complex; HDAC: Histone deacetylase.
Ikaros may also function as a transcriptional activator. Ikaros can activate transcription of reporter plasmids[39], cd8a transcription in developing T cells[40], and adult globin genes in developing erythrocytes[21]. Interestingly, the histone acetyltransferase that contains the SWI/SNF chromatin remodeling complex interacts with Ikaros in both T cells[31] and erythrocyte precursors[41] and is associated with transcriptional activation[42]. Furthermore, Ikaros also interacts with the positive transcriptional elongation factor complex in yolk sac erythroid cells, recruiting it during the induction of globin genes[43]. Thus, Ikaros may activate the transcription of certain target genes, possibly by recruiting SWI/SNF, or promoting transcriptional elongation.
Post-translational modifications of Ikaros itself provide an additional layer of complexity to Ikaros-mediated transcriptional regulation. Ikaros can be phosphorylated and dephosphorylated at multiple residues, by casein kinase 2 and protein phosphatase 1, respectively[30,44-46]. Phosphorylation of Ikaros in turn inhibits its DNA binding, ability to block the cell cycle and repress genes such as tdt, and recruitment to peri-centromeric heterochromatin[44-46]. In addition to phosphorylation, SUMOylation at two separate residues (K58 and K240) antagonizes interactions between Ikaros and Sin3A, Sin3B, Mi-2b and CtBP, and relieves Ikaros mediated repression of reporter plasmids[47]. Thus, Ikaros can repress or activate transcription through a variety of mechanisms depending on post-translational modifications, cell type, protein partners and target gene.
IKAROS IN B CELL DEVELOPMENT
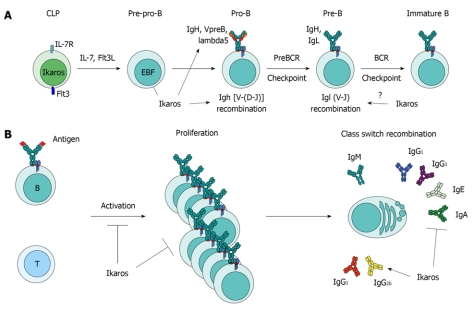
B cell development in the bone marrow takes place in sequential steps that are characterized by gene expression programs, and developmental checkpoints centered on antigen receptor rearrangement (Figure 1A, Table 2)[48,49]. To start, hematopoietic stem cells become progressively more restricted to the lymphoid lineage, differentiating into lymphoid primed multi-potent progenitors (LMPPs) and then common lymphoid progenitors (CLPs)[48]. The transcription factor E2A and interleukin-7 receptor (IL-7R) signaling in CLPs induce the expression of the B lineage specifying transcription factor, EBF1[50-52], and together with Flt3 signaling, differentiation into the earliest committed B cell developmental stage, the pre-pro B cell[53]. EBF1 allows further progression to the pro-B stage and induces the expression of B lineage genes. Crucially, one of these genes is Pax5[54,55], which is required for further development[56] and locks in the B lineage by repressing other cell fates[57,58]. At the pro-B stage, cells undergo immunoglobulin heavy chain (Igh) rearrangements, and successfully rearranged heavy chains pair with the surrogate light chain proteins Lambda5 and VpreB1/2 to provide a maturation signal[59,60]. This pre-B cell receptor (BCR) signaling, combined with IL-7R signals, induces differentiation into pre-B cells, several rounds of division, and rearrangement of the Ig light chain[61-63]. Those cells that a express functional BCR, consisting of Ig heavy and light chains, suppress further rearrangements (allelic exclusion) and migrate to the spleen to undergo final maturation steps.
Figure 1.
Ikaros controls multiple levels of B cell lymphopoiesis and function. A: Ikaros plays a crucial role in the specification of the B cell lineage by promoting the expression of the IL-7R and Flt3 receptors in common lymphoid progenitors (CLPs) and of the EBF transcription factor in pre-pro-B cells. Later, Ikaros regulates Igh recombination by activating Rag gene expression and contracting the Igh locus. After the preBCR check point, Ikaros also downregulates the expression of the preBCR component, Lambda5. During light-chain rearrangment, it is thought that Ikaros regulates allelic exclusion; B: In the periphery, Ikaros sets the B cell activation threshold to antigen and T cell co-stimulation, and inhibits hyper-proliferation of activated B cells. Finally, during class switch recombination, Ikaros controls isotype choice by inhibiting switching to IgG2b and IgG2a and promoting switching to all other isotypes.
Table 2.
Critical members of the transcription factor network controlling B cell development
| Gene | Ref. | Roles |
| Ikaros (Ikzf1) | [64] | Required for earliest B cell progenitors |
| [66,67] | Required for efficient pro-B to pre-B transition | |
| [67] | Required for repression of non-B cell fates | |
| [65,67] | Promotes Igh rearrangment and Rag expression | |
| E2A (Tcfe2a) | [51] | Required for earliest B cell progenitors |
| [86] | Required for EBF1 expression | |
| EBF1 (Ebf1) | [87] | Required for expression of B lineage genes development past pro-B stage |
| Pax5 (Pax5) | [56] | Required for differentiation past pro-B stage |
| [57,58] | Required for commitment to B cell lineage (e.g. repression of alternative fates) |
EBF1: Early B cell factor 1.
