Abstract
Background
The extent to which cardiovascular disease (CVD) risk factors cluster in youth with a diagnosis of type 1 (T1DM) or type 2 diabetes mellitus (T2DM) is unclear. Therefore, we aimed to evaluate potential clustering of traditional CVD risk factors that may reflect an unmeasured but unifying single pathology that may explain the phenomenon of the metabolic syndrome in these youths.
Methods
Youths who participated in the SEARCH for Diabetes in Youth study with diabetes diagnosed <20 years, with current age >10 years (maximum current age, 22 years) were included. Confirmatory factor analysis (CFA) was performed to determine statistical associations among CVD risk factors, including obesity, blood pressure, triglycerides, and high-density lipoprotein cholesterol (HDL-C). Diabetes type was defined by diabetes autoantibodies (DAA) and fasting C-peptide (FCP); type 1 (T1DM, DAA positive, and FCP <0.8 ng/mL, n = 1198) and type 2 (T2DM, DAA negative, and FCP >2.9 ng/mL, n = 95). For T1DM, the sample was split randomly and analyses were conducted separately in each split sample.
Results
Among five prespecified data structures ranging from a single underlying factor to a hierarchical structure of factors, the worst-fitting model for both of the T1DM split samples was the single-factor structure and the best-fitting model was a three-correlated-factor structure. The three correlated factors identified were obesity, lipids, and blood pressure. Results were very similar for youths with T2DM.
Conclusion
There is little evidence that a single factor underlies the CVD risk factor pattern in youths with diabetes. The concept of the metabolic syndrome provides a useful description of clinical characteristics but does not efficiently capture a single target for etiologic research among youths with diabetes.
Introduction
Previously, we reported from the SEARCH for Diabetes in Youth study (SEARCH) that about 15% of youths with type 1a diabetes mellitus (T1DM) and about 90% of youths with type 2 DM (T2DM) have two or more traditional cardiovascular disease (CVD) risk factors present in addition to glucose intolerance,1 compared with a metabolic syndrome prevalence of approximately 6% in nondiabetic youths.2 Thus, it is of utmost importance to advance knowledge regarding the early determinants of CVD risk factors among youths with diabetes. The SEARCH study is a multicenter epidemiologic study that began conducting population-based ascertainment of nongestational cases of diagnosed diabetes in youths less than 20 years of age in 2001 for prevalent cases and continuing with case ascertainment for incident cases through the present.3 One of the major aims of SEARCH is to evaluate risk factors for the chronic complications of diabetes, including risk factors for CVD.
Definitions of the metabolic syndrome have been promoted as a way to identify individuals efficiently who have multiple CVD risk factors; however, the merits of such definitions have been vigorously debated.4–9 It is not clear whether the metabolic syndrome captures a unique, unmeasured, but etiologically important, phenomenon that explains future risk for vascular events. If such a unifying etiologic process does exist, this would be an extremely important target for research. Therefore, using data collected in the SEARCH cohort, we aimed to evaluate statistically the phenomenon of clustering of the traditional CVD risk factors included in the definition of metabolic syndrome put forth by the National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III) criteria,10 as applied to children and youths.
Preliminary analyses from SEARCH used exploratory factor analysis and yielded a three-factor solution for both T1 and T2DM, with only 42.9% and 35.9% of the total variance for T1 and T2DM, respectively,11 thereby providing little evidence for a single underlying etiology. Most previous factor analyses are from studies of adults4; nondiabetic youth have produced similar results.12,13 Lawlor et al.14 suggested that confirmatory factor analysis (CFA) may be preferable over more commonly used exploratory factor analysis for evaluation of metabolic syndrome because of the capacity of CFA to evaluate explicitly the goodness of fit of a particular structure (e.g., a single factor) to the observed data. Specifically, analyses presented herein evaluate whether a single-factor model provided the best fit to the data, compared to alternative models of more than one factor. In the present report, CFA was employed in parallel in youth with T1DM and those with T2DM. These analyses provided the opportunity to evaluate systematically whether clustering may operate differently depending on the prevalence of the CVD risk factors in the population of interest, with one group (T2DM) known to be severely insulin resistant.
