Abstract
Through alternate splicing, the Ikaros gene produces multiple proteins. Ikaros is essential for normal hematopoiesis and possesses tumor suppressor activity. Ikaros isoforms interact to form dimers and potentially multimeric complexes. Diverse Ikaros complexes produced by the presence of different Ikaros isoforms are hypothesized to confer distinct functions. Small dominant-negative Ikaros isoforms have been shown to inhibit the tumor suppressor activity of full-length Ikaros. Here, we describe how Ikaros activity is regulated by the coordinated expression of the largest Ikaros isoforms IK-1 and IK-H. Although IK-1 is described as full-length Ikaros, IK-H is the longest Ikaros isoform. IK-H, which includes residues coded by exon 3B (60 bp that lie between exons 3 and 4), is abundant in human but not murine hematopoietic cells. Specific residues that lie within the 20 amino acids encoded by exon 3B give IK-H DNA-binding characteristics that are distinct from those of IK-1. Moreover, IK-H can potentiate or inhibit the ability of IK-1 to bind DNA. IK-H binds to the regulatory regions of genes that are upregulated by Ikaros, but not genes that are repressed by Ikaros. Although IK-1 localizes to pericentromeric heterochromatin, IK-H can be found in both pericentromeric and non-pericentromeric locations. Anti-silencing activity of gamma satellite DNA has been shown to depend on the binding of IK-H, but not other Ikaros isoforms. The unique features of IK-H, its influence on Ikaros activity, and the lack of IK-H expression in mice suggest that Ikaros function in humans may be more complex and possibly distinct from that in mice.
Keywords: Ikaros; Chromatin, Pericentromeric; Transcription; IK-H; Leukemia; γ satellite
IKAROS ISOFORM EXPRESSION AND IKAROS ACTIVITY
An important factor in the functional diversity of individual genes is their ability to encode different proteins. Many genes produce multiple proteins that can have dissimilar functions through alternate splicing. This process is responsible for the generation of complex structural and regulatory networks that control normal cellular function. Determination of the functional similarities and differences of individual isoforms is essential for understanding the role of a gene in a particular process.
The Ikaros gene encodes a zinc finger protein that is essential for normal hematopoiesis and that acts as a tumor suppressor[1-3]. The Ikaros protein binds DNA at the upstream regulatory elements of its target genes and regulates their transcription via chromatin remodeling. Soon after discovery of the Ikaros gene, it was determined that it encodes multiple isoforms via alternate splicing[4-6]. In mice, the full-length Ikaros isoform comprises three domains: (1) a DNA-binding domain (at the N-terminal end of the protein) that consists of four C2H2 Kruppel-like zinc finger motifs; (2) a protein-interaction domain (at the C-terminal end of the protein) that consists of two zinc finger motifs with an unusual structure that is most similar to those described in the hunchback gene in Drosophila; and (3) an activation domain that is a loosely defined domain that is located between the DNA-binding and protein-interaction domains[4]. Every Ikaros isoform described thus far contains the protein-interaction domain, but they differ in the presence of the DNA-binding domain, or other regions of the gene. Ikaros associates in vivo with its various isoforms, as well as with other Ikaros family members that contain a protein-interaction domain similar to that of Ikaros[7]. Ikaros binds DNA as a dimer and possibly as a multimer, therefore, it has been hypothesized that diverse complexes that comprise full-length Ikaros together with different types of isoforms could have distinct functions[8,9]. The ability of the different Ikaros isoforms to interact with each other and with other Ikaros family members[7,10,11] creates many possible combinations of proteins with the potential for diverse functions: four major possibilities are summarized in Figure 1.
Figure 1.
