Abstract
Background
The use of chromium-containing dietary supplements is widespread among patients with type 2 diabetes. Chromium's effects in patients at high risk for developing diabetes, especially those with metabolic syndrome, is unknown. The objective of this study was to determine the effects of chromium picolinate (CrPic) on glucose metabolism in patients with metabolic syndrome.
Method
A double-blind, placebo-controlled, randomized trial was conducted at a U.S. academic medical center. Sixty three patients with National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III)-defined metabolic syndrome were included. The primary end point was a change in the insulin sensitivity index derived from a frequently sampled intravenous glucose tolerance test. Prespecified secondary end points included changes in other measurements of glucose metabolism, oxidative stress, fasting serum lipids, and high sensitivity C-reactive protein.
Results
After 16 weeks of CrPic treatment, there was no significant change in insulin sensitivity index between groups (P = 0.14). However, CrPic increased acute insulin response to glucose (P = 0.02). CrPic had no significant effect on other measures of glucose metabolism, body weight, serum lipids, or measures of inflammation and oxidative stress.
Conclusion
CrPic at 1000 μg/day does not improve key features of the metabolic syndrome in obese nondiabetic patients.
Introduction
The use of chromium dietary supplements is widespread with estimated sales reaching $101 million in 2004.1 Chromium is second only to calcium in most commonly used mineral.1 Its use is especially popular among patients with type 2 diabetes (T2DM) or those attempting to lose weight.2 Many of these patients have metabolic syndrome, a cluster of metabolic abnormalities characterized by abdominal obesity, impaired fasting glucose (IFG), dyslipidemia, and elevated blood pressure.3 It is estimated that 47 million Americans have metabolic syndrome in some form.4 Importantly, metabolic syndrome is associated with a three-fold increase in the risk of developing T2DM and also significantly increases the risk of atherosclerotic cardiovascular disease (ASCVD).5 Insulin resistance, a central component of metabolic syndrome, has been identified as a potential therapeutic target of both dietary and pharmacologic interventions. Whereas a few studies have explored the effect of chromium supplementation on insulin sensitivity, the data remain inconclusive.6,7 Chromium yeast treatment had no effects in patients with T2DM,8 whereas chromium picolinate (CrPic) supplementation in subjects with T2DM on sulphonylurea agents significantly improves insulin resistance and glucose control.9 Supplemental trivalent chromium (Cr+3) has been shown to improve insulin sensitivity in some patients with T2DM, but its effect in patients at high risk of developing T2DM is largely unknown.10
The primary purpose of this study was to test the hypothesis that Cr+3 supplementation improves insulin sensitivity in patients with metabolic syndrome. Secondary outcomes included additional measurements of insulin secretion, disposition index, changes in body composition, lipid parameters, and markers of inflammation and oxidative stress.
Materials and Methods
Sixty three nondiabetic subjects between the ages of 18 and 75 with abdominal obesity and metabolic syndrome as diagnosed according to the criteria of the National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATPIII) were recruited from the Philadelphia metropolitan area and enrolled in the study between February, 2004, and March, 2005. Subject eligibility required waist circumference ≥102 cm for men and ≥89 cm for women and at least two of the following: systolic blood pressure ≥130 or diastolic blood pressure ≥85 mmHg or taking ≥1 antihypertensive agent; fasting blood glucose (FBG) ≥6.1 mmol/L, but <7 mmol/L; fasting triglycerides (TGs) ≥1.68, but ≤8.96 mmol/L; or high-density lipoprotein cholesterol (HDL-C) ≤1 mmol/L for males and ≤1.29 mmol/L for females. Subjects with FBG ≥6.1 mmol/L, had a 75-gram oral glucose tolerance test performed under fasting conditions to rule out the presence of underlying diabetes. Per guidelines, a 2-hour plasma glucose value of ≥11.1 mmol/L was exclusionary. Other major exclusion criteria included: ASCVD, low-density lipoprotein cholesterol (LDL-C) >4.9 mmol/L, liver transaminases three times the upper limit of normal, renal insufficiency, fibrates, or dietary supplements (excluding a multivitamin with <100 μg chromium). Subjects on stable (≥6 weeks) statin therapy were included. Of the 153 volunteers assessed for eligibility, 78 were excluded based on entry criteria and 12 chose not to participate. The protocol was approved by the Institutional Review Boards at the University of Pennsylvania and the Philadelphia VA Medical Center. All study subjects provided written, informed consent.
