Abstract
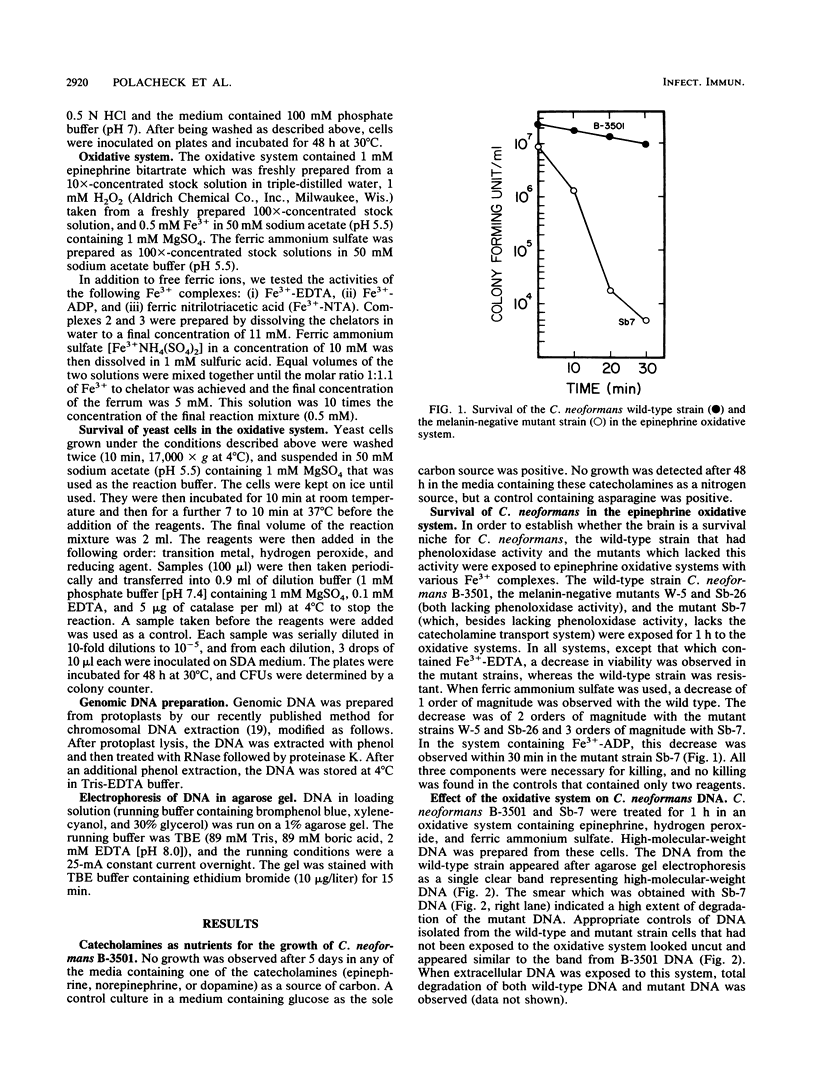
Cryptococcus neoformans was unable to utilize catecholamines (epinephrine, norepinephrine, or dopamine) as sole carbon or nitrogen sources. Therefore, catecholamines are not essential growth factors for this fungus and the brain is not a preferred nutritional niche for its growth with regard to catecholamines. To establish whether the brain is a survival niche for C. neoformans and to explain the role of phenoloxidase as a virulence factor, a wild-type strain that had phenoloxidase activity and mutants which lacked it were exposed to an epinephrine oxidative system, and the survival of both strains was tested. The oxidative system contained epinephrine as an electron donor, Fe3+ as the catalytic transition metal ion, and hydrogen peroxide as an electron acceptor. The wild-type strain was found to be resistant to this oxidative system, whereas under the same conditions the mutant strain was susceptible and its survival decreased at a rate of 4 logs per h. Damage to high-molecular-weight DNA seems to be a causative factor of cell death after exposure of the mutants to the oxidative system. These results suggest that C. neoformans may survive in the brain because of its ability to utilize catecholamines for melanogenesis and thus neutralize the harmful effects of catecholamines which are manifested in the presence of hydrogen peroxide and transition metal ions. The role of phenoloxidase in resistance to the epinephrine oxidative system is also discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ecker D. J., Emery T. Iron uptake from ferrichrome A and iron citrate in Ustilago sphaerogena. J Bacteriol. 1983 Aug;155(2):616–622. doi: 10.1128/jb.155.2.616-622.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein S., Czapski G. The role and mechanism of metal ions and their complexes in enhancing damage in biological systems or in protecting these systems from the toxicity of O2-. J Free Radic Biol Med. 1986;2(1):3–11. doi: 10.1016/0748-5514(86)90117-0. [DOI] [PubMed] [Google Scholar]
- Graf E., Mahoney J. R., Bryant R. G., Eaton J. W. Iron-catalyzed hydroxyl radical formation. Stringent requirement for free iron coordination site. J Biol Chem. 1984 Mar 25;259(6):3620–3624. [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984 Apr 1;219(1):1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon-Chung K. J., Hill W. B., Bennett J. E. New, special stain for histopathological diagnosis of cryptococcosis. J Clin Microbiol. 1981 Feb;13(2):383–387. doi: 10.1128/jcm.13.2.383-387.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon-Chung K. J., Polacheck I., Popkin T. J. Melanin-lacking mutants of Cryptococcus neoformans and their virulence for mice. J Bacteriol. 1982 Jun;150(3):1414–1421. doi: 10.1128/jb.150.3.1414-1421.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon-Chung K. J., Rhodes J. C. Encapsulation and melanin formation as indicators of virulence in Cryptococcus neoformans. Infect Immun. 1986 Jan;51(1):218–223. doi: 10.1128/iai.51.1.218-223.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Day E. D., Jr Superoxide-dependent production of hydroxyl radical catalyzed by iron-EDTA complex. FEBS Lett. 1978 Feb 1;86(1):139–142. doi: 10.1016/0014-5793(78)80116-1. [DOI] [PubMed] [Google Scholar]