Control of B lineage specification and commitment by Ikaros
Ikaros plays crucial roles in B lineage specification and commitment. Ik-/- mice lack pre-pro-B cells and exhibit a complete block in B lymphopoiesis[64]. Similarly, there is a striking reduction in pre-pro-B cells in mice bearing a hypomorphic mutation in Ikzf1 (IkL/L), which results in Ikaros expression at about 10% of normal levels[65]. Together, these data indicate that Ikaros is crucial for B lineage specification. This was originally thought to be due to a role for Ikaros in promoting Flt3 and/or IL-7R expression on early hematopoietic progenitors because: (1) Flt3 and IL-7R signaling are required for pre-pro-B cell development[53]; (2) Ik-/- LSK (Lin-Sca1+c-kit+) cells lack Flt3 mRNA expression[16]; and (3) Ik-/- LMPPs express reduced levels of Il7r mRNA[66]. Retroviral expression of IL-7R or Flt3 independently in Ik-/- LSK cells however, does not rescue B cell development, indicating that (1) it is the reduced expression of both receptors together (not either one individually) which blocks Ik-/- B lymphopoiesis, and/or (2) that Ikaros has other important functions in B lineage specification[67]. In support of the latter view, retroviral expression of EBF in Ik-/- LSK cells does rescue pro-B cell development, indicating that Ikaros contributes to B lineage specification by promoting the expression of EBF[67]. Ikaros likely contributes to EBF expression in part by activating IL-7R expression, as IL-7 signals are required for EBF transcription[50,52]. However, as retroviral expression of IL-7R could not rescue Ik-/- B cell development, there is likely an IL-7 signaling independent function for Ikaros in EBF upregulation[67]; these functions are not understood at this time. Finally, it should be noted that EBF mediated rescue of Ik-/- pro-B cell development was inefficient, indicating that Ikaros is likely to play further roles in B lineage specification that are independent of EBF regulation.
Beyond the activation of B cell specific gene programs, Ikaros is also critical for B lineage commitment. In comparison to Ikaros-sufficient pro-B cell lines, EBF-induced Ik-/- pro-B cell lines exhibit promiscuous myeloid gene expression (e.g. csf1r). More strikingly, like Pax-5-/- pro-B lines, Ik-/- pro-B lines can be differentiated into macrophages when cultured with macrophage colony-stimulating factor[67]. Thus, in addition to contributing to B lineage specification through the activation of IL-7R, EBf1 and Flt3 expression, Ikaros also locks in the B lineage by shutting off alternative cell fates.
Ikaros in Ig gene recombination
Ikaros clearly plays critical roles in B cell development beyond specification and commitment. Ikaros hypomorphic mice (IkL/L) exhibit a partial block in differentiation between the pro-B and pre-B stages, indicating that Ikaros promotes this transition[65]. Similarly, EBF-induced Ik-/- pro-B cell lines do not mature into pre-B cells, further demonstrating that Ikaros contributes to developmental checkpoints in pro-B cells[67]. Interestingly, early experiments has found that compared with wild type (WT), IkL/L pro-B cells express lower levels of Rag1 and Rag2, which mediate VDJ recombination[65], indicating that Ikaros may contribute to pre-B development by promoting heavy-chain rearrangements. This was confirmed by the observations that EBF-induced Ik-/- pro-B cells lack Rag1 and Rag2 expression and Ikaros binds directly to their promoters[67]. Thus, it appears that Ikaros controls pre-B development by activating Rag gene expression and Igh rearrangments. Indeed, Reynaud and colleagues have found that DH-JH and especially VH-DJH recombination is perturbed or absent in EBF-induced Ik-/- pro-B lines. Interestingly, retroviral expression of Rag1 and 2 in these cell lines does not rescue VH-DJH recombination, and VH and DJH gene segments are found further from each other in the nucleus than in Ikaros-sufficient pro-B cell lines[67]. Thus, in addition to promoting Rag gene expression, Ikaros contributes to Igh locus contraction, a critical step required for V-DJ recombination[68], and for pro-B to pre-B differentiation.
Beyond the pro-B stage and Igh rearrangement, Ikaros is likely to play continued roles in B cell development. Ikaros is thought to down-regulate preBCR signaling by repressing Igll1 (Lambda5) transcription in preB cells[69,70]. Ikaros may also contribute to light-chain rearrangment and allelic exclusion. Deletion of an Ikaros-binding, cis-acting regulatory element in the Igk locus, abolishes monoallelic silencing of V-J rearrangement, suggesting that Ikaros participates in allelic exclusion[71,72]. Ikaros-mediated control of this process has not been rigorously tested, however, as the entire silencer (> 4 kb) rather than specific Ikaros binding sites, is deleted, and allelic exclusion has yet to be studied in Ikaros-deficient B cells. Finally, considering that Ikaros activates Rag gene expression in pro-B cells during Igh recombination, this role may well be reprised at the pre-B stage to allow for light-chain recombination. Taken together, these studies demonstrate that Ikaros is crucial to multiple early steps of B cell development.
IKAROS IN MATURE B CELLS
Mature B cells respond to antigen with co-stimulation from T cells, eventually undergoing multiple divisions, and producing high-affinity antibodies with various constant domains that provide unique effector functions. Ikaros controls both the threshold at which B cells respond, as well as the choice of antibody isotype they will express (Figure 1B).
Ikaros sets the threshold for B cell activation
Ikaros is a crucial regulator of B cell activation. Ikaros-deficient IkL/L B cells exhibit lower activation thresholds to stimulation than WT cells (e.g. proliferate to lower concentrations of anti-IgM stimulation)[65]. Similarly, B cells from mice bearing a B cell specific transgene encoding the dominant negative (DN) Ikaros 7 isoform, are hyper-responsive to stimulation by mitogens such as lipopolysaccharide[73]. Thus, Ikaros sets B cell activation thresholds for antigen and mitogen stimuli. Interestingly, Ikaros plays a similar role in setting activation thresholds for TCR signals in T cells[5,74].