Study design
We conducted a randomized, double-blind, placebo-controlled study of the safety and efficacy of 16 weeks of CrPic therapy. Subjects who met entry criteria returned to the clinic within 30 days where a frequently sampled intravenous glucose tolerance test (FSIGT) was performed and were then randomized in a 1:1 double-blind fashion to receive either CrPic or matching placebo. Subjects received 500-μg capsules of CrPic supplied as Chromax®, or placebo, both of which were manufactured by Nutrition21 (Purchase, NY) and independently tested for disintegration, dissolution, Cr+3 and CrPic content by Covance Laboratories prior to randomization.
Subjects were instructed to consume one capsule in the morning and one in the evening daily with water for 16 weeks. Subjects returned to the clinic at 8 and 16 weeks for follow up.
Adherence was assessed by pill count. The 24-hour urine samples were analyzed for urinary chromium at baseline, 8 weeks, and 16 weeks. Dietary adherence was assessed by 3-day food records and analyzed by Esha Food Processor (version 8.22). Physical activity was assessed by a modified version of physical activity frequency questionnaire (PAFQ) with permission of the author.11,12
Measurements
Clinical parameters
Blood pressure was measured with an automatic electronic sphygmomanometer, with the subject in the sitting position after resting for at least 5 minutes. Body weight was measured on a calibrated scale (Tronix digital scale, Carol Stream, IL) with shoes off to the nearest 0.1 kg and height was measured to the nearest 0.1 cm. True waist circumference was measured midway between the 10th rib and the iliac crest with the subjects in the standing position. Bioimpedance analysis (BIA) was performed fasting, in the supine position using a Quantum II Analyzer (RJL Systems, MI).
Glucose and insulin metabolism
FSIGT was performed at baseline and at 16 weeks. After a 12-hour fast, two intravenous catheters, one in each arm, were placed in the subject's antecubital vein. One line was used for blood withdrawal and one for the infusion of glucose and insulin. Baseline blood samples were collected at 5-minute intervals (−10, −5, 0). A bolus of 50% glucose (0.3 gram/kg) was injected at time 0 followed by a bolus of insulin (0.03 U/kg) at 20 minutes. Blood samples were collected at 2, 3, 4, 5, 6, 8, 10, 12, 14, 16, 18, 20, 22, 25, 30, 40, 50, 70, 100, 140, and 180 minutes. Blood samples were placed on ice and centrifuged at within 1 hour, and the separated plasma was frozen and stored at −70°C until assayed. Plasma immunoreactive insulin was measured in duplicate using a human specific double-antibody radioimmunoassay from Linco Research (St. Charles, Missouri). Plasma glucose was analyzed in duplicate by an enzymatic calorimetric assay from Wako Diagnostics (Richmond, VA). The Bergman minimal model method was used to evaluate glucose-induced insulin release and insulin sensitivity.13 Insulin sensitivity (SI) and glucose effectiveness index (Sg), acute insulin response to glucose (AIRg), and disposition index (DI) were calculated from the FSIGT results using MINMOD MILLENIUM program version 3.0.14 Hemoglobin A1c was measured by high-permformance liquid chromatography (HPLC; Primus, Kansas City, MO).
Lipids and lipoprotein analysis and inflammatory markers
Lipid parameters were analyzed from ethylenediaminetetraacetic acid (EDTA) plasma collected after a 12-hour fast in a Centers for Disease Control (CDC)-standardized lipid laboratory. Plasma total cholesterol (TC), HDL-C, and TG were measured enzymatically on a Cobas Fara II autoanalyzer (Roche Diagnostic Systems Inc., NJ) using Sigma reagents (Sigma Chemical Co., MO). LDL-C and very-low-density lipoprotein cholesterol (VLDL-C) levels were determined after ultracentrifugation.