- Miranda M., Botti D., Di Cola M. Possible genotoxicity of melanin synthesis intermediates: tyrosinase reaction products interact with DNA in vitro. Mol Gen Genet. 1984;193(3):395–399. doi: 10.1007/BF00382074. [DOI] [PubMed] [Google Scholar]
- Polacheck I., Hearing V. J., Kwon-Chung K. J. Biochemical studies of phenoloxidase and utilization of catecholamines in Cryptococcus neoformans. J Bacteriol. 1982 Jun;150(3):1212–1220. doi: 10.1128/jb.150.3.1212-1220.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polacheck I., Kwon-Chung K. J. Melanogenesis in Cryptococcus neoformans. J Gen Microbiol. 1988 Apr;134(4):1037–1041. doi: 10.1099/00221287-134-4-1037. [DOI] [PubMed] [Google Scholar]
- Polacheck I., Lebens G. A. Electrophoretic karyotype of the pathogenic yeast Cryptococcus neoformans. J Gen Microbiol. 1989 Jan;135(1):65–71. doi: 10.1099/00221287-135-1-65. [DOI] [PubMed] [Google Scholar]
- Quinn T. C., Mann J. M., Curran J. W., Piot P. AIDS in Africa: an epidemiologic paradigm. Science. 1986 Nov 21;234(4779):955–963. doi: 10.1126/science.3022379. [DOI] [PubMed] [Google Scholar]
- Rhodes J. C., Polacheck I., Kwon-Chung K. J. Phenoloxidase activity and virulence in isogenic strains of Cryptococcus neoformans. Infect Immun. 1982 Jun;36(3):1175–1184. doi: 10.1128/iai.36.3.1175-1184.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ro J. Y., Lee S. S., Ayala A. G. Advantage of Fontana-Masson stain in capsule-deficient cryptococcal infection. Arch Pathol Lab Med. 1987 Jan;111(1):53–57. [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Role of iron and ethylenediaminetetraacetic acid in the bactericidal activity of a superoxide anion-generating system. Arch Biochem Biophys. 1981 May;208(2):512–519. doi: 10.1016/0003-9861(81)90539-7. [DOI] [PubMed] [Google Scholar]
- Samuni A., Aronovitch J., Godinger D., Chevion M., Czapski G. On the cytotoxicity of vitamin C and metal ions. A site-specific Fenton mechanism. Eur J Biochem. 1983 Dec 1;137(1-2):119–124. doi: 10.1111/j.1432-1033.1983.tb07804.x. [DOI] [PubMed] [Google Scholar]
- Sugioka K., Nakano H., Nakano M., Tero-Kubota S., Ikegami Y. Generation of hydroxyl radicals during the enzymatic reductions of the Fe3+-ADP-phosphate-adriamycin and Fe3+-ADP-EDTA systems. Less involvement of hydroxyl radical and a great importance of proposed perferryl ion complexes in lipid peroxidation. Biochim Biophys Acta. 1983 Oct 11;753(3):411–421. doi: 10.1016/0005-2760(83)90065-6. [DOI] [PubMed] [Google Scholar]
- Zuger A., Louie E., Holzman R. S., Simberkoff M. S., Rahal J. J. Cryptococcal disease in patients with the acquired immunodeficiency syndrome. Diagnostic features and outcome of treatment. Ann Intern Med. 1986 Feb;104(2):234–240. doi: 10.7326/0003-4819-104-2-234. [DOI] [PubMed] [Google Scholar]