While it is clear that Ikaros regulates B cell responses to stimulation, the mechanism remains a mystery. Data from T cells have suggested that Ikaros maintains activation thresholds by integrating the inputs of multiple signaling cascades into a transcriptional response to stimulation[5,74]. The rationale for this is two fold: (1) in comparison with WT cells, Ik+/- and Ik+/DN T cells are resistant to inhibitors of signaling pathways that lie downstream of the TCR (MAPK, Ras, PI3K/Akt, LCK/FYN, PKC, calcineurin), indicating that no one pathway is responsible for increased proliferation; and (2) Ikaros, colocalizes with heterochromatin and replication foci, and thus its loss might result in widespread gene deregulation[5,74]. This latter point may be especially relevant in cycling cells, which must synthesize their DNA and re-establish heterochromatin with each cycle. To date however, it is unclear if Ikaros deficiency grossly changes the transcriptional response to BCR, TCR or mitogen stimulation in lymphocytes, and thus, this model lacks strong experimental support.
There are other intriguing possibilities to explain how Ikaros controls B cell activation thresholds. First, retroviral Ikaros expression in Ik-/- T lymphoma cell lines upregulates the cell cycle inhibitor p27kip and blocks cell cycle progression[75]. Thus Ikaros might set activation thresholds in B cells by maintaining the expression of specific cell cycle inhibitors. Another intriguing possibility focuses on the Notch pathway. Ikaros represses Notch target genes in T cells[23,38] and Notch activity can synergize with BCR and CD40 signaling to enhance B cell activation[76]. Thus, Ikaros could control BCR-induced activation by suppressing Notch pathway activity. Finally, it should be noted that while Ca++ mobilization is similar between WT and IkL/L B cells after stimulation, the activation of other signaling pathways has not been examined[65]. Furthermore, these comparisons have not been rigorously made in T cells. Interestingly, Ikaros appears to regulate BCR signaling positively in chicken DT40 cells by directly suppressing SHIP phosphatase expression[77,78]. While the hypo-responsive phenotype of Ik-/- DT40 cells contrasts with the hyper-responsive phenotype of Ikaros-deficient murine B and T cells, work in Ik-/- DT40 cells clearly demonstrates that Ikaros can control the sensitivity of signaling pathways downstream of antigen receptors. Thus, it is possible that Ikaros deficiency controls lymphocyte activation thresholds by regulating cell cycle inhibitors, Notch signals, or BCR, TCR or Toll-like receptor signaling pathways.
It is important to note that the abnormal activation threshold in Ikaros-deficient B cells may have real consequences for tolerance. Mutations that compromise B cell activation thresholds often lead to autoantibody production[79,80]. In keeping with this, mice with B cells expressing DN Ikaros express higher levels of auto antibodies than do WT mice[73]. Similarly, Ikaros hypomorphic IkL/L mice express auto antibodies (Sellars M, Kastner P and Chan S unpublished data). While this suggests a possible role for Ikaros in the induction of B cell tolerance, it is unclear from current studies if this would be B cell intrinsic role for Ikaros and not one of its related family members. DN Ikaros isoforms retain the ability to interact with and inhibit other Ikaros family members, including Aiolos, which also regulates B cell activation thresholds[81]. Thus elevated auto-antibodies in mice expressing a B cell restricted DN Ikaros transgene[73], could be due in part to Aiolos inhibition. Furthermore, IkL/L T cells are hyper-responsive[23], and thus auto-antibodies in these mice could be the result of defective T cell tolerance. Further studies with conditional knockouts of Ikaros will be necessary to understand its fully role in B cell tolerance.
Ikaros regulates isotype selection during immunoglobulin class switch recombination
Class switch recombination (CSR) allows the humoral immune response to clear pathogens effectively by pairing a single antibody variable region gene with different constant region genes (CH) responsible for unique effector functions[82]. Recombination occurs between induced double stranded breaks in repetitive DNA sequences called switch (S) regions; which are located upstream of each CH gene (except δ). These breaks are initiated by activation-induced cytidine deaminase (AID) in a transcription-dependent manner, the mechanism of which is not fully understood[82,83]. Importantly, it is transcription across specific S regions in response to antigen, cytokine, and co-stimulatory signals that targets those S regions for CSR[84]. Despite its importance, the factors controlling S region transcription have remained largely unidentified.
Recent work has demonstrated that Ikaros is a central regulator of S region transcription and thereby of isotype selection during CSR. The first clue to this came when IkL/L mice were found to exhibit abnormal serum antibody titers, characterized by striking > 50% reductions in IgG3 and IgG1, and > 50% increases in IgG2b and IgG2a[65]. In vitro culture assays then revealed that Ikaros deficiency results in increased and ectopic CSR to IgG2b and IgG2a, and reduced CSR to all other isotypes[85]. Mechanistically, Ikaros binds directly to the Igh locus, including the 3’ enhancer and S region promoters and suppresses activating epigenetic marks (e.g. histone acetylation), transcription and AID accessibility across Sγ2b and Sγ2a. In fact, this transcriptional repression at a subset of S regions allows other S regions to compete for AID-induced CSR. Thus, Ikaros is a master regulator of isotype specification during CSR, and mediates this function by modulating transcriptional competition between S regions.
CONCLUSION
Ikaros controls major aspects of B cell development and B cell responses to antigen. It contributes to B lineage specification, commitment and maturation (Figure 1A, Table 2). Ikaros activates the expression of IL7R and Flt3, signals through which are critical for the development of the earliest B cell progenitors. Ikaros further promotes B cell differentiation by inducing EBF1, which itself activates a B cell transcriptional program. Beyond specification, Ikaros plays roles similar to Pax5 in repressing alternative (especially myeloid) fates in B cell progenitors. Finally, Ikaros contributes to further maturation by activating Rag gene expression and constricting the Igh locus to allow for antigen receptor recombination. Clearly Ikaros is a critical regulator of early B cell development.