Markers of inflammation and oxidative stress
High-sensitivity C-reactive protein (hs-CRP) was measured with an ultra-high-sensitivity latex turbidimetric immunoassay (Wako Chemicals USA Inc., Richmond, VA) on a Cobas Fara II analyzer (Roche Diagnostics, Indianapolis). Oxidative stress was assessed by urinary isoprostane 8,12-iso-iPF2α-VI as measured by liquid chromatography/tandem mass spectrometry.15 The levels of 8,12-iso-iPF2α-VI were normalized to urinary creatinine levels and reported as ng 8,12-iso-iPF2α-VI/mg Cr).
Assessment of chromium status
Urinary chromium was assessed from a 24-hour urine collection using a microcomputer-controlled atomic absorption spectrometer (Model 5000, Perkin-Elmer Corp., Norwalk, CT), equipped with a programmable microcomputer-controlled graphite furnace (Model HGA 500, Perkin-Elmer). All determinations were made using argon as the furnace gas and pyrolytically coated furnace tubes as described by Anderson et al.16
Outcomes and sample size calculations
The primary end point of the study was change in SI. For this variable, we anticipated net increases in SI of 20% between the CrPic and placebo groups at 16 weeks. Accounting for a 15% estimated drop-out rate, we estimated that a sample size of 30 per group would provide at least 80% power to detect this difference between the two groups, using a two-tailed α of 0.05 and an estimated within group SD of ± 25%.
Statistical analysis
The primary analysis of glucose metabolism comes from a modified intent-to-treat analysis of 57 subjects. We excluded 6 randomized subjects from this analysis because either baseline or 16-week FSIGT could not be performed (n = 4) or SI values were extreme outliers (n = 2) (1 subject had a pre-SI > 50 [mU/L]−1min−1 whereas another had a post-SI of <0.01, both of which were considered not biologically plausible). Ordinary least squares (OLS) linear regression was used to analyze all FSIGT parameters to allow to adjust for baseline variables and treatment by baseline level interactions. These same analyses were also applied to secondary outcomes, including AIRg, Sg, and disposition index (DI). For nonglucose variable analyses, unpaired Student t-tests or the Kruskal–Wallis test were used as appropriate. In addition, an analysis of covariance (ANCOVA) model was used for LDL-C to adjust for baseline differences. For discrete variables, group differences were assessed using a chi-squared test.
Data were analyzed using STATA (Version 9.2, College Station, TX) and P < 0.05 was used to determine statistical significance. All values are presented as mean ± SD or median and interquartile range (IQR). All P values were two-tailed.
Results
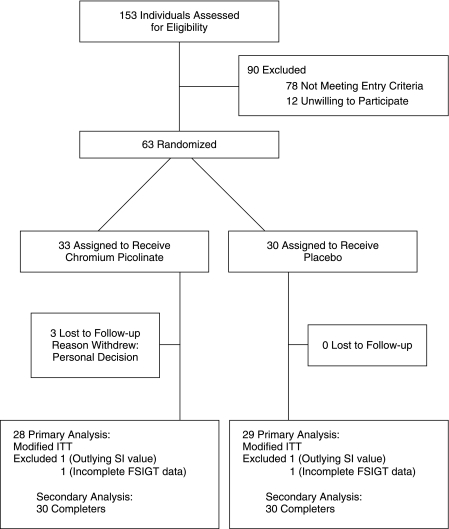
Of the 63 individuals randomized, 60 completed the study and 3 individuals in the CrPic group withdrew for personal reasons (Fig. 1). The participants were well matched for key baseline characteristics except for race (Table 1). The placebo group had a greater proportion of participants who were white as compared to the CrPic group (P < 0.001). There were no changes in weight (−0.12 ± 2.5 kg for CrPic and 0.72 ± 3.0 kg for placebo) (P = 0.62) or waist circumference (0.38 ± 2.3 cm for CrPic and 0.26 ± .4 cm for placebo) (P = 0.87) in response to treatment. Diet and physical activity as measured by questionnaire stayed constant throughout the study (data not shown).
FIG. 1.
Participant flow diagram.
Table 1.
