Abstract
Pneumatosis intestinalis (PI) is defined as gas within the gastrointestinal wall and is associated with a variety of disorders. As a concurrent occurrence with pneumoperitoneum, it can easily to be mistaken for bowel ischemia with perforated peritonitis. In fact, air dissection or rupture from subserosal cysts may be the cause of intraperitoneal and intraluminal free air, with clinical symptoms such as abdominal pain and fullness occurring as a result. We hereby report a case of an 82-year-old male with a history of chronic obstructive pulmonary disease who was diagnosed with bowel ischemia and received emergency laparotomy because of the appearance of PI and pneumoperitoneum on abdominal computed tomography scan. However, no perforated hollow organ or necrotic bowel segment was found, only diffusely distributed massive intraperitoneal air and PI of gastrointestinal tract. The laparotomy seemed non-therapeutic for this patient. This is significant warning for clinicians to differentiate the associated conditions of PI, and to evaluate whether or not emergency surgery is necessary.
Keywords: Pneumatosis intestinalis, Pneumoperitoneum, Computed tomography
INTRODUCTION
Pneumatosis intestinalis (PI) is indicated, radiologically or pathologically, as gas within the gastrointestinal wall. Air dissection or rupture from subserosal cysts may be the cause of intraperitoneal and intraluminal free air, which causes clinical symptoms such as abdominal pain and fullness[1,2]. In the past, this was regarded as a sign of intestinal ischemia, which was indicated for surgical intervention, especially when it coexisted with the presence of pneumoperitoneum. However, new evidence indicates that a conservative approach may be sufficient in certain cases presenting with PI[3,4].
We hereby report a case of an 82-year-old male with a history of chronic obstructive pulmonary disease (COPD) who presented with abdominal pain. The computed tomography (CT) scan showed intramural gas of the gastrointestinal (GI) tract and massive pneumoperitoneum, which mimicked intestinal ischemia and perforation. The diagnosis of PI with pneumoperitoenum was confirmed via exploratory laparotomy and subsequent pathological analysis, though the etiology remained uncertain. An operation was probably unnecessary for this patient as there are other ways to determine the possible need for laparotomy, such as repeated laboratory and radiological tests. Conservative treatment is probably more suitable for the relief of PI.
CASE REPORT
An 82-year-old man with a past medical history of COPD visited our emergency department because of generalized abdominal pain with fullness and intermittent vomiting for three days. Physical examinations revealed tenderness over the whole abdomen and his hemodynamic status was relatively stable. C-reactive protein was 1.0 mg/dL, marginally elevated from the normal upper limit of 0.8 mg/dL, but other laboratory data were all within normal limits. The abdominal CT scan revealed generalized bowel distention, intramural air within stomach, small and large intestines, and massive intraperitoneal free air (Figure 1). Laparotomy was performed due to the suspected diagnosis of bowel ischemia and hollow organ perforation. Pneumoperitoneum, bowel wall congestion and edematous cystic changes were identified in a CT scan, whereas no bowel perforation was detected. The most prominent pneumatosed jejunal segment around 50 cm in length was resected with primary anastomosis because of the suspicion of bowel ischemia and necrosis. In addition, loop ileostomy was conducted for decompression of the dilated large bowel. Pathologically, the sections of intestinal wall showed diffuse gas-filled cysts of variable size (Figure 2A and B), leading to the diagnosis of PI. Autoimmune or rheumatological diseases were excluded by unremarkable results from laboratory analysis of markers including rheumatoid factor, antinuclear antibody and subtypes (antibodies to dsDNA, Sm, Ro, La), anti-cardiolipin antibody, and serum immunoglobulins, as well as normal results from physical examinations. The possible cause of PI may be associated with underlying COPD. In the following days, the patient received chest physical therapy and medications including bronchodilaters and mucolytics for exacerbated COPD and superimposed pneumonia. Repeated abdominal CT scan 2 mo later confirmed the resolution of PI. The patient was discharged uneventfully with no further complaints.