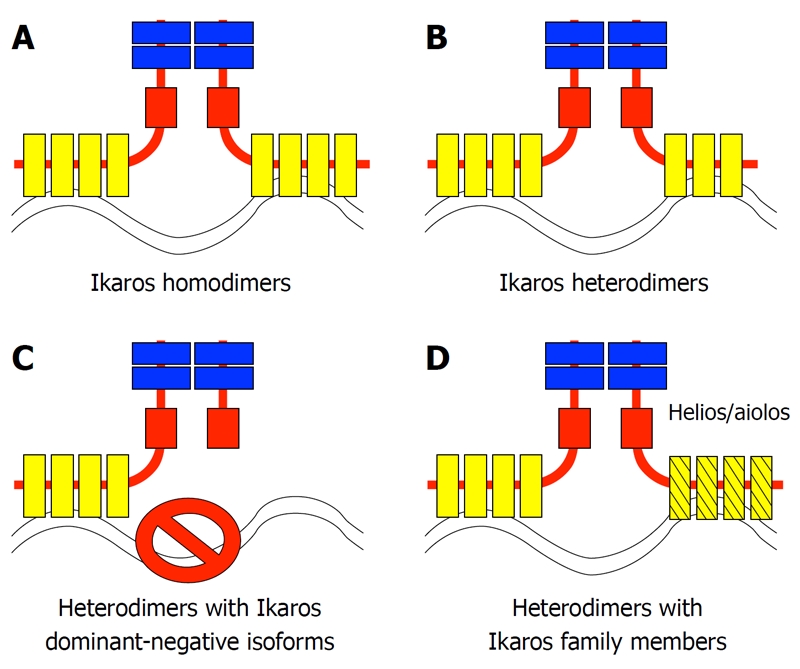
The association of Ikaros proteins with Ikaros isoforms and Ikaros family members controls the activity of the Ikaros protein complex. The four main types of Ikaros family-containing protein complexes are shown. A: Homodimers of the most abundant Ikaros isoform (IK-1); B: Heterodimers of IK-1 with DNA-binding Ikaros isoforms; C: Heterodimers of IK-1 and DNA-nonbinding (DN) Ikaros isoforms - this complex does not bind DNA; D: Heterodimers of Ikaros with its other family members (e.g. Helios or Aiolos).
Studies of mice with mutations in the Ikaros gene, together with biochemical experiments, have revealed that small Ikaros isoforms that contain a protein-interaction domain and that lack a DNA-binding domain exert a dominant-negative (DN) effect by inhibiting the activity of the full-length Ikaros protein[6]. This is demonstrated in Ikaros knockout mice that express small DN isoforms. These mice have a more severe phenotype when compared to “true null” Ikaros knockout mice[1,12,13]. Overexpression of DN isoforms disrupts normal hematopoiesis and has been shown to inhibit normal Ikaros function in numerous in vitro and in vivo systems[14,15]. In biochemical experiments, overexpression of DN isoforms inhibits DNA-binding of full-length Ikaros. High expression of DN Ikaros isoforms has been associated with hematopoietic malignancies in humans and mice, as well as with pituitary gland tumors[3,15-20]. It has been hypothesized that this is most likely due to inhibition of the tumor suppressor function of Ikaros and possibly other family members. Thus, the first established functional relationship between Ikaros isoforms was relatively clear cut -full-length Ikaros is functional and a tumor suppressor, whereas small DN isoforms are inhibitory and pro-oncogenic. A mechanistic explanation for this involved inhibition of the DNA-binding ability of the full-length Ikaros isoform by small DN ones. This involved the possibility depicted in Figure 1C.
Deciphering the functional significance of other large Ikaros isoforms has proved to be more complicated. One particular problem is the abundance of endogenous full-length Ikaros isoforms in most hematopoietic cells, which makes it difficult to study the function of individual large Ikaros isoforms in vivo. However, over time, data have emerged to reveal the potential functional significance of other Ikaros isoforms. Ikaros-2 (Ikaros-V in the nomenclature of the Smale group), which lacks the first N-terminal DNA-binding zinc finger, has been shown to produce a footprint distinct from that of IK-1 in DNase protection assays[6], although the functional significance of this has not been studied. A report by Payne et al[21] has revealed that the Ikaros-X isoform has a unique expression pattern compared to other large Ikaros isoforms, and has suggested that this isoform plays a role in myeloid differentiation. This study was limited to expression analysis and did not provide detailed functional analysis of the Ikaros-X isoform.