In the periphery, Ikaros controls activation thresholds and isotype choice during CSR. Ikaros appears to control CSR by directly regulating activating epigenetic marks and transcription at constant region gene promoters (Figure 1B). Notably however, the mechanism by which Ikaros regulates B cell activation remains largely undefined. Does Ikaros shape the transcriptional response to stimulation, repress Notch-mediated proliferation signals, directly control the expression of cell cycle regulators, and/or modulate B cell receptor signaling itself? Or does Ikaros act through some other mechanism to control B cell activation? Future research will be needed to resolve these questions.
Footnotes
Supported by La Ligue Contre le Cancer (équipe labellisée), l’Agence Nationale de la Recherche and La Fondation pour la Recherche Médicale, with institute funding from INSERM, CNRS and l’Université de Strasbourg
Peer reviewers: Sin-Hyeog Im, PhD, Associate Professor, Department of Life Sciences, Gwangju Institute of Science and Technology, 1 Oryong-dong, Buk-gu, Gwangju 500-712, South Korea; Mate Tolnay, PhD, Division of Monoclonal Antibodies, 10903 New Hampshire Ave, HFD-123, NIH Bldg. 29B , Silver Spring, MD 20993, United States; Dirk Saerens, PhD, Department of Cellular and Molecular Immunology, Vrije Universiteits Brussel-Vlaams Instituut voor Biotechnology, Pleinlaan 2, B-1050 Brussel, Belgium
S- Editor Cheng JX L- Editor Kerr C E- Editor Zheng XM
References
- 1.Lo K, Landau NR, Smale ST. LyF-1, a transcriptional regulator that interacts with a novel class of promoters for lymphocyte-specific genes. Mol Cell Biol. 1991;11:5229–5243. doi: 10.1128/mcb.11.10.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Georgopoulos K, Moore DD, Derfler B. Ikaros, an early lymphoid-specific transcription factor and a putative mediator for T cell commitment. Science. 1992;258:808–812. doi: 10.1126/science.1439790. [DOI] [PubMed] [Google Scholar]
- 3.Georgopoulos K, Bigby M, Wang JH, Molnar A, Wu P, Winandy S, Sharpe A. The Ikaros gene is required for the development of all lymphoid lineages. Cell. 1994;79:143–156. doi: 10.1016/0092-8674(94)90407-3. [DOI] [PubMed] [Google Scholar]
- 4.Winandy S, Wu L, Wang JH, Georgopoulos K. Pre-T cell receptor (TCR) and TCR-controlled checkpoints in T cell differentiation are set by Ikaros. J Exp Med. 1999;190:1039–1048. doi: 10.1084/jem.190.8.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avitahl N, Winandy S, Friedrich C, Jones B, Ge Y, Georgopoulos K. Ikaros sets thresholds for T cell activation and regulates chromosome propagation. Immunity. 1999;10:333–343. doi: 10.1016/s1074-7613(00)80033-3. [DOI] [PubMed] [Google Scholar]
- 6.Urban JA, Winandy S. Ikaros null mice display defects in T cell selection and CD4 versus CD8 lineage decisions. J Immunol. 2004;173:4470–4478. doi: 10.4049/jimmunol.173.7.4470. [DOI] [PubMed] [Google Scholar]
- 7.Klug CA, Morrison SJ, Masek M, Hahm K, Smale ST, Weissman IL. Hematopoietic stem cells and lymphoid progenitors express different Ikaros isoforms, and Ikaros is localized to heterochromatin in immature lymphocytes. Proc Natl Acad Sci U S A. 1998;95:657–662. doi: 10.1073/pnas.95.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelley CM, Ikeda T, Koipally J, Avitahl N, Wu L, Georgopoulos K, Morgan BA. Helios, a novel dimerization partner of Ikaros expressed in the earliest hematopoietic progenitors. Curr Biol. 1998;8:508–515. doi: 10.1016/s0960-9822(98)70202-7. [DOI] [PubMed] [Google Scholar]
- 9.Payne KJ, Nicolas JH, Zhu JY, Barsky LW, Crooks GM. Cutting edge: predominant expression of a novel Ikaros isoform in normal human hemopoiesis. J Immunol. 2001;167:1867–1870. doi: 10.4049/jimmunol.167.4.1867. [DOI] [PubMed] [Google Scholar]
- 10.Molnár A, Wu P, Largespada DA, Vortkamp A, Scherer S, Copeland NG, Jenkins NA, Bruns G, Georgopoulos K. The Ikaros gene encodes a family of lymphocyte-restricted zinc finger DNA binding proteins, highly conserved in human and mouse. J Immunol. 1996;156:585–592. [PubMed] [Google Scholar]
- 11.Tonnelle C, Bardin F, Maroc C, Imbert AM, Campa F, Dalloul A, Schmitt C, Chabannon C. Forced expression of the Ikaros 6 isoform in human placental blood CD34(+) cells impairs their ability to differentiate toward the B-lymphoid lineage. Blood. 2001;98:2673–2680. doi: 10.1182/blood.v98.9.2673. [DOI] [PubMed] [Google Scholar]
- 12.Payne KJ, Huang G, Sahakian E, Zhu JY, Barteneva NS, Barsky LW, Payne MA, Crooks GM. Ikaros isoform x is selectively expressed in myeloid differentiation. J Immunol. 2003;170:3091–3098. doi: 10.4049/jimmunol.170.6.3091. [DOI] [PubMed] [Google Scholar]
- 13.Nakayama H, Ishimaru F, Katayama Y, Nakase K, Sezaki N, Takenaka K, Shinagawa K, Ikeda K, Niiya K, Harada M. Ikaros expression in human hematopoietic lineages. Exp Hematol. 2000;28:1232–1238. doi: 10.1016/s0301-472x(00)00530-0. [DOI] [PubMed] [Google Scholar]