Figure 1.
Use of lung window setting in abdominal computed tomography scan revealed massive intraperitoneal free air (arrowheads) and diffuse air collected within the bowel wall (arrows).
Figure 2.
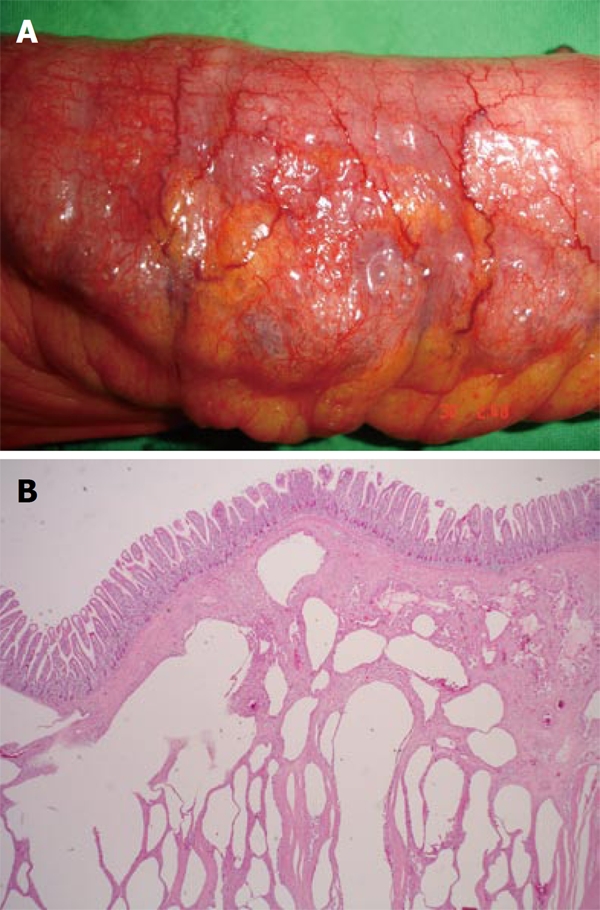
Intestinal wall was grossly thickened, congested, with bubbles on the surface (A) and microscopically the section of small intestine showed diffuse variable sized gas-filled cysts in the submucosa and serosa (B).
DISCUSSION
Conventional PI has been classified as primary (idiopathic) and secondary[5]. Primary PI is referred to as the cystic collection of air in the colonic wall with an unknown cause. Secondary PI has been associated with numerous clinical conditions. The most common sources of PI possibly are intraluminal GI gas, bacterial production of gas, and pulmonary gas[1,6]. The increase in the intraluminal pressure and extent of mucosal injury, as seen in intestinal obstruction, endoscopic exam, trauma, mucosal injury incited by autoimmune diseases, acquired immunodeficiency, immunosuppressive therapy, and cytotoxic therapy[1,2], may lead to intralumoinal gas dissection into the injured GI tract intramurally. The invasion of gas-producing bacteria into the injured GI mucosa may be responsible for the bacterial theory of PI. Pulmonary gas formation may arise from alveolar rupture, which results in the dissection of air along vascular channels in the mediastinum, tracking caudally to the retroperitoneum and then to the vascular supply of the viscera[1,6]. A review from Boerner and colleagues revealed that 20% out of the 123 patients have had COPD[7].
The overall incidence of PI may be as low as 0.03%, according to an autopsy series[8]. In recent times, due to the increased use of the CT scan, the reported incidence of PI has increased to 0.3%[9]. Of those patients diagnosed with PI, 30%-40% have bowel ischemia/necrosis, and another 30% have bowel obstruction[3,9]. In another study, of 97 patients diagnosed with PI by CT scan, approximately 50% could have been successfully managed non-operatively, indicating that CT scan is non-specific and should not be used as the sole indicator for laparotomy[4]. Conventionally, exploratory laparotomies were performed for patients with PI and pneumoperitoneum because the CT scan indicated possible bowel necrosis and perforation, although the appearance of pneumoperitoneum on CT scan may be attributed to the rupture of PI-associated subserosal cysts[10-12]. However, there are no large-scale reports of the incidence of pneumoperitoneum in patients with PI.