Most functional studies of Ikaros isoforms have been performed using murine transcripts and proteins. An early murine study has detected the presence of Ikaros protein and cDNA that includes coding sequence that lies between currently designated Ikaros exons 3 and 4[6]. Human Ikaros gene transcripts that incorporate the human homolog of this additional exon (which we designate exon 3B) have been subsequently detected in leukemia samples[19]. Studies of Ikaros protein expression in normal human hematopoietic cells have identified several Ikaros splice forms that include exon 3B, including one larger in size than any of the previously described murine Ikaros isoforms[21,22]. Comparisons of Ikaros expression in mice and humans have shown that strong protein level expression of this isoform occurs only in human cells[21,23]. For this reason, the largest Ikaros splice variant is termed Ikaros-H (H for human). Due to the high sequence homology between the murine and human Ikaros genes, the activity of individual isoforms has been assumed to be very similar. The high level protein expression of Ikaros-H in human, but not murine cells is an intriguing observation that suggests that the regulation of Ikaros activity in human cells might be more complex than in mouse cells. Other than Ikaros-1 (IK-1, and in the nomenclature of the Smale group, Ikaros-VI), Ikaros-H has been the subject of the most extensive functional studies to date. The following summarizes the functional characteristics of the Ikaros-H isoform.
EXPRESSION PATTERN AND DNA BINDING AFFINITY OF IK-H
The Ikaros-H isoform (IK-H, also designated IK-1+ in alternate nomenclature[22]) contains the sequence of the “full-length” Ikaros isoform (IK-1) plus an additional 20 amino acids N-terminal to the DNA-binding zinc fingers (Exon 3B in Figure 2A). In mice, the most abundant Ikaros isoforms in primary T and B lineage cells and in lymphoid leukemia cells are IK-1 and IK-2, while IK-H is essentially absent[21,23]. In contrast, in humans, IK-1, IK-2 and IK-H are expressed at comparable levels in primary leukemia cells and in lymphoid cell lines. IK-H is also abundant in primary human T and B cells[21]. Thus, IK-H exhibits a species-specific expression pattern.
Figure 2.
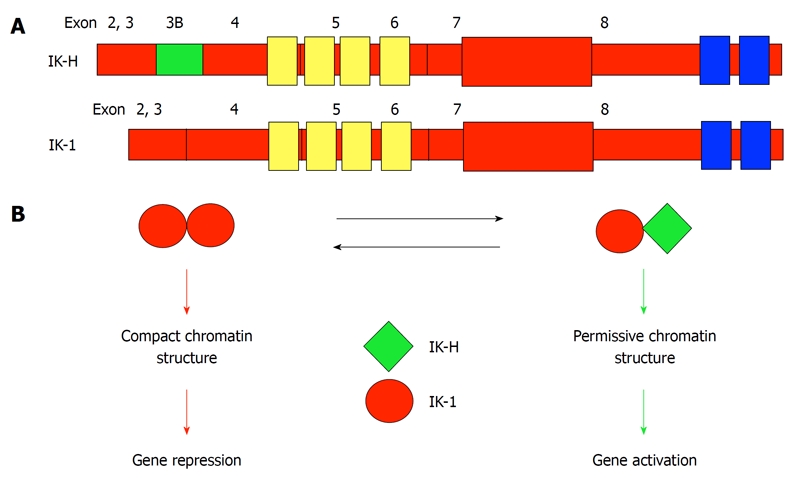
A model for IK-H regulation of human gene expression. A: Exon structure of IK-1 and IK-H. The 20 amino acids encoded by Exon 3B distinguish IK-H from IK-1. Zinc fingers responsible for DNA binding are shown in yellow. Zinc fingers responsible for dimerization are shown in blue; B: A protein complex that contains IK-1 homodimers binds DNA tightly, leading to a compact chromatin structure and gene repression. Binding of Ikaros protein complexes that contain IK-H changes chromatin into a permissive structure leading to activation of gene expression.
Ikaros contains four C2H2 Kruppel-like zinc finger motifs at its N-terminal end (exons 4-6) that directly interact with DNA. Zinc fingers 2 and 3 are essential for DNA binding of Ikaros protein, although other zinc fingers probably contribute to DNA-binding specificity of Ikaros[24]. Ikaros isoforms bind to the Ikaros consensus sequence TGGGAA/T with the core sequence comprising GGGA[24]. DNA binding analysis has revealed that the two largest human isoforms, IK-1 and IK-H, have different DNA-binding affinities[23]. Both isoforms can bind to the DNA sequences that contain the TGGGAA/T consensus site, as well as to high-affinity sites where two core consensus GGGA sequences are present within 40 bp of each other. However, only IK-1 is able to bind efficiently to DNA sequences that contain a single core sequence, GGGA; the absence of the second GGGA consensus sequence abolishes DNA binding by IK-H. This result was surprising and suggests that the presence of the 20 amino acids that are located upstream from the DNA-binding zinc fingers in IK-H have an inhibitory effect on DNA binding of Ikaros protein. Substitution mutational analysis[23]. Has identified three specific amino acids within the N region that are responsible for the differences in DNA binding of IK-H and IK-1. This suggests that it is not the presence of the additional 20 amino acids N-terminal of the DNA-binding zinc fingers that affects the ability of the Ikaros protein to bind DNA, but rather interaction of specific residues within the 20-amino-acid region with unknown proteins.