Baseline Characteristics of Study Population
| Subject characteristicsa | CrPic (n = 33) | Placebo (n = 30) |
|---|---|---|
| Demographics | ||
| Age, years | 47.7 ± 10 | 51.1 ± 13 |
| Male sex, n | 13 | 18 |
| Female sex, n | 20 | 12 |
| Race | ||
| White, n | 16 | 27 |
| Non-white, n | 17 | 3 |
| Current smokers, n | 4 | 6 |
| Current ethanol users, n | 22 | 20 |
| Metabolic syndrome features | ||
| Waist circumference, cm | 109 ± 13 | 112 ± 13 |
| Men | 116 ± 8 | 119 ± 15 |
| Women | 105 ± 15 | 104 ± 13 |
| Fasting TG mmol/L | 2.0 ± 1 | 2.1 ± 0.8 |
| Fasting HDL-C mmol/L | 1.03 ± 0.2 | 1.04 ± 0.2 |
| Men | 0.97 ± 0.2 | 0.97 ± 0.2 |
| Women | 1.06 ± 0.2 | 1.15 ± 0.2 |
| Systolic blood pressure, mmHg | 130 ± 12 | 129 ± 15 |
| Diastolic blood pressure, mmHg | 81 ± 10 | 79 ± 10 |
| Fasting glucose, mmol/L | 4.74 ± 0.8 | 4.54 ± 0.6 |
| Impaired fasting glucose, n | 4 | 2 |
| Impaired glucose tolerance, n | 14 | 13 |
| Body mass index, kg/m2 | 37.8 ± 9 | 35.2 ± 6 |
| Percent body fat | 39.9 ± 12 | 36.7 ± 11 |
| Use of antihypertensive drugs, n (%) | 22 (66.7) | 17 (56.7) |
| Use of statin drugs | 8 | 12 |
| Glucose metabolism measures | ||
| SI (mU/L)1min−1 | 2.20 ± 2.7 | 1.68 ± 1.1 |
| Sg min−1 | 0.02 ± 0.01 | 0.08 ± 0.3 |
| AIRg mUL−1 | 653 ± 557 | 806 ± 505 |
| DI | 1294 ± 1620 | 1312 ± 1144 |
All values are mean ± SD; comparisons nonsignificant except for race, P < 0.001.
Abbreviations: CrPic, chromium picolinate; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; SI, insulin sensitivity; Sg, glucose effectiveness index; AIRg, acute insulin response to glucose; DI, disposition index.
Glucose metabolism
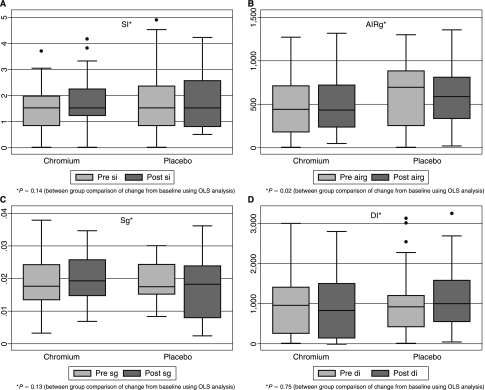
There were no significant baseline differences in measures of insulin resistance or insulin secretion (SI, AIRg, Sg, or DI) between the groups (Table 1). After 16 weeks of CrPic treatment, there was no significant change in SI index (Fig. 2). CrPic significantly increased AIRg compared to placebo (Fig. 2). There were no effects of CrPic on Sg or DI. The findings of increased AIRg and unchanged SI were unchanged, even after inclusion of the 2 subjects with significant outlying values. Additionally, hemoglobin A1c did not change in either group ([6.01 ± 0.4 to 6.09 ± 0.6% for CrPic] and [5.79 ± 0.5 to 5.85 ± 0.4% for placebo]). Fasting glucose was also unchanged ([4.66 ± 0.81 to 4.92 ± 0.91 mmol/L for CrPic [P = 0.09]) and (4.54 ± 0.64 to 4.5 ± 0.85 mg/dL for placebo [P = 0.89]). Subgroup analysis was also carried out for subjects with impaired glucose tolerance (IGT). CrPic had no effect on change in SI for either group [(+1.17 ± 3.6 (mU/L)−1min−1 for CrPic (P = 0.39]) and (−0.19 ± 0.71 [mU/L]−1min−1 for placebo (P = 0.35]). AIRg was also unchanged in patients with IGT ([+77.77 ± 112.7 mU L−1min−1 for CrPic (P = 0.09]) and (−105.39 ± 539.7 mU L−1min−1 for placebo (P = 0.49]).
FIG. 2.