Materials and Methods
Study design
Data for this analysis derive from the SEARCH for Diabetes in Youth Study.3 SEARCH has six centers that are located in Ohio, Colorado, Washington, South Carolina, Hawaii, and Southern California. In all sites, diabetes cases were considered to be valid if they were diagnosed by a health-care provider. The study was reviewed and approved by the local Institutional Review Boards (IRB) that had jurisdiction over the local study population. Using Health Insurance Portability and Accountability Act-compliant procedures, youths with diabetes identified by the SEARCH recruiting network were asked to complete a survey that collected information on age, sex, age at diagnosis, diabetes treatment history, and race/ethnicity. Youths (excluding those with secondary diabetes or diabetes known to be due to genetic causes of β-cell failure) who replied to the survey were then invited to a study visit. Before the study visit, written informed consent was obtained according to the guidelines established by the local IRB from subjects who were 18 years of age and older or from the parent or guardian if the subject was less than 18 years of age. Written assent was also obtained from the subjects who were less than 18 years of age as governed by local IRB instructions.
Variable measurement
Standardized physical examinations were conducted by trained and certified study staff members. Height and weight measurements were used to calculate body mass index (BMI, kg/m2), and age- and gender-specific BMI z-scores were derived based on the U.S. Centers for Disease Control and Prevention.15 Waist circumference was measured using the National Health and Nutrition Examination Survey (NHANES) protocol,16 which uses the anatomic landmarks of the iliac crest and mid-axillary line. Three blood pressure measurements were obtained using a portable mercury manometer.15
Blood was drawn after fasting for at least 8 hours for measurement of diabetes autoantibodies (glutamic acid decarboxylase [GAD] 65, insulinoma antigen-2 [IA-2], and lipids (total cholesterol (TC), low-density lipoprotein cholesterol [LDL-C], high-density lipoprotein cholesterol [HDL-C], triglycerides, and very-low-density lipoprotein cholesterol [VLDL-C]). Laboratory samples were obtained only if there was no episode of diabetic ketoacidosis within the prior month. Specimens were processed locally at the sites and then shipped within 24 hours to the central laboratory (Northwest Lipid Metabolism and Diabetes Research Laboratories, University of Washington, Seattle, WA), where they were analyzed. Measurements of TC, HDL-C, and triglycerides were performed enzymatically on a Hitachi 917 autoanalyzer (Boehringer Mannheim Diagnostics, Indianapolis, Indiana). LDL-C levels were calculated by the Friedewald equation for individuals with triglyceride levels less than 400 mg/dL,17 and by Lipid Research Clinics Beta Quantification18 for those with triglyceride levels at least 400 mg/dL.
Samples were analyzed for glutamic acid decarboxyl-ase-65 (GAD65) and IA-2 diabetes autoantibodies (DAA) in radioligand-binding assays.19 The levels were expressed as relative indices, using positive and negative control samples. The positive control sample was the World Health Organization (WHO) standard for islet cell antibodies. The negative control samples were prepared from a pool of normal sera. A signal-to-noise ratio of 10 or above was required.
For the present report, we wished to evaluate potential clustering of CVD risk factors in two distinct groups of individuals: those with biochemical evidence of T1DM and those with biochemical evidence of T2DM, using the same definitions as were applied in the previous SEARCH report on prevalence of CVD risk factors.1 Thus, diabetes type was classified based on GAD65 and IA-2 DAA and fasting C-peptide (FCP) as follows: T1DM = DAA+ and FCP <0.8 ng/mL and T2DM = DAA− and FCP ≥2.9 ng/mL. Youths who did not meet either strict definition were excluded from the present analysis.
For descriptive purposes, CVD risk factors were defined according to the NCEP ATP III definition modified for age10 as follows: HDL-C < 40 mg/dL; waist circumference >90th percentile for age and sex (Cook S, NHANES III, personal communication); systolic (SBP) or diastolic blood pressure (DBP) >90th percentile for age, sex, and height.20 The metabolic syndrome was defined for youth with diabetes as those who have at least two of these CVD risk factors, in addition to having T1DM or T2DM.
Subject inclusion and exclusion
To facilitate the descriptive comparisons between youth with T1DM and those with T2DM, we restricted analyses to SEARCH participants who were prevalent in 2001 or incident in 2002 or 2003, and whose current age at the time of the SEARCH visit was >10 years (maximum current age, 22 years). Youths younger than 10 years of age were excluded from this analysis because of the exceedingly small number of subjects under age 10 with T2DM.