IK-H REGULATES DNA BINDING OF OTHER IKAROS ISOFORMS
Evidence that the two largest human Ikaros isoforms have different DNA-binding affinities has led to us to speculate that the DNA-binding affinity of Ikaros proteins for a specific DNA sequence could depend on the relative expression levels of IK-1 and IK-H isoforms. We hypothesize that IK-H could either synergize with IK-1 or act to inhibit its DNA-binding, depending on its unique affinity for a particular DNA sequence. This hypothesis has been tested in vitro by mixing recombinant IK-1 and IK-H isoforms and comparing the DNA-binding affinity of the IK-1/IK-H mixture with the same quantity of IK-1 protein. IK-1 and IK-H act synergistically to bind DNA that contains two Ikaros binding sites. However, the IK-H isoform inhibits the binding of IK-1 to DNA that contains a single Ikaros binding site[23]. Thus, IK-H can either potentiate or inhibit the DNA binding of the IK-1 isoform in vitro.
It has been demonstrated that Ikaros binds DNA in vivo as a dimer and possibly as a multimeric complex[25]. To test whether the interaction of IK-1 and IK-H regulates the DNA-binding affinity of Ikaros in vivo, the ability of Ikaros to bind DNA has been compared using electrophoretic mobility shift assay and chromatin immunoprecipitation (ChIP) to assess cells that expressed only IK-1, only IK-H, or both isoforms. Expression of IK-H selectively affects the DNA-binding of Ikaros proteins - DNA-binding to the DNA sequences that contain a single Ikaros binding site is reduced, but binding to DNA with two Ikaros consensus binding sites is strong. These experiments were performed in activated T cells, thus confirming the physiological relevance of IK-H interactions with IK-1. These results demonstrate that IK-H does not function as a typical DN isoform (as described for the small Ikaros isoforms that lack DNA binding zinc fingers), but rather as a unique control mechanism that determines DNA-binding specificity of Ikaros proteins in vivo. This provides confirmation that Ikaros binds DNA in vivo as a dimer or multimer, and for the first time has demonstrated the functional significance of the coexpression of different large Ikaros isoforms: to provide precise control of the DNA-binding specificity of Ikaros in cells.
SUBCELLULAR LOCALIZATION OF THE LARGEST IKAROS ISOFORMS
The condensed chromatin that flanks centromeres is known as pericentromeric heterochromatin (PC-HC). Genes localized to PC-HC are generally transcriptionally inactive. The subcellular localization of Ikaros to PC-HC has been shown to correlate directly with its DNA-binding affinity towards the repetitive sequences that are part of PC-HC[24]. Confocal microscopy has revealed that IK-1 and IK-H exhibit distinct subcellular localization. The IK-1 isoform displays the typical punctate distribution pattern consistent with PC-HC localization. In contrast, IK-H exhibits dual - non-centromeric and centromeric - localization. The non-centromeric localization of IK-H is not in the form of the diffuse nuclear staining that is typically observed in other transcriptional factors, but rather localization to other specific nuclear structures[23]. More intriguing is that the non-centromeric localization of IK-H is observed in hematopoietic cells, but not when this isoform is transduced into fibroblasts. When transduced in murine hematopoietic cells, IK-H retains the same localization pattern as in human cells. This suggests that the distinct localization pattern of IK-H is tissue-specific and that it is a result of the unique properties of the IK-H protein, and not due to the differences in heterochromatin between different species. This also provides evidence that IK-H exists in complexes that do not contain the IK-1 isoform and thus, it may have its own unique cellular function. This provides the first evidence to suggest that different Ikaros isoforms may function in distinct subcellular regions.