Box and whisker plots of change from baseline in measures of glucose metabolism after 16 weeks of CrPic versus placebo. There was no significant change in SI (mU/L)1 min−1, P = 0.14 (A); Sg min−1, P = 0.13 (C); or DI P = 0.75 (D). CrPic significantly increased AIRg mUL−1min−1 compared to placebo, P = 0.02 (B).
Lipids, inflammatory markers, and oxidative stress
Baseline LDL-C was significantly higher in the CrPic group as compared to the placebo group (P = 0.007). A small nonsignificant reduction in LDL-C in the CrPic group and an increase in the placebo group was observed. Concomitant statin therapy had no significant impact on this reduction. However, when adjusted for the baseline differences, LDL-C was unchanged in response to treatment (P = 0.15) (Table 2). There was no effect of CrPic treatment on HDL-C, TGs, or TG/HDL cholesterol ratio (Table 2). There was no significant change in urinary isoprostanes ([−0.08 ± 4.1 ng 8,12-iso-iPF2α-VI/mg creatinine for CrPic] and [−0.11 ± 3.3 ng 8,12-iso-iPF2α-VI/mg creatinine for placebo] [P = 0.91]) after 16 weeks of treatment. Median hs-CRP changed from 2.98 (IQR 2.1–5.9) to 3.01 (IQR 2.1–5.4) mg/L in the CrPic group and from 1.93 (IQR 1.0–4.0) to 1.97 (IQR 1.1–4.8) mg/L in the placebo group (P = 0.38).
Table 2.
Raw and Percent Changes in Serum Lipoproteins After 16 Weeks of Therapy
|
Variables |
Chromium (n = 31) |
Placebo (n = 31) |
|
Between group comparisons |
||||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Baseline | Week 16 | % change from baseline | Baseline | Week 16 | % change from baseline | Net % difference (Cr vs. plc) | P-valuea |
| TC (mmol/L) | 5.30 (1.1) | 5.15 (1.1) | −2.5% | 4.78 (0.9) | 4.86 (0.9) | 2.0% | −4.4% | .15 |
| Non-HDL (mmol/L) | 4.29 (1.1) | 4.14 (1.0) | −2.9% | 3.75 (0.9) | 3.78 (0.9) | 2.1% | −5.1% | .20 |
| LDL-C (mmol/L)b | 3.39 (0.9) | 3.23 (1.0) | −2.4% | 2.79 (0.8) | 3.03 (0.9) | 9.0% | −11.4% | .12 |
| VLDL-C (mmol/L)c | 0.70 (0.3–2.4) | 0.70 (0.2–2.7) | 0.0% | 0.88 (0.2–2.3) | 0.72 (0.1–1.7) | −7.1% | −1.85% | .07 |
| TG (mmol/L)c | 1.72 (0.9–4.6) | 1.58 (0.5–6.1) | −0.3% | 2.08 (0.8–4.8) | 1.76 (0.6–3.3) | −17.7% | −10.6% | .11 |
| HDL-C (mmol/L) | 1.0 (0.2) | 1.0 (0.2) | −0.2% | 1.1 (0.2) | 1.0 (0.2) | −0.3% | −0.11% | .98 |
P values are for unpaired t-test except for LDL-C where an ANCOVA analysis was used to account for baseline difference.
Baseline LDL-C was significantly different between treatment groups (P = 0.007).
Median (IQR 25-75).
Chromium adherence and study blinding
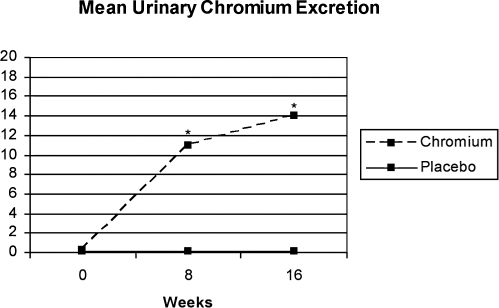
Both groups had mean adherence by pill count of >90%. There were no differences in urinary chromium excretion between the groups at baseline. Chromium supplementation resulted in a marked increase in chromium urinary excretion at both week 8 (10.93 ± 6.9 μg/L [P < 0.001]) and week 16 (14.06 ± 8.4 μg/L [P < 0.001]) whereas placebo had no effect ([0.14 ± 0.2 μg/L at week 8) and (0.11 ± 0.1 μg/L at week 16) (NS) (Fig. 3). There was no correlation between urinary chromium excretion and change in AIRg (P = 0.45) or SI (P = 0.69).