For patients diagnosed radiologically with PI and a complete history should be taken and physical examinations carried out, especially where there are pulmonary diseases such as COPD[1,2], systemic diseases as scleroderma, AIDS and inflammatory bowel diseases[1,2] or medications such as chemotherapeutics, steroids or immunosuppressive agents[1,2,13]. Appropriate medical treatment should be adopted according to pre-existing illness. In fact, around 50% of patients with PI can be successfully managed non-operatively[4].
Nonetheless, following the identification of PI urgent surgery may be essential, especially in conditions such as strangulated bowel obstruction or ischemia. Abdominal rebound tenderness, sepsis and failure to respond to conservative treatment are clear clinical indications for surgical treatment. The presence of metabolic acidosis, higher APACHE II score and serum lactic acid level > 2.0 mmol/L at the time of diagnosis are indicators of poor prognosis[2,4,9]. The appearance of PI on abdominal CT scan gives a definitive diagnosis of bowel ischemia in only 60% of cases[14]. Signs of the appearances of intramural gas, thromboembolism in the mesenteric vessels, portal venous gas, absence of bowel wall enhancement, or ischemic signs in other organs are considered more specific indications of bowel ischemia[15]. A broad spectrum of conditions appear as PI on CT scan, and it is reported that patients with PI and other CT findings of ischemia are more likely to have gangrenous bowel[14], especially where there is portal venous gas, or portal mesenteric gas, which is associated with 81% of patients with transmural bowel infarction[16].
Meticulous integration of the laboratory data, the appearance on abdominal CT scan and clinical presentations permit clinicians to distinguish benign from life-threatening PI and to decide whether or not urgent surgical intervention is necessary. As described in this case report, it is sometimes difficult to deal with ambiguous findings. For example, the coexistence of PI and intraperitoneal free air on CT scan can be easily mistaken for bowel ischemia and perforation peritonitis[17]. Since normal laboratory results are not typically consistent with the symptoms of bowel ischemia, surgical intervention would be non-therapeutic, in such cases. To manage patients with uncertain diagnoses, diagnostic peritoneal lavage or laparoscopy could be performed as an adjunct to confirm bowel necrosis or hollow visceral perforation.
Footnotes
Peer reviewers: Ned Abraham, MBBS, FRACS, FRCS, PhD, Coffs Colorectal and Capsule Endoscopy Centre, University of New South Wales, 187 Rose Avenue (POB 2244), Coffs Harbour, NSW 2450, Australia; Hubert Scheidbach, MD, Professor, Department of Surgery, Otto von Guericke University, Junoweg 31, D-39118 Magdeburg, Magdeburg, Germany
S- Editor Wang JL L- Editor Hughes D E- Editor Zheng XM
References
- 1.St Peter SD, Abbas MA, Kelly KA. The spectrum of pneumatosis intestinalis. Arch Surg. 2003;138:68–75. doi: 10.1001/archsurg.138.1.68. [DOI] [PubMed] [Google Scholar]
- 2.Greenstein AJ, Nguyen SQ, Berlin A, Corona J, Lee J, Wong E, Factor SH, Divino CM. Pneumatosis intestinalis in adults: management, surgical indications, and risk factors for mortality. J Gastrointest Surg. 2007;11:1268–1274. doi: 10.1007/s11605-007-0241-9. [DOI] [PubMed] [Google Scholar]