- 14.Ezzat S, Asa SL. The emerging role of the Ikaros stem cell factor in the neuroendocrine system. J Mol Endocrinol. 2008;41:45–51. doi: 10.1677/JME-08-0045. [DOI] [PubMed] [Google Scholar]
- 15.Boggs SS, Trevisan M, Patrene K, Geogopoulos K. Lack of natural killer cell precursors in fetal liver of Ikaros knockout mutant mice. Nat Immun. 1998;16:137–145. doi: 10.1159/000069438. [DOI] [PubMed] [Google Scholar]
- 16.Nichogiannopoulou A, Trevisan M, Neben S, Friedrich C, Georgopoulos K. Defects in hemopoietic stem cell activity in Ikaros mutant mice. J Exp Med. 1999;190:1201–1214. doi: 10.1084/jem.190.9.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papathanasiou P, Attema JL, Karsunky H, Hosen N, Sontani Y, Hoyne GF, Tunningley R, Smale ST, Weissman IL. Self-renewal of the long-term reconstituting subset of hematopoietic stem cells is regulated by Ikaros. Stem Cells. 2009;27:3082–3092. doi: 10.1002/stem.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allman D, Dalod M, Asselin-Paturel C, Delale T, Robbins SH, Trinchieri G, Biron CA, Kastner P, Chan S. Ikaros is required for plasmacytoid dendritic cell differentiation. Blood. 2006;108:4025–4034. doi: 10.1182/blood-2006-03-007757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dumortier A, Kirstetter P, Kastner P, Chan S. Ikaros regulates neutrophil differentiation. Blood. 2003;101:2219–2226. doi: 10.1182/blood-2002-05-1336. [DOI] [PubMed] [Google Scholar]
- 20.Wu L, Nichogiannopoulou A, Shortman K, Georgopoulos K. Cell-autonomous defects in dendritic cell populations of Ikaros mutant mice point to a developmental relationship with the lymphoid lineage. Immunity. 1997;7:483–492. doi: 10.1016/s1074-7613(00)80370-2. [DOI] [PubMed] [Google Scholar]
- 21.Lopez RA, Schoetz S, DeAngelis K, O'Neill D, Bank A. Multiple hematopoietic defects and delayed globin switching in Ikaros null mice. Proc Natl Acad Sci U S A. 2002;99:602–607. doi: 10.1073/pnas.022412699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winandy S, Wu P, Georgopoulos K. A dominant mutation in the Ikaros gene leads to rapid development of leukemia and lymphoma. Cell. 1995;83:289–299. doi: 10.1016/0092-8674(95)90170-1. [DOI] [PubMed] [Google Scholar]
- 23.Dumortier A, Jeannet R, Kirstetter P, Kleinmann E, Sellars M, dos Santos NR, Thibault C, Barths J, Ghysdael J, Punt JA, et al. Notch activation is an early and critical event during T-Cell leukemogenesis in Ikaros-deficient mice. Mol Cell Biol. 2006;26:209–220. doi: 10.1128/MCB.26.1.209-220.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papathanasiou P, Perkins AC, Cobb BS, Ferrini R, Sridharan R, Hoyne GF, Nelms KA, Smale ST, Goodnow CC. Widespread failure of hematolymphoid differentiation caused by a recessive niche-filling allele of the Ikaros transcription factor. Immunity. 2003;19:131–144. doi: 10.1016/s1074-7613(03)00168-7. [DOI] [PubMed] [Google Scholar]
- 25.Mullighan CG, Miller CB, Radtke I, Phillips LA, Dalton J, Ma J, White D, Hughes TP, Le Beau MM, Pui CH, et al. BCR-ABL1 lymphoblastic leukaemia is characterized by the deletion of Ikaros. Nature. 2008;453:110–114. doi: 10.1038/nature06866. [DOI] [PubMed] [Google Scholar]
- 26.Olivero S, Maroc C, Beillard E, Gabert J, Nietfeld W, Chabannon C, Tonnelle C. Detection of different Ikaros isoforms in human leukaemias using real-time quantitative polymerase chain reaction. Br J Haematol. 2000;110:826–830. doi: 10.1046/j.1365-2141.2000.02297.x. [DOI] [PubMed] [Google Scholar]
- 27.Nakase K, Ishimaru F, Avitahl N, Dansako H, Matsuo K, Fujii K, Sezaki N, Nakayama H, Yano T, Fukuda S, et al. Dominant negative isoform of the Ikaros gene in patients with adult B-cell acute lymphoblastic leukemia. Cancer Res. 2000;60:4062–4065. [PubMed] [Google Scholar]
- 28.Brown KE, Guest SS, Smale ST, Hahm K, Merkenschlager M, Fisher AG. Association of transcriptionally silent genes with Ikaros complexes at centromeric heterochromatin. Cell. 1997;91:845–854. doi: 10.1016/s0092-8674(00)80472-9. [DOI] [PubMed] [Google Scholar]
- 29.Koipally J, Renold A, Kim J, Georgopoulos K. Repression by Ikaros and Aiolos is mediated through histone deacetylase complexes. EMBO J. 1999;18:3090–3100. doi: 10.1093/emboj/18.11.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sridharan R, Smale ST. Predominant interaction of both Ikaros and Helios with the NuRD complex in immature thymocytes. J Biol Chem. 2007;282:30227–30238. doi: 10.1074/jbc.M702541200. [DOI] [PubMed] [Google Scholar]
- 31.Kim J, Sif S, Jones B, Jackson A, Koipally J, Heller E, Winandy S, Viel A, Sawyer A, Ikeda T, et al. Ikaros DNA-binding proteins direct formation of chromatin remodeling complexes in lymphocytes. Immunity. 1999;10:345–355. doi: 10.1016/s1074-7613(00)80034-5. [DOI] [PubMed] [Google Scholar]