Of the 6792 validated, age-eligible cases, 2144 participated in the SEARCH in-person clinic examination and had complete data as required for assignment of diabetes type and conduct of the factor analyses. We included 1249 youths with T1DM and 123 youths with T2DM. We excluded 772 youths who did not meet our strict criteria for T1DM or T2DM; these included 241 with “possible T1DM” (DAA+ but intermediate levels of C-peptide), 43 “hybrid” (DAA+ and high levels of C-peptide), 345 “possible T1DM” (DAA− and low C-peptide), and 143 “possible T2DM” (DAA− and intermediate C-peptide). Because generally accepted definitions of diabetes typology for youths with such phenotypes are not currently available, we preferred not to include these individuals in the present analyses and instead opted to include only youths whose DAA and C-peptide data provided strong evidence for either T1DM or T2DM. Additionally, we excluded youths who reported use of antihypertensive or lipid-lowering medication, and those taking thiazolidinediones (TZD), which enhance insulin sensitivity (n = 51 excluded with T1DM; n = 28 excluded with T2DM). The final analysis sample included 1198 with T1DM and 95 with T2DM. These were the same subjects included in preliminary analyses published previously utilizing exploratory factor analysis.11
Statistical analyses
The CALIS procedure of SAS 8.2 was used to conduct CFA, in which the maximum likelihood estimation technique was used to estimate the parameters for pre-specified data structures. For the six CVD risk factors (BMI, waist, HDL, log triglycerides, systolic blood pressure, and diastolic blood pressure), we prespecified five structures designed to reflect results in the previous literature as well as to address concerns that inclusion of variables known to be highly correlated on the basis of prior knowledge including biology (e.g., SBP and DBP) would drive observation of a separate factor representing those correlated variables. Thus, we specified the following models: (1) one factor underlies all risk factors; (2) two correlated factors: one factor reflects BMI, waist, HDL, and log triglycerides, and the other reflects systolic and diastolic blood pressure; (3) three correlated factors: obesity, lipids, and blood pressure; (4) one factor with correlated residuals between BMI and waist, HDL, and log triglycerides, and systolic and diastolic blood pressure; and (5) a hierarchical structure with three first-order factors comprising obesity, lipid, and blood pressure and one second-order factor reflecting insulin resistance or the syndrome itself.
Specification of these models reflect our a priori intent to compare models 2–5 to the first model (one factor), as well as our expectation that each of alternative models 2–5 could appropriately capture underlying biological processes. For example, it seemed reasonable to suspect that the obesity measures (BMI and waist) would load together, that lipid measures (triglycerides and HDL) would load together, and that blood pressure measures (SBP and DBP) would load together. Furthermore, we considered that a latent variable might exist, namely insulin resistance; this was considered in specifying model 1, and also in model 5 via the second-order factor.
We evaluated the fit of each structure to the data by examining the following fit indices: the root mean squared residual (RMR), Akaike's Information Criterion (AIC), Bayesian Information Criterion (BIC), also known as Schwarz's Bayesian Criterion. These fit statistics are those considered sensitive to models that lack necessary parameters and which are relatively insensitive to small sample size (such as n < 150).21,22
For subjects with T1DM, we had a sufficient sample size to allow conduct of the CFA on a split sample. Specifically, Bernoulli random numbers (n = 1198) from Bernoulli distribution with p = 0.5 were simulated. After merging these random numbers to youths with T1DM, those with a random number value of 0 were in the first-half sample (n = 600), and those with a random number value 1 were in the second-half sample (n = 598). The relatively small sample size of youths with T2DM (n = 95) precluded the split sample approach in this group.
Results
Descriptive characteristics of subjects with T1DM and T2DM are shown in Table 1. Youths with T2DM were more commonly of minority race/ethnicity, and had lower HDL values as well as higher triglycerides, BMI, waist circumference, and blood pressure values. The prevalence of metabolic syndrome in T1DM was 21.2%, compared with 92.6% among youths with T2DM.
Table 1.