ROLE OF IK-H IN GENE ACTIVATION AND CHROMATIN REMODELING
It has been demonstrated that Ikaros can act both as a positive and a negative regulator of gene expression. This is likely related to the ability of Ikaros to associate with the SWI/SNF activator complex or with the histone deacetylase repressor complex, NuRD[26-28]. The evidence that Ikaros can both activate and repress expression of its target genes by recruiting them to PC-HC suggests a role for chromatin remodeling in this process[29]. The molecular mechanisms that determine whether Ikaros functions as an activator or repressor of transcription are unknown.
Experiments that have tested the DNA-binding specificities of IK-1 and IK-H isoforms to regulatory sequences of known Ikaros target genes have revealed differential binding of these isoforms to regulatory elements of repressed vs activated Ikaros targets. Binding of both IK-H and IK-1 to the upstream regulatory elements of IKCa1, Granzyme B, STAT4, and FAAH has been demonstrated by ChIP and electromobility supershift assay. In contrast, IK-1 and other Ikaros isoforms, but not IK-H, bind the upstream regulatory element of the VPAC-1 receptor gene. In vivo, Ikaros proteins downregulate transcription of the VPAC1 receptor gene[30], while positively regulating expression of IKCa1[31], Granzyme B[32], STAT4[33] and FAAH. The observation that the IK-H isoform binds to the regulatory region of genes upregulated by Ikaros, but not to the regulatory region of genes repressed by Ikaros has led to the intriguing hypothesis that the IK-H isoform acts as an activator, while other Ikaros isoforms act as transcriptional repressors of Ikaros target genes[23]. According to this hypothesis, IK-H would have the opposite function of other Ikaros isoforms in regulating transcription. The mechanism by which Ikaros would activate its target genes upon their recruitment to PC-HC has not been resolved.
A detailed analysis of the function of human gamma-satellite DNA repeats reveals that gamma-satellite DNA has anti-silencing activity[34]. ChIP analysis has documented binding of both IK-H and IK-1 to the gamma-satellite DNA. Mutational analysis reveals that the anti-silencing activity of gamma-satellite DNA is directly dependent on the presence of two consensus Ikaros binding sites that are in close proximity (bipartite motif), and on the binding of IK-H. The mutation of one Ikaros consensus binding site that abolishes the binding of IK-H, but not binding of other Ikaros isoforms, leads to the loss of the anti-silencing activity of gamma-satellite DNA[34]. These results clearly showed that: (1) human gamma-satellite DNA allows a transcriptionally permissive chromatin conformation, thus it can have an anti-silencing function; and (2) binding of the IK-H Ikaros isoform (but not other Ikaros isoforms) is essential for the anti-silencing activity of gamma-satellite DNA. This sheds light on the molecular mechanisms by which Ikaros can both activate or repress its target genes via recruitment to PC-HC and establishes distinct (and possibly opposing) roles for IK-H and IK-1 isoforms in the regulation of gene expression and chromatin remodeling. The current (simplified) model of transcriptional regulation by IK-H and IK-1 is outlined in Figure 2B.
CONCLUSION
In summary, the Ikaros gene encodes a large number of different proteins via alternate splicing. Ikaros-encoded proteins form dimers and multimers that regulate gene expression and chromatin remodeling. The presented analysis emphasizes that the function of Ikaros complexes depends on the presence of particular Ikaros isoforms, and that individual isoforms likely have unique functions. High expression of the IK-H isoform in human lymphoid cells, but not their murine counterparts, suggests that the regulatory function of Ikaros in humans is more complex and possibly distinct from that in mice.
The discovery of functional differences between IK-1 and IK-H isoforms raises many questions that need to be resolved. Future research will be directed to achieve the following goals: (1) dissection of the DNA-binding specificity of IK-1 and IK-H using global functional genomic approaches; and (2) identification of additional genes that are regulated by Ikaros and a correlation of the binding of IK-1 and IK-H to upstream regulatory regions with changes in target gene expression. Further analysis of the function of Ikaros proteins is essential for understanding their role in normal human hematopoiesis and their tumor suppressor function in human leukemia.