- 32.Koipally J, Georgopoulos K. A molecular dissection of the repression circuitry of Ikaros. J Biol Chem. 2002;277:27697–27705. doi: 10.1074/jbc.M201694200. [DOI] [PubMed] [Google Scholar]
- 33.Cobb BS, Morales-Alcelay S, Kleiger G, Brown KE, Fisher AG, Smale ST. Targeting of Ikaros to pericentromeric heterochromatin by direct DNA binding. Genes Dev. 2000;14:2146–2160. doi: 10.1101/gad.816400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koipally J, Georgopoulos K. Ikaros interactions with CtBP reveal a repression mechanism that is independent of histone deacetylase activity. J Biol Chem. 2000;275:19594–19602. doi: 10.1074/jbc.M000254200. [DOI] [PubMed] [Google Scholar]
- 35.Koipally J, Georgopoulos K. Ikaros-CtIP interactions do not require C-terminal binding protein and participate in a deacetylase-independent mode of repression. J Biol Chem. 2002;277:23143–23149. doi: 10.1074/jbc.M202079200. [DOI] [PubMed] [Google Scholar]
- 36.Thompson EC, Cobb BS, Sabbattini P, Meixlsperger S, Parelho V, Liberg D, Taylor B, Dillon N, Georgopoulos K, Jumaa H, et al. Ikaros DNA-binding proteins as integral components of B cell developmental-stage-specific regulatory circuits. Immunity. 2007;26:533. doi: 10.1016/j.immuni.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 37.Trinh LA, Ferrini R, Cobb BS, Weinmann AS, Hahm K, Ernst P, Garraway IP, Merkenschlager M, Smale ST. Down-regulation of TDT transcription in CD4(+)CD8(+) thymocytes by Ikaros proteins in direct competition with an Ets activator. Genes Dev. 2001;15:1817–1832. doi: 10.1101/gad.905601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kleinmann E, Geimer Le Lay AS, Sellars M, Kastner P, Chan S. Ikaros represses the transcriptional response to Notch signaling in T-cell development. Mol Cell Biol. 2008;28:7465–7475. doi: 10.1128/MCB.00715-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koipally J, Heller EJ, Seavitt JR, Georgopoulos K. Unconventional potentiation of gene expression by Ikaros. J Biol Chem. 2002;277:13007–13015. doi: 10.1074/jbc.M111371200. [DOI] [PubMed] [Google Scholar]
- 40.Harker N, Naito T, Cortes M, Hostert A, Hirschberg S, Tolaini M, Roderick K, Georgopoulos K, Kioussis D. The CD8alpha gene locus is regulated by the Ikaros family of proteins. Mol Cell. 2002;10:1403–1415. doi: 10.1016/s1097-2765(02)00711-6. [DOI] [PubMed] [Google Scholar]
- 41.O'Neill DW, Schoetz SS, Lopez RA, Castle M, Rabinowitz L, Shor E, Krawchuk D, Goll MG, Renz M, Seelig HP, et al. An ikaros-containing chromatin-remodeling complex in adult-type erythroid cells. Mol Cell Biol. 2000;20:7572–7582. doi: 10.1128/mcb.20.20.7572-7582.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang W. The SWI/SNF family of ATP-dependent chromatin remodelers: similar mechanisms for diverse functions. Curr Top Microbiol Immunol. 2003;274:143–169. doi: 10.1007/978-3-642-55747-7_6. [DOI] [PubMed] [Google Scholar]
- 43.Bottardi S, Zmiri FA, Bourgoin V, Ross J, Mavoungou L, Milot E. Ikaros interacts with P-TEFb and cooperates with GATA-1 to enhance transcription elongation. Nucleic Acids Res. 2011;39:3505–3519. doi: 10.1093/nar/gkq1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gómez-del Arco P, Maki K, Georgopoulos K. Phosphorylation controls Ikaros's ability to negatively regulate the G(1)-S transition. Mol Cell Biol. 2004;24:2797–2807. doi: 10.1128/MCB.24.7.2797-2807.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gurel Z, Ronni T, Ho S, Kuchar J, Payne KJ, Turk CW, Dovat S. Recruitment of ikaros to pericentromeric heterochromatin is regulated by phosphorylation. J Biol Chem. 2008;283:8291–8300. doi: 10.1074/jbc.M707906200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Popescu M, Gurel Z, Ronni T, Song C, Hung KY, Payne KJ, Dovat S. Ikaros stability and pericentromeric localization are regulated by protein phosphatase 1. J Biol Chem. 2009;284:13869–13880. doi: 10.1074/jbc.M900209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gómez-del Arco P, Koipally J, Georgopoulos K. Ikaros SUMOylation: switching out of repression. Mol Cell Biol. 2005;25:2688–2697. doi: 10.1128/MCB.25.7.2688-2697.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nutt SL, Kee BL. The transcriptional regulation of B cell lineage commitment. Immunity. 2007;26:715–725. doi: 10.1016/j.immuni.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 49.Mandel EM, Grosschedl R. Transcription control of early B cell differentiation. Curr Opin Immunol. 2010;22:161–167. doi: 10.1016/j.coi.