Characteristics of Youth With Type 1 and Type 2 Diabetes: SEARCH for Diabetes in Youth 2001–2003
| Variables | Type 1 (n = 1198) | Type 2 (n = 95) |
|---|---|---|
| Gender (%) | ||
| Male | 47.8 | 31.6 |
| Female | 52.2 | 68.4 |
| Race/Ethnicity (%) | ||
| White | 78.1 | 20.0 |
| African American | 6.0 | 26.3 |
| Hispanic | 10.9 | 19.0 |
| American Indian | 0.9 | 23.2 |
| Multiple | 2.4 | 3.2 |
| API | 1.6 | 8.4 |
| Others | 0.2 | 0.0 |
| Age (years), mean (SD) | 14.83 (3.13) | 16.61 (2.67) |
| Duration of diabetes (years), mean (SD) | 5.2 (3.9) | 2.1 (1.6) |
| HDL-C (mg/dL), mean (SD) | 55.30 (12.59) | 37.95 (8.92) |
| Triglycerides (mg/dL), meana (SD) | 70.93 (1.68) | 145.31 (1.95) |
| BMI z-score (kg/m2), mean (SD) | 0.61 (0.86) | 2.18 (0.78) |
| Waist (cm), mean (SD) | 78.84 (11.60) | 113.91 (15.89) |
| DBP (mmHg), mean (SD) | 67.96 (9.43) | 75.10 (10.32) |
| SBP (mmHg), mean (SD) | 106.06 (10.55) | 118.03 (11.15) |
| Prevalence of CVD risk factors (%)b | ||
| High blood pressure | 27.24 | 65.63 |
| High triglycerides | 18.11 | 65.63 |
| Low HDL-C | 10.05 | 61.46 |
| High waist circumference | 21.26 | 95.85 |
| Metabolic syndrome | 21.4 | 92.7 |
Geometric mean.
Based on the definitions used to establish prevalence of the metabolic syndrome in nondiabetic youth in the NHANES III population,10 with metabolic syndrome defined for youth with diabetes as those who have at least two additional CVD risk factors listed, in addition to having Type 1 or Type 2 diabetes.
Abbreviations: API, Asian-Pacific Islander; SD, standard deviation; HDL-C, high-density lipoprotein; BMI, body mass index; DBP, diastolic blood pressure; SBP, systolic blood pressure; CVD, cardiovascular disease.
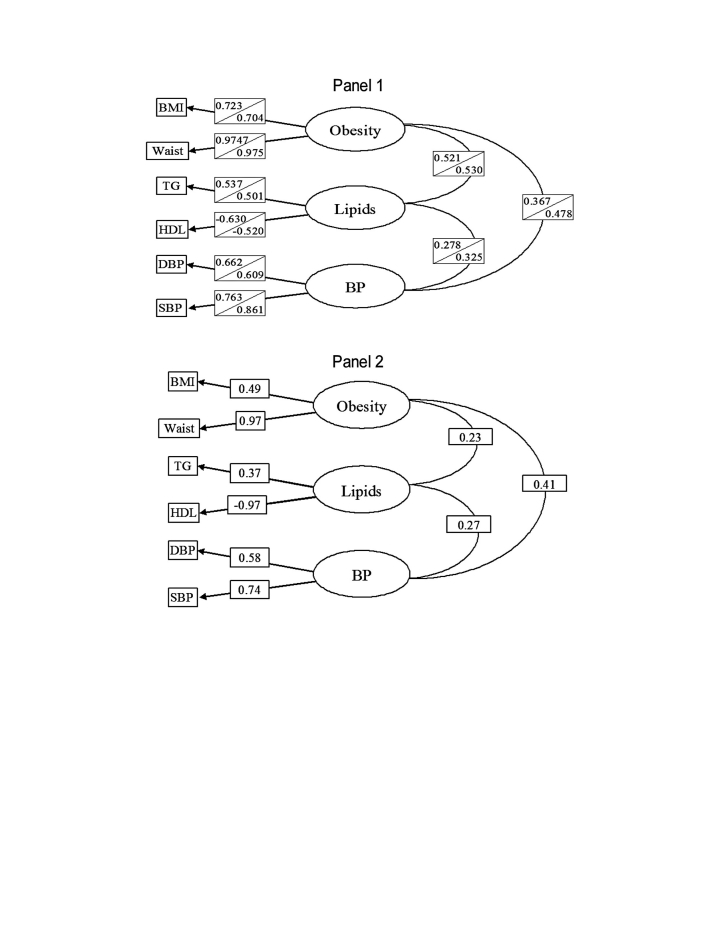
The CFA results are given in Table 2. For the T1DM samples, the best fit was obtained for the model of three correlated factors; the worst fit was obtained for the model of one factor. Findings were similar for T2DM, with the strongest evidence for the best fit observed for the model with three correlated factors. Figure 1 shows the parameter estimates for the models of three correlated factors, for both samples of T1DM and for T2DM, respectively. Results were similar for all three analyses.