Footnotes
Supported by (in part) An R01 HL095120 grant, a St. Baldrick’s Foundation Career Development Award, the Four Diamonds Fund of the Pennsylvania State University, College of Medicine, and the John Wawrynovic Leukemia Research Scholar Endowment (SD)
Peer reviewers: Carlo Ventura, MD, PhD, Full Professor of Molecular Biology, Chief, Laboratory of Molecular Biology and Stem Cell Engineering-National Institute of Biostructures and Biosystems, University of Bologna, S. Orsola - Malpighi Hospital, Cardiovascular Department, Pavilion 21 Via Massarenti 9 Bologna 40138, Italy; Guillermo Montoya, Professor, Structural Biology and Biocomputing, Spanish National Cancer Research Centre, Melchor Fdez. Almagro 3, 28029 Madrid, Spain; Koji Hisatake, Professor, University of Tsukuba, 1-1-1 Tennodai, Tsukuba 305-8575, Japan
S- Editor Cheng JX L- Editor Kerr C E- Editor Zheng XM
References
- 1.Georgopoulos K, Bigby M, Wang JH, Molnar A, Wu P, Winandy S, Sharpe A. The Ikaros gene is required for the development of all lymphoid lineages. Cell. 1994;79:143–156. doi: 10.1016/0092-8674(94)90407-3. [DOI] [PubMed] [Google Scholar]
- 2.Georgopoulos K, Moore DD, Derfler B. Ikaros, an early lymphoid-specific transcription factor and a putative mediator for T cell commitment. Science. 1992;258:808–812. doi: 10.1126/science.1439790. [DOI] [PubMed] [Google Scholar]
- 3.Winandy S, Wu P, Georgopoulos K. A dominant mutation in the Ikaros gene leads to rapid development of leukemia and lymphoma. Cell. 1995;83:289–299. doi: 10.1016/0092-8674(95)90170-1. [DOI] [PubMed] [Google Scholar]
- 4.Molnár A, Georgopoulos K. The Ikaros gene encodes a family of functionally diverse zinc finger DNA-binding proteins. Mol Cell Biol. 1994;14:8292–8303. doi: 10.1128/mcb.14.12.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun L, Liu A, Georgopoulos K. Zinc finger-mediated protein interactions modulate Ikaros activity, a molecular control of lymphocyte development. EMBO J. 1996;15:5358–5369. [PMC free article] [PubMed] [Google Scholar]
- 6.Hahm K, Ernst P, Lo K, Kim GS, Turck C, Smale ST. The lymphoid transcription factor LyF-1 is encoded by specific, alternatively spliced mRNAs derived from the Ikaros gene. Mol Cell Biol. 1994;14:7111–7123. doi: 10.1128/mcb.14.11.7111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hahm K, Cobb BS, McCarty AS, Brown KE, Klug CA, Lee R, Akashi K, Weissman IL, Fisher AG, Smale ST. Helios, a T cell-restricted Ikaros family member that quantitatively associates with Ikaros at centromeric heterochromatin. Genes Dev. 1998;12:782–796. doi: 10.1101/gad.12.6.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morgan B, Sun L, Avitahl N, Andrikopoulos K, Ikeda T, Gonzales E, Wu P, Neben S, Georgopoulos K. Aiolos, a lymphoid restricted transcription factor that interacts with Ikaros to regulate lymphocyte differentiation. EMBO J. 1997;16:2004–2013. doi: 10.1093/emboj/16.8.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klug CA, Morrison SJ, Masek M, Hahm K, Smale ST, Weissman IL. Hematopoietic stem cells and lymphoid progenitors express different Ikaros isoforms, and Ikaros is localized to heterochromatin in immature lymphocytes. Proc Natl Acad Sci U S A. 1998;95:657–662. doi: 10.1073/pnas.95.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dovat S, Montecino-Rodriguez E, Schuman V, Teitell MA, Dorshkind K, Smale ST. Transgenic expression of Helios in B lineage cells alters B cell properties and promotes lymphomagenesis. J Immunol. 2005;175:3508–3515. doi: 10.4049/jimmunol.175.6.3508. [DOI] [PubMed] [Google Scholar]