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 50.Dias S, Silva H, Cumano A, Vieira P. Interleukin-7 is necessary to maintain the B cell potential in common lymphoid progenitors. J Exp Med. 2005;201:971–979. doi: 10.1084/jem.20042393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bain G, Maandag EC, Izon DJ, Amsen D, Kruisbeek AM, Weintraub BC, Krop I, Schlissel MS, Feeney AJ, van Roon M. E2A proteins are required for proper B cell development and initiation of immunoglobulin gene rearrangements. Cell. 1994;79:885–892. doi: 10.1016/0092-8674(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 52.Kikuchi K, Lai AY, Hsu CL, Kondo M. IL-7 receptor signaling is necessary for stage transition in adult B cell development through up-regulation of EBF. J Exp Med. 2005;201:1197–1203. doi: 10.1084/jem.20050158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sitnicka E, Brakebusch C, Martensson IL, Svensson M, Agace WW, Sigvardsson M, Buza-Vidas N, Bryder D, Cilio CM, Ahlenius H, et al. Complementary signaling through flt3 and interleukin-7 receptor alpha is indispensable for fetal and adult B cell genesis. J Exp Med. 2003;198:1495–1506. doi: 10.1084/jem.20031152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hirokawa S, Sato H, Kato I, Kudo A. EBF-regulating Pax5 transcription is enhanced by STAT5 in the early stage of B cells. Eur J Immunol. 2003;33:1824–1829. doi: 10.1002/eji.200323974. [DOI] [PubMed] [Google Scholar]
- 55.O'Riordan M, Grosschedl R. Coordinate regulation of B cell differentiation by the transcription factors EBF and E2A. Immunity. 1999;11:21–31. doi: 10.1016/s1074-7613(00)80078-3. [DOI] [PubMed] [Google Scholar]
- 56.Nutt SL, Urbánek P, Rolink A, Busslinger M. Essential functions of Pax5 (BSAP) in pro-B cell development: difference between fetal and adult B lymphopoiesis and reduced V-to-DJ recombination at the IgH locus. Genes Dev. 1997;11:476–491. doi: 10.1101/gad.11.4.476. [DOI] [PubMed] [Google Scholar]
- 57.Delogu A, Schebesta A, Sun Q, Aschenbrenner K, Perlot T, Busslinger M. Gene repression by Pax5 in B cells is essential for blood cell homeostasis and is reversed in plasma cells. Immunity. 2006;24:269–281. doi: 10.1016/j.immuni.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 58.Souabni A, Cobaleda C, Schebesta M, Busslinger M. Pax5 promotes B lymphopoiesis and blocks T cell development by repressing Notch1. Immunity. 2002;17:781–793. doi: 10.1016/s1074-7613(02)00472-7. [DOI] [PubMed] [Google Scholar]
- 59.Kitamura D, Kudo A, Schaal S, Müller W, Melchers F, Rajewsky K. A critical role of lambda 5 protein in B cell development. Cell. 1992;69:823–831. doi: 10.1016/0092-8674(92)90293-l. [DOI] [PubMed] [Google Scholar]
- 60.Mundt C, Licence S, Shimizu T, Melchers F, Mårtensson IL. Loss of precursor B cell expansion but not allelic exclusion in VpreB1/VpreB2 double-deficient mice. J Exp Med. 2001;193:435–445. doi: 10.1084/jem.193.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fleming HE, Paige CJ. Pre-B cell receptor signaling mediates selective response to IL-7 at the pro-B to pre-B cell transition via an ERK/MAP kinase-dependent pathway. Immunity. 2001;15:521–531. doi: 10.1016/s1074-7613(01)00216-3. [DOI] [PubMed] [Google Scholar]
- 62.Hess J, Werner A, Wirth T, Melchers F, Jäck HM, Winkler TH. Induction of pre-B cell proliferation after de novo synthesis of the pre-B cell receptor. Proc Natl Acad Sci U S A. 2001;98:1745–1750. doi: 10.1073/pnas.041492098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marshall AJ, Fleming HE, Wu GE, Paige CJ. Modulation of the IL-7 dose-response threshold during pro-B cell differentiation is dependent on pre-B cell receptor expression. J Immunol. 1998;161:6038–6045. [PubMed] [Google Scholar]
- 64.Wang JH, Nichogiannopoulou A, Wu L, Sun L, Sharpe AH, Bigby M, Georgopoulos K. Selective defects in the development of the fetal and adult lymphoid system in mice with an Ikaros null mutation. Immunity. 1996;5:537–549. doi: 10.1016/s1074-7613(00)80269-1. [DOI] [PubMed] [Google Scholar]
- 65.Kirstetter P, Thomas M, Dierich A, Kastner P, Chan S. Ikaros is critical for B cell differentiation and function. Eur J Immunol. 2002;32:720–730. doi: 10.1002/1521-4141(200203)32:3<720::AID-IMMU720>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 66.Yoshida T, Ng SY, Zuniga-Pflucker JC, Georgopoulos K. Early hematopoietic lineage restrictions directed by Ikaros. Nat Immunol. 2006;7:382–391. doi: 10.1038/ni1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reynaud D, Demarco IA, Reddy KL, Schjerven H, Bertolino E, Chen Z, Smale ST, Winandy S, Singh H. Regulation of B cell fate commitment and immunoglobulin heavy-chain gene rearrangements by Ikaros. Nat Immunol. 2008;9:927–936. doi: 10.1038/ni.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fuxa M, Skok J, Souabni A, Salvagiotto G, Roldan E, Busslinger M. Pax5 induces V-to-DJ rearrangements and locus contraction of the immunoglobulin heavy-chain gene. Genes Dev. 2004;18:411–422. doi: 10.1101/gad.291504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thompson EC, Cobb BS, Sabbattini P, Meixlsperger S, Parelho V, Liberg D, Taylor B, Dillon N, Georgopoulos K, Jumaa H, et al. Ikaros DNA-binding proteins as integral components of B cell developmental-stage-specific regulatory circuits. Immunity. 