FIG. 3.
Levels of urinary chromium (reported as means ± SD) obtained over a 24-hour collection significantly increased in obese subjects with metabolic syndrome randomized to CrPic at both 8 and 16 weeks compared to negligible levels over time in the placebo-randomized group (*P < 0.001 for 8 and 16 week time points).
Safety and adverse events
Overall, CrPic was well tolerated. There were no serious adverse events (SAEs) in either group.
Discussion
The results of what we believe is the first randomized placebo controlled trial of chromium in metabolic syndrome did not demonstrate an effect of pharmacologic dosing of CrPic on insulin sensitivity or other features of metabolic syndrome. CrPic did, however, increase the early phase of insulin secretion in response to glucose as measured by AIRg.
Multiple prior studies have examined the effects of chromium supplementation on glucose metabolism with inconsistent results. This is most likely due to significant variability among these studies with respect to the dose and preparation of chromium given, the duration of use, the measurement techniques, and the study population. Whereas most of the studies have been in patients with T2DM, few studies have been performed in patients at high risk for developing the disease, such as those with IGT, obesity, or a family history of T2DM.17,18 An improvement in insulin sensitivity as assessed by the minimal model was reported in patients with obesity and a family history of T2DM after receiving CrPic (1000 μgrams/day) for 32 weeks.18 However neither the exact magnitude of this effect nor the effect on AIRg data were reported. Similar findings were made using a hyperglycemic clamp to assess SI after administering CrCl3 for 12 weeks to patients IGT in an open-label study.17 Others have not shown any change in insulin sensitivity after Cr+3 supplementation in a nondiabetic elderly population and in patients with IGT.19,20 Specifically, a recent smaller study targeted patients with IGT and reported no improvement in insulin sensitivity after treatment with CrPic (800 μg/day) using the homeostasis model assessment (homeostasis model assessment [HOMA] index).20
Studies have demonstrated lower plasma insulin levels in both the fasting and glucose stimulated state in response to chromium treatment.18,21,22 However, none of these studies used AIRg as a measure of insulin secretion. Joseph et al. demonstrated a decrease in insulin AUC without an increase in insulin secretion in patients treated with chromium and resistance training for 12 weeks.23 Our study also describes the effect of chromium supplementation on insulin secretion as measured by AIRg. AIRg is a measure of the acute β-cell response to rising glucose through insulin secretion24 and has been shown to be an independent predictor of the development of T2DM, even after adjusting for SI.25 Although accumulating evidence from randomized trials support the role of targeting IR as a means of preventing T2DM, data on insulin secretagogues are lacking, although the short-acting sulfonylurea nateglidine is being prospectively evaluated alone and with the angiotensin receptor blocker valsartan in the NAVIGATOR trial to study whether these drugs can delay or prevent T2DM in patients with IGT.26 Although our finding is intriguing, it should be interpreted with caution because the relative effect was small and could have been a chance finding, and the clinical relevance of the observed increase AIRg may be limited.
This study also explored the effects of chromium on other metabolic syndrome features. Despite previous literature suggesting a weight loss effect of CrPic,27 we found no changes in body weight or waist circumference over 16 weeks. Additionally, CrPic did not affect fasting serum TG or HDL-C as previously suggested.18,19,28 Our results did not demonstrate any changes in markers of vascular inflammation or oxidative stress after chromium supplementation. Whereas we were admittedly underpowered to detect changes in hs-CRP, we are reassured by the fact that CrPic did not increase oxidative stress as has previously been suggested by others.29 Additionally, we did not find that CrPic supplementation led to adverse events or to a worsening of renal function or a reduction in iron stores as has been previously been reported,30,31 suggesting that up to 16 weeks of a supraphysiologic dose of CrPic is safe.