- 11.Alinikula J, Kohonen P, Nera KP, Lassila O. Concerted action of Helios and Ikaros controls the expression of the inositol 5-phosphatase SHIP. Eur J Immunol. 2010;40:2599–2607. doi: 10.1002/eji.200940002. [DOI] [PubMed] [Google Scholar]
- 12.Wu L, Nichogiannopoulou A, Shortman K, Georgopoulos K. Cell-autonomous defects in dendritic cell populations of Ikaros mutant mice point to a developmental relationship with the lymphoid lineage. Immunity. 1997;7:483–492. doi: 10.1016/s1074-7613(00)80370-2. [DOI] [PubMed] [Google Scholar]
- 13.Wang JH, Nichogiannopoulou A, Wu L, Sun L, Sharpe AH, Bigby M, Georgopoulos K. Selective defects in the development of the fetal and adult lymphoid system in mice with an Ikaros null mutation. Immunity. 1996;5:537–549. doi: 10.1016/s1074-7613(00)80269-1. [DOI] [PubMed] [Google Scholar]
- 14.Tonnelle C, Bardin F, Maroc C, Imbert AM, Campa F, Dalloul A, Schmitt C, Chabannon C. Forced expression of the Ikaros 6 isoform in human placental blood CD34(+) cells impairs their ability to differentiate toward the B-lymphoid lineage. Blood. 2001;98:2673–2680. doi: 10.1182/blood.v98.9.2673. [DOI] [PubMed] [Google Scholar]
- 15.Ezzat S, Yu S, Asa SL. Ikaros isoforms in human pituitary tumors: distinct localization, histone acetylation, and activation of the 5' fibroblast growth factor receptor-4 promoter. Am J Pathol. 2003;163:1177–1184. doi: 10.1016/S0002-9440(10)63477-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mullighan CG, Goorha S, Radtke I, Miller CB, Coustan-Smith E, Dalton JD, Girtman K, Mathew S, Ma J, Pounds SB, et al. Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature. 2007;446:758–764. doi: 10.1038/nature05690. [DOI] [PubMed] [Google Scholar]
- 17.Mullighan CG, Miller CB, Radtke I, Phillips LA, Dalton J, Ma J, White D, Hughes TP, Le Beau MM, Pui CH, et al. BCR-ABL1 lymphoblastic leukaemia is characterized by the deletion of Ikaros. Nature. 2008;453:110–114. doi: 10.1038/nature06866. [DOI] [PubMed] [Google Scholar]
- 18.Kuiper RP, Schoenmakers EF, van Reijmersdal SV, Hehir-Kwa JY, van Kessel AG, van Leeuwen FN, Hoogerbrugge PM. High-resolution genomic profiling of childhood ALL reveals novel recurrent genetic lesions affecting pathways involved in lymphocyte differentiation and cell cycle progression. Leukemia. 2007;21:1258–1266. doi: 10.1038/sj.leu.2404691. [DOI] [PubMed] [Google Scholar]
- 19.Sun L, Heerema N, Crotty L, Wu X, Navara C, Vassilev A, Sensel M, Reaman GH, Uckun FM. Expression of dominant-negative and mutant isoforms of the antileukemic transcription factor Ikaros in infant acute lymphoblastic leukemia. Proc Natl Acad Sci U S A. 1999;96:680–685. doi: 10.1073/pnas.96.2.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yagi T, Hibi S, Takanashi M, Kano G, Tabata Y, Imamura T, Inaba T, Morimoto A, Todo S, Imashuku S. High frequency of Ikaros isoform 6 expression in acute myelomonocytic and monocytic leukemias: implications for up-regulation of the antiapoptotic protein Bcl-XL in leukemogenesis. Blood. 2002;99:1350–1355. doi: 10.1182/blood.v99.4.1350. [DOI] [PubMed] [Google Scholar]
- 21.Payne KJ, Huang G, Sahakian E, Zhu JY, Barteneva NS, Barsky LW, Payne MA, Crooks GM. Ikaros isoform x is selectively expressed in myeloid differentiation. J Immunol. 2003;170:3091–3098. doi: 10.4049/jimmunol.170.6.3091. [DOI] [PubMed] [Google Scholar]
- 22.Payne KJ, Nicolas JH, Zhu JY, Barsky LW, Crooks GM. Cutting edge: predominant expression of a novel Ikaros isoform in normal human hemopoiesis. J Immunol. 2001;167:1867–1870. doi: 10.4049/jimmunol.167.4.1867. [DOI] [PubMed] [Google Scholar]