- 3.Knechtle SJ, Davidoff AM, Rice RP. Pneumatosis intestinalis. Surgical management and clinical outcome. Ann Surg. 1990;212:160–165. doi: 10.1097/00000658-199008000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morris MS, Gee AC, Cho SD, Limbaugh K, Underwood S, Ham B, Schreiber MA. Management and outcome of pneumatosis intestinalis. Am J Surg. 2008;195:679–82; discussion 682-3. doi: 10.1016/j.amjsurg.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 5.KOSS LG. Abdominal gas cysts (pneumatosis cystoides intestinorum hominis); an analysis with a report of a case and a critical review of the literature. AMA Arch Pathol. 1952;53:523–549. [PubMed] [Google Scholar]
- 6.Pear BL. Pneumatosis intestinalis: a review. Radiology. 1998;207:13–19. doi: 10.1148/radiology.207.1.9530294. [DOI] [PubMed] [Google Scholar]
- 7.Boerner RM, Fried DB, Warshauer DM, Isaacs K. Pneumatosis intestinalis. Two case reports and a retrospective review of the literature from 1985 to 1995. Dig Dis Sci. 1996;41:2272–2285. doi: 10.1007/BF02071412. [DOI] [PubMed] [Google Scholar]
- 8.Heng Y, Schuffler MD, Haggitt RC, Rohrmann CA. Pneumatosis intestinalis: a review. Am J Gastroenterol. 1995;90:1747–1758. [PubMed] [Google Scholar]
- 9.Hawn MT, Canon CL, Lockhart ME, Gonzalez QH, Shore G, Bondora A, Vickers SM. Serum lactic acid determines the outcomes of CT diagnosis of pneumatosis of the gastrointestinal tract. Am Surg. 2004;70:19–23; discussion 23-24. [PubMed] [Google Scholar]
- 10.Mularski RA, Ciccolo ML, Rappaport WD. Nonsurgical causes of pneumoperitoneum. West J Med. 1999;170:41–46. [PMC free article] [PubMed] [Google Scholar]
- 11.Mularski RA, Sippel JM, Osborne ML. Pneumoperitoneum: a review of nonsurgical causes. Crit Care Med. 2000;28:2638–2644. doi: 10.1097/00003246-200007000-00078. [DOI] [PubMed] [Google Scholar]
- 12.Maltz C. Benign pneumoperitoneum and pneumatosis intestinalis. Am J Emerg Med. 2001;19:242–243. doi: 10.1053/ajem.2001.22669. [DOI] [PubMed] [Google Scholar]
- 13.Ho LM, Paulson EK, Thompson WM. Pneumatosis intestinalis in the adult: benign to life-threatening causes. AJR Am J Roentgenol. 2007;188:1604–1613. doi: 10.2214/AJR.06.1309. [DOI] [PubMed] [Google Scholar]
- 14.Kernagis LY, Levine MS, Jacobs JE. Pneumatosis intestinalis in patients with ischemia: correlation of CT findings with viability of the bowel. AJR Am J Roentgenol. 2003;180:733–736. doi: 10.2214/ajr.180.3.1800733. [DOI] [PubMed] [Google Scholar]
- 15.Saba L, Mallarini G. Computed tomographic imaging findings of bowel ischemia. J Comput Assist Tomogr. 2008;32:329–340. doi: 10.1097/RCT.0b013e3180dc8cb1. [DOI] [PubMed] [Google Scholar]
- 16.Wiesner W, Mortelé KJ, Glickman JN, Ji H, Ros PR. Pneumatosis intestinalis and portomesenteric venous gas in intestinal ischemia: correlation of CT findings with severity of ischemia and clinical outcome. AJR Am J Roentgenol. 2001;177:1319–1323. doi: 10.2214/ajr.177.6.1771319. [DOI] [PubMed] [Google Scholar]
- 17.Sakurai Y, Hikichi M, Isogaki J, Furuta S, Sunagawa R, Inaba K, Komori Y, Uyama I. Pneumatosis cystoides intestinalis associated with massive free air mimicking perforated diffuse peritonitis. World J Gastroenterol. 2008;14:6753–6756. doi: 10.3748/wjg.14.6753. [DOI] [PMC free article] [PubMed] [Google Scholar]