Table 2.
CVD Risk Factors Evaluated by Confirmatory Factor Analysis in Youths With Type 1 and Type 2 Diabetes
| Fit statisticsa | One factor | Two correlated factors | Three correlated factors | One factor with correlated residuals | Hierarchical three factors |
|---|---|---|---|---|---|
| Subjects with type 1 diabetes: first half-sample (n = 600) | |||||
| RMR | 0.116 | 0.066 | 0.035 | 0.058 | 0.035 |
| AIC | 205.63 | 58.88 | 15.37 | 50.77 | 21.37 |
| BIC | 161.65 | 19.31 | −15.41 | 15.59 | 3.78 |
| Subjects with type 1 diabetes: second half-sample (n = 598) | |||||
| RMR | 0.102 | 0.054 | 0.033 | 0.049 | 0.033 |
| AIC | 176.25 | 34.93 | 12.90 | 32.83 | 18.90 |
| BIC | 132.314.61 | −17.85 | −2.32 | 1.33 | |
| Subjects with type 2 diabetes (n = 95) | |||||
| RMR | 0.131 | 0.139 | 0.082 | 0.073 | 0.082 |
| AIC | 26.73 | 26.72 | −4.19 | −2.92 | 1.81 |
| BIC | 3.75 | 6.29 | −24.62 | −18.24 | −10.96 |
Bold values identify the best-fitting model, SEARCH for Diabetes in Youth 2001–2003.
Abbreviations: RMR, root mean squared residual; AIC, Akaike's information criteria; BIC, Bayesian information criterion, also known as Schwarz's Bayesian Criterion.
FIG. 1.
Maximum likelihood parameter estimates from the best-fitting CVD risk factor model based on confirmatory factor analysis: three correlated factors. (Panel 1) Youths with T1DM first-half sample (n = 600) (top), second-half sample (n = 598) (bottom). (Panel 2) T2DM (n = 95), SEARCH for Diabetes in Youth 2001–2003.
Discussion
CFA findings for youths with either T1 or T2DM were that a single-factor model yielded the worst fit of the CVD risk factor data, compared to four other prespecified models. The best-fitting model incorporated three correlated factors: obesity, lipids, and blood pressure. We suggest that etiologically driven studies of CVD risk factors in youth with diabetes consider obesity, lipids, and blood pressure as separate (but potentially correlated) variables, rather than focus on the metabolic syndrome.
CFA allows for tests of specific hypothesis that a prespecified model (e.g., one latent factor) provides a good fit of the CVD risk factor data, compared to other models.14 Additionally, it has been argued that forcing factors to be uncorrelated, as is done via orthogonal rotation in principal factor analysis, is inconsistent with a priori knowledge of correlated biologic processes. Here, CFA is useful because the method avoids the need to force independence among factors. Shen and colleagues23 used CFA to test the goodness of fit for a four-factor model. Results confirmed the hypothesis of four factors (insulin resistance, obesity, lipids, and blood pressure), and this was established for men and women across three ethnic groups. In contrast, Pladevall et al.8 used CFA to test the hypothesis that components of the metabolic syndrome were best described by a single common factor versus a four-factor model, and results favored the single common factor.
Pladevall et al.8 criticized prior work due to correlations among variables such as SBP and DBP, triglycerides and HDL, and waist and BMI, suggesting that such correlations would drive results away from finding a single common factor because those highly correlated variables representing essentially the same phenomenon would load together to yield the respective separate phenomenon (e.g., blood pressure, lipids, obesity) rather than loading on a single factor overall. Therefore, unlike previous work, we systematically prespecified five models that allowed not only for the single-factor possibility but also for a priori knowledge of underlying biology including correlation between measures. Still, consistent with the results of the initial exploratory principal factor analysis, CFA ruled out a single common factor and identified three correlated factors as the best-fitting data structure for both T1DM and T2DM.
Reaven recently suggested that clustering of risk factors would only occur in the presence of insulin resistance.9 Interestingly, in the present data, the three-correlated-factor structure emerged both for youth with T1DM and those with T2DM. It is possible that despite the starkly different prevalence of the risk factors between T1DM and T2DM, the correlation among the three factors in the best-fitting model may be due to unmeasured insulin resistance in both populations. It is of note, however, that the hierarchical model that included one second-order factor (presumably representing insulin resistance given Reaven's argument) also did not fit the data as well as the model of three correlated factors.