2007;26:335–344. doi: 10.1016/j.immuni.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 70.Sabbattini P, Lundgren M, Georgiou A, Chow C, Warnes G, Dillon N. Binding of Ikaros to the lambda5 promoter silences transcription through a mechanism that does not require heterochromatin formation. EMBO J. 2001;20:2812–2822. doi: 10.1093/emboj/20.11.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu Z, Widlak P, Zou Y, Xiao F, Oh M, Li S, Chang MY, Shay JW, Garrard WT. A recombination silencer that specifies heterochromatin positioning and ikaros association in the immunoglobulin kappa locus. Immunity. 2006;24:405–415. doi: 10.1016/j.immuni.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 72.Goldmit M, Ji Y, Skok J, Roldan E, Jung S, Cedar H, Bergman Y. Epigenetic ontogeny of the Igk locus during B cell development. Nat Immunol. 2005;6:198–203. doi: 10.1038/ni1154. [DOI] [PubMed] [Google Scholar]
- 73.Wojcik H, Griffiths E, Staggs S, Hagman J, Winandy S. Expression of a non-DNA-binding Ikaros isoform exclusively in B cells leads to autoimmunity but not leukemogenesis. Eur J Immunol. 2007;37:1022–1032. doi: 10.1002/eji.200637026. [DOI] [PubMed] [Google Scholar]
- 74.Cortes M, Wong E, Koipally J, Georgopoulos K. Control of lymphocyte development by the Ikaros gene family. Curr Opin Immunol. 1999;11:167–171. doi: 10.1016/s0952-7915(99)80028-4. [DOI] [PubMed] [Google Scholar]
- 75.Kathrein KL, Lorenz R, Innes AM, Griffiths E, Winandy S. Ikaros induces quiescence and T-cell differentiation in a leukemia cell line. Mol Cell Biol. 2005;25:1645–1654. doi: 10.1128/MCB.25.5.1645-1654.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thomas M, Calamito M, Srivastava B, Maillard I, Pear WS, Allman D. Notch activity synergizes with B-cell-receptor and CD40 signaling to enhance B-cell activation. Blood. 2007;109:3342–3350. doi: 10.1182/blood-2006-09-046698. [DOI] [PubMed] [Google Scholar]
- 77.Nera KP, Alinikula J, Terho P, Narvi E, Törnquist K, Kurosaki T, Buerstedde JM, Lassila O. Ikaros has a crucial role in regulation of B cell receptor signaling. Eur J Immunol. 2006;36:516–525. doi: 10.1002/eji.200535418. [DOI] [PubMed] [Google Scholar]
- 78.Alinikula J, Kohonen P, Nera KP, Lassila O. Concerted action of Helios and Ikaros controls the expression of the inositol 5-phosphatase SHIP. Eur J Immunol. 2010;40:2599–2607. doi: 10.1002/eji.200940002. [DOI] [PubMed] [Google Scholar]
- 79.Gauld SB, Merrell KT, Cambier JC. Silencing of autoreactive B cells by anergy: a fresh perspective. Curr Opin Immunol. 2006;18:292–297. doi: 10.1016/j.coi.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 80.Tuscano JM, Harris GS, Tedder TF. B lymphocytes contribute to autoimmune disease pathogenesis: current trends and clinical implications. Autoimmun Rev. 2003;2:101–108. doi: 10.1016/s1568-9972(02)00148-9. [DOI] [PubMed] [Google Scholar]
- 81.Wang JH, Avitahl N, Cariappa A, Friedrich C, Ikeda T, Renold A, Andrikopoulos K, Liang L, Pillai S, Morgan BA, et al. Aiolos regulates B cell activation and maturation to effector state. Immunity. 1998;9:543–553. doi: 10.1016/s1074-7613(00)80637-8. [DOI] [PubMed] [Google Scholar]
- 82.Stavnezer J, Guikema JE, Schrader CE. Mechanism and regulation of class switch recombination. Annu Rev Immunol. 2008;26:261–292. doi: 10.1146/annurev.immunol.26.021607.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ramiro A, Reina San-Martin B, McBride K, Jankovic M, Barreto V, Nussenzweig A, Nussenzweig MC. The role of activation-induced deaminase in antibody diversification and chromosome translocations. Adv Immunol. 2007;94:75–107. doi: 10.1016/S0065-2776(06)94003-6. [DOI] [PubMed] [Google Scholar]
- 84.Chaudhuri J, Alt FW. Class-switch recombination: interplay of transcription, DNA deamination and DNA repair. Nat Rev Immunol. 2004;4:541–552. doi: 10.1038/nri1395. [DOI] [PubMed] [Google Scholar]
- 85.Sellars M, Reina-San-Martin B, Kastner P, Chan S. Ikaros controls isotype selection during immunoglobulin class switch recombination. J Exp Med. 2009;206:1073–1087. doi: 10.1084/jem.20082311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kee BL, Murre C. Induction of early B cell factor (EBF) and multiple B lineage genes by the basic helix-loop-helix transcription factor E12. J Exp Med. 1998;188:699–713. doi: 10.1084/jem.188.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lin H, Grosschedl R. Failure of B-cell differentiation in mice lacking the transcription factor EBF. Nature. 1995;376:263–267. doi: 10.1038/376263a0. [DOI] [PubMed] [Google Scholar]