Our study has several limitations. First, our study was single center and short term and included a heterogeneous patient population. Patients with metabolic syndrome, while easy to identify clinically, are quite heterogeneous with regard to their level of insulin sensitivity as demonstrated by our baseline FSIGT data. This is documented by the fact that only 52% of our metabolic syndrome subjects had impaired glucose metabolism (IFG or IGT) at baseline, a variable commonly used in diabetes prevention intervention trials. Recent data in patients with T2DM suggests baseline insulin resistance as assessed by the hyperinsulinemic eyglycemic clamp may predict response of CrPic on insulin sensitivity.32 Our study was not designed to answer this question; however, our data do not support this finding in obese patients with IGT as assessed by FIGTT. Another possibility is that we initially overestimated the effect size for chromium on SI, resulting in an underpowered analysis for this variable. Our intervention was for 16 weeks and it is possible (though unlikely) that a longer duration of chromium use is needed to observe any clinically beneficial effects. Finally, it is possible that CrPic supplementation is more effective in chromium-deficient patients and that our population had normal baseline chromium balance based on urinary chromium, thus limiting an effect, although we do not have any data available on the prevalence of chromium deficiency in adults with metabolic syndrome.20
In conclusion, the study does not support the use of chromium as a treatment for patients with metabolic syndrome. Despite its widespread use, we were unable to demonstrate any beneficial effect of CrPic supplementation on detailed measures of insulin sensitivity, body weight, lipids, and inflammatory markers in obese nondiabetic patients with metabolic syndrome. Currently, there are clinically proven alternatives (diet and exercise) for the prevention of diabetes in this patient population that are highly effective and should remain the mainstay of treatment. The unanticipated effect of CrPic on insulin secretion may warrant further study in larger samples of pre-diabetic patients to reproduce this finding and further elucidate its mechanism.
Acknowledgments
We thank Anna Lillethun, Linda Morrell, and Kimberly McMahon for technical support; Lisa Basel-Brown for diet instruction and analysis; and Helen Teng for assistance with coordination of the study. We would also like to thank Noella Bryden (Beltsville Human Nutrition Research Center, USDA) for the chromium determinations and Dr. Anne Cappola for her review of this manuscript.
This work was supported by the following grants: R21DK067241, K-23 AT-00058, and M01-RR00040 (Translational Research Center [TRC]). Nutrition21 provided active drug and placebo. Dr. Boston is the principal author of the modeling software MinMod Millenium.
Trial registration: Study ID numbers, AT001147-01; last updated, December 8, 2005; record first received, August 17, 2006; ClinicalTrials.gov Identifier, NCT00128154; health authority, United States: Federal Government; www.clinicaltrials.gov/ct/show/NCT00128154?order=1/.
The results of this study were included in the abstract publication for the 66th Scientific Sessions of the American Diabetes Association in Washington, DC, June, 2006.
References
- 1.NBJ's Supplement Business Report 2005. Nutrition Business Journal. San Diego, CA: Penton Media; 2005. [Google Scholar]
- 2.Shapiro K. Gong WC. Natural products used for diabetes. J Am Pharm Assoc. 2002;42:217–226. doi: 10.1331/108658002763508515. [DOI] [PubMed] [Google Scholar]
- 3.Expert Panel on Detection EaToHBCiA. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 4.Ford ES. Giles WH. Dietz WH. Prevalence of the metabolic syndrome among US adults: findings From the Third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 5.Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care. 2005;28:1769–1778. doi: 10.2337/diacare.28.7.1769. [DOI] [PubMed] [Google Scholar]
- 6.Cefalu WT. Hu FB. Role of chromium in human fealth and in diabetes. Diabetes Care. 2004;27:2741–2751. doi: 10.2337/diacare.27.11.2741. [DOI] [PubMed] [Google Scholar]
- 7.Ethan M B. Tatsioni A. Lichtenstein AH. Lau J. Pittas AG. Effect of chromium supplementation on glucose metabolism and lipids: a systematic review of randomized trials. Diabetes Care. 2007;30:2154–2163. doi: 10.2337/dc06-0996. [DOI] [PubMed] [Google Scholar]
- 8.Klefstra N. Houweling ST. Bakker SJ. Verhoeven S. Gans RO. Jong BM. Bilo H. Chromium treatment has no effect in patients with type 2 diabetes in a western population. Diabetes Care. 2007;30:1092–1096. doi: 10.2337/dc06-2192. [DOI] [PubMed] [Google Scholar]
- 9.Martin J. Wang ZQ. Zhang XH. Wachtel