Our findings in no way argue against the importance of insulin resistance and traditional components of the metabolic syndrome in the development of cardiovascular disease in either T1DM or T2DM. Among over 200 youths with T1DM, a wide range of insulin resistance as measured by euglycemic clamp has been demonstrated; in this sample, insulin resistance was associated significantly with measures of overall and central adiposity, dyslipidemia, and blood pressure.24 Increased risk for diabetes-related complications and mortality has been associated with metabolic syndrome components and insulin resistance in two large T1DM cohorts.25,26 Interestingly, in the Pittsburgh Epidemiology of Diabetes Complications Study cohort,25 components of three different definitions of metabolic syndrome predicted major diabetes-related complications better than the overall syndrome. In a large group (n = 1366) T2DM patients, insulin resistance measured by homeostasis model assessment–insulin resistance (HOMA-IR) was independently associated with lipids, obesity, and hypertension,27 and in the Verona Diabetes Complications Study, the presence of the metabolic syndromewas associated with nearly a five-fold increase in CVD risk.28
Limitations
We had a limited number of individuals with T2DM, so we were unable to conduct a split sample replication of the CFA among youth with T2DM. This reflects the epidemiology of diabetes in youths in which the major form of diabetes that occurs is T1DM.29 Also, the response rate for youths registered as valid cases in SEARCH to attend the SEARCH in-person visit was not optimal, as detailed in Methods. However, for the issue of nonresponse to impact substantially on the results, one would need to suppose that the multivariate associations within subjects who did not attend the clinic visit differed from the multivariate associations within subjects who did attend. This seems unlikely; we consider the internal validity of these findings to be acceptable.
Under the SEARCH protocol, individuals with clinically diagnosed maturity-onset diabetes of the young (MODY) were not included in the SEARCH in-person visit and therefore were excluded. It is possible that a small number of individuals classified as T2DM under our criteria have MODY, although with the high C-peptide required under our strict definition, this is unlikely. It is also possible that some youths who tested negative for both IA2 and GAD may be positive for an unmeasured diabetes-related autoantibody. Given we also required a very high fasting C-peptide level for those classified as T2DM, we suspect that misclassification due to unmeasured (but positive) DAA would be negligent. Finally, given previously published findings from SEARCH that CVD risk factor prevalence differs according to race/ethnicity even after adjustment for diabetes type,1 it is possible that race/ethnicity played an important role with respect to the emergence of the three-correlated-factor solution identified in the present analyses. We did not have sufficient numbers within specific race/ethnic subgroups according to DM type to evaluate the issue of race/ethnicity rigorously within the analytic methods employed here; however, future work in SEARCH will focus on developing this area as additional subjects are added to the cohort over time.
In summary, for youths with either T1DM or T2DM, the individual components of metabolic syndrome and the metabolic syndrome itself can be used to describe CVD risk status for clinical or public health purposes. However, the present results suggest that for etiologically driven studies of CVD risk profile, metabolic syndrome may not be useful due to heterogeneous phenomena underlying this construct. Studies of the determinants of individually measured CVD risk factors, and of vascular end points, are critically needed to address long-term risk for CVD in youth with diabetes.
Acknowledgments
SEARCH for Diabetes in Youth is funded by the Centers for Disease Control and Prevention (PA number 00097 and DP-05-069) and supported by the National Institute of Diabetes and Digestive and Kidney Diseases. Site Contract Numbers: California (U01 DP000246); Colorado (U01 DP000247); Hawaii (U01 DP000245); Ohio (U01 DP000248); South Carolina (U01 DP000254); Washington (U01 DP000244); Coordinating Center (U01 DP000250)
The authors wish to acknowledge the involvement of General Clinical Research Centers (GCRC) at the following institutions in the SEARCH for Diabetes in Youth Study: Medical University of South Carolina (grant number M01 RR01070); Cincinnati Children's Hospital (grant number M01 RR08084); Children's Hospital and Regional Medical Center and the University of Washington School of Medicine (grant number M01RR00037 and M01RR001271); Colorado Pediatric General Clinical Research Center (grant number M01 RR00069)
The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention.