- 23.Ronni T, Payne KJ, Ho S, Bradley MN, Dorsam G, Dovat S. Human Ikaros function in activated T cells is regulated by coordinated expression of its largest isoforms. J Biol Chem. 2007;282:2538–2547. doi: 10.1074/jbc.M605627200. [DOI] [PubMed] [Google Scholar]
- 24.Cobb BS, Morales-Alcelay S, Kleiger G, Brown KE, Fisher AG, Smale ST. Targeting of Ikaros to pericentromeric heterochromatin by direct DNA binding. Genes Dev. 2000;14:2146–2160. doi: 10.1101/gad.816400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trinh LA, Ferrini R, Cobb BS, Weinmann AS, Hahm K, Ernst P, Garraway IP, Merkenschlager M, Smale ST. Down-regulation of TDT transcription in CD4(+)CD8(+) thymocytes by Ikaros proteins in direct competition with an Ets activator. Genes Dev. 2001;15:1817–1832. doi: 10.1101/gad.905601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koipally J, Renold A, Kim J, Georgopoulos K. Repression by Ikaros and Aiolos is mediated through histone deacetylase complexes. EMBO J. 1999;18:3090–3100. doi: 10.1093/emboj/18.11.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim J, Sif S, Jones B, Jackson A, Koipally J, Heller E, Winandy S, Viel A, Sawyer A, Ikeda T, et al. Ikaros DNA-binding proteins direct formation of chromatin remodeling complexes in lymphocytes. Immunity. 1999;10:345–355. doi: 10.1016/s1074-7613(00)80034-5. [DOI] [PubMed] [Google Scholar]
- 28.O'Neill DW, Schoetz SS, Lopez RA, Castle M, Rabinowitz L, Shor E, Krawchuk D, Goll MG, Renz M, Seelig HP, et al. An ikaros-containing chromatin-remodeling complex in adult-type erythroid cells. Mol Cell Biol. 2000;20:7572–7582. doi: 10.1128/mcb.20.20.7572-7582.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koipally J, Heller EJ, Seavitt JR, Georgopoulos K. Unconventional potentiation of gene expression by Ikaros. J Biol Chem. 2002;277:13007–13015. doi: 10.1074/jbc.M111371200. [DOI] [PubMed] [Google Scholar]
- 30.Dorsam G, Goetzl EJ. Vasoactive intestinal peptide receptor-1 (VPAC-1) is a novel gene target of the hemolymphopoietic transcription factor Ikaros. J Biol Chem. 2002;277:13488–13493. doi: 10.1074/jbc.M107922200. [DOI] [PubMed] [Google Scholar]
- 31.Ghanshani S, Wulff H, Miller MJ, Rohm H, Neben A, Gutman GA, Cahalan MD, Chandy KG. Up-regulation of the IKCa1 potassium channel during T-cell activation. Molecular mechanism and functional consequences. J Biol Chem. 2000;275:37137–37149. doi: 10.1074/jbc.M003941200. [DOI] [PubMed] [Google Scholar]
- 32.Wargnier A, Lafaurie C, Legros-Maïda S, Bourge JF, Sigaux F, Sasportes M, Paul P. Down-regulation of human granzyme B expression by glucocorticoids. Dexamethasone inhibits binding to the Ikaros and AP-1 regulatory elements of the granzyme B promoter. J Biol Chem. 1998;273:35326–35331. doi: 10.1074/jbc.273.52.35326. [DOI] [PubMed] [Google Scholar]
- 33.Yap WH, Yeoh E, Tay A, Brenner S, Venkatesh B. STAT4 is a target of the hematopoietic zinc-finger transcription factor Ikaros in T cells. FEBS Lett. 2005;579:4470–4478. doi: 10.1016/j.febslet.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 34.Kim JH, Ebersole T, Kouprina N, Noskov VN, Ohzeki J, Masumoto H, Mravinac B, Sullivan BA, Pavlicek A, Dovat S, et al. Human gamma-satellite DNA maintains open chromatin structure and protects a transgene from epigenetic silencing. Genome Res. 2009;19:533–544. doi: 10.1101/gr.086496.108. [DOI] [PMC free article] [PubMed] [Google Scholar]