References
- 1.Rodriguez BL. Fujimoto W. Mayer-Davis E. Imperatore G. Williams DE. Bell RA. Wadwa RP. Palla SL. Liu L. Kershnar A. Daniels S. Linder B. Prevalence of Cardiovascular Disease Risk Factors in U.S. Children and Adolescents with Diabetes: The SEARCH for Diabetes in Youth Study. Diabetes Care. 2006;29:1891–1896. doi: 10.2337/dc06-0310. [DOI] [PubMed] [Google Scholar]
- 2.Duncan GE. Li SM. Zhou XH. Prevalence and trends of a metabolic syndrome phenotype among U.S. adolescents, 1999–2000. Diabetes Care. 2004;27:2438–2443. doi: 10.2337/diacare.27.10.2438. [DOI] [PubMed] [Google Scholar]
- 3.SEARCH for Diabetes in Youth. a multicenter study of the prevalence, incidence and classification of diabetes mellitus in youth. Control Clin Trials. 2004;25:458–471. doi: 10.1016/j.cct.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Kahn R. Buse J. Ferrannini E. Stern M. The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2005;28:2289–2304. doi: 10.2337/diacare.28.9.2289. [DOI] [PubMed] [Google Scholar]
- 5.Reaven GM. The metabolic syndrome: requiescat in pace. Clin Chem. 2005;51:931–938. doi: 10.1373/clinchem.2005.048611. [DOI] [PubMed] [Google Scholar]
- 6.Grundy SM. Does the metabolic syndrome exist? Diabetes Care. 2006;29:1689–1692. doi: 10.2337/dc05-2307. [DOI] [PubMed] [Google Scholar]
- 7.Kahn R. The metabolic syndrome (emperor) wears no clothes. Diabetes Care. 2006;29:1693–1696. doi: 10.2337/dc06-1453. [DOI] [PubMed] [Google Scholar]
- 8.Pladevall M. Singal B. Williams LK. Brotons C. Guyer H. Sadurni J. Falces C. Serrano-Rios M. Gabriel R. Shaw JE. Zimmet PZ. Haffner S. A single factor underlies the metabolic syndrome: a confirmatory factor analysis 2. Diabetes Care. 2006;29:113–122. doi: 10.2337/diacare.29.1.113. [DOI] [PubMed] [Google Scholar]
- 9.Reaven GM. The metabolic syndrome: is this diagnosis necessary? Am J Clin Nutr. 2006;83:1237–1247. doi: 10.1093/ajcn/83.6.1237. [DOI] [PubMed] [Google Scholar]
- 10.Cook S. Weitzman M. Auinger P. Nguyen M. Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988–1994. Arch Pediatr Adolesc Med. 2003;157:821–827. doi: 10.1001/archpedi.157.8.821. [DOI] [PubMed] [Google Scholar]
- 11.Mayer-Davis EJ. Ma B. Lawson AB. D'Agostino R. Liese AD. Bell RA. Do Cardiovascular Disease (CVD) Risk Factors Cluster in Youth with Type 1A or Type 2 Diabetes? (Abstract) Diabetes. 2006;55:A220–A221. [Google Scholar]
- 12.Goodman E. Dolan LM. Morrison JA. Daniels SR. Factor analysis of clustered cardiovascular risks in adolescence: obesity is the predominant correlate of risk among youth. Circulation. 2005;111:1970–1977. doi: 10.1161/01.CIR.0000161957.34198.2B. [DOI] [PubMed] [Google Scholar]
- 13.Chen W. Srinivasan S. Elkasabany A. Berenson G. Cardiovascular risk factors clustering features of insulin resistance syndrome (Syndrome X) in a biracial (Black-White) population of children, adolescents, and young adults: the Bogalusa Heart Study. Am J Epidemiol. 1999;150:667–674. doi: 10.1093/oxfordjournals.aje.a010069. [DOI] [PubMed] [Google Scholar]
- 14.Lawlor DA. Ebrahim S. May M. Davey SG. (Mis)use of factor analysis in the study of insulin resistance syndrome. Am J Epidemiol. 2004;159:1013–1018. doi: 10.1093/aje/kwh150. [DOI] [PubMed] [Google Scholar]
- 15.Obesity and cardiovascular disease risk factors in black and white girls: the NHLBI Growth and Health Study. Am J Public Health. 1992;82:1613–1620. doi: 10.2105/ajph.82.12.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernandez JR. Redden DT. Pietrobelli A. Allison DB. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J Pediatr. 2004;145:439–444. doi: 10.1016/j.jpeds.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 17.Friedewald WT. Levy RI. Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 18.Hainline A., Jr. Miller DT. Mather A. The Coronary Drug Project. Role and methods of the Central Laboratory. Control Clin Trials. 1983;4:377–387. doi: 10.1016/0197-2456(83)90023-5. [DOI] [PubMed] [Google Scholar]
- 19.Marcovina SM. Landin-Olsson M. Essen-Moller A. Palmer JP. Lernmark A. Evaluation of a novel radioimmunoassay using 125I-labelled human recombinant GAD65 for the determination of glutamic acid decarboxylase (GAD65) autoantibodies. Int J Clin Lab Res. 2000;30:21–26. doi: 10.1007/s005990070029. [DOI] [PubMed] [Google Scholar]
- 20.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children Adolescents. Fourth report on the diagnosis, evaluation, and treatment of high blood pressure in childrem and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- 21.Hu LT. Bentler PM. Evaluating model fit. In: Hoyle RH, editor. Structural Equation Modeling: Concepts, Issues, and Applications. Thousand Oaks, CA: Sage; 1995. pp. 76–99. [Google Scholar]
- 22.Hu LT. Bentler PM. Fit indices in covariance structure modeling: Sensitivity to underparameterized model misspecification. Psycholog Methods. 1998;3:424–453. [Google Scholar]
- 23.Shen BJ. Goldberg RB. Llabre MM. Schneiderman N. Is the factor structure of the metabolic syndrome comparable between men and women and across three ethnic groups: the Miami Community Health Study. Ann Epidemiol. 2006;16:131–137. doi: 10.1016/j.annepidem.2005.06.049. [DOI] [PubMed] [Google Scholar]
- 24.Szadkowska A. Pietrzak I. Mianowska B. Bodalska-Lipinska J. Keenan HA. Toporowska-Kowalska E. Mlynarski W. Bodalski J. Insulin sensitivity in Type 1 diabetic children and adolescents. Diabet Med. 2008;25:282–288. doi: 10.1111/j.1464-5491.2007.02357.x. [DOI] [PubMed] [Google Scholar]
- 25.Pambianco G. Costacou T. Orchard TJ. The prediction of major outcomes of type 1 diabetes: a 12-year prospective evaluation of three separate definitions of the metabolic syndrome and their components and estimated glucose disposal rate: the Pittsburgh Epidemiology of Diabetes Complications Study experience. Diabetes Care. 2007;30:1248–1254. doi: 10.2337/dc06-2053. [DOI] [PubMed] [Google Scholar]
- 26.Makinen VP. Forsblom C. Thorn LM. Waden J. Gordin D. Heikkila O. Hietala K. Kyllonen L. Kyto J. Rosengard-Barlund M. Saraheimo M. Tolonen N. Parkkonen M. Kaski K. la-Korpela M. Groop PH. Metabolic phenotypes, vascular complications and premature deaths in a population of 4,197 patients with type 1 diabetes. Diabetes. 2008;57:2480–2487. doi: 10.2337/db08-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonora E. Targher G. Alberiche M. Formentini G. Calcaterra F. Lombardi S. Marini F. Poli M. Zenari L. Raffaelli A. Perbellini S. Zenere MB. Saggiani F. Bonadonna RC. Muggeo M. Predictors of insulin sensitivity in Type 2 diabetes mellitus. Diabet Med. 2002;19:535–542. doi: 10.1046/j.1464-5491.2002.00764.x. [DOI] [PubMed] [Google Scholar]
- 28.Bonora E. Targher G. Formentini G. Calcaterra F. Lombardi S. Marini F. Zenari L. Saggiani F. Poli M. Perbellini S. Raffaelli A. Gemma L. Santi L. Bonadonna RC. Muggeo M. The Metabolic Syndrome is an independent predictor of cardiovascular disease in Type 2 diabetic subjects. Prospective data from the Verona Diabetes Complications Study. Diabet Med. 2004;21:52–58. doi: 10.1046/j.1464-5491.2003.01068.x. [DOI] [PubMed] [Google Scholar]
- 29.Liese AD. D'Agostino RB., Jr. Hamman RF. Kilgo PD. Lawrence JM. Liu LL. Loots B. Linder B. Marcovina S. Rodriguez B. Standiford D. Williams DE. The burden of diabetes mellitus among US youth: prevalence estimates from the SEARCH for Diabetes in Youth Study. Pediatrics. 2006;118:1510–1518. doi: 10.1542/peds.2006-0690. [DOI] [PubMed] [Google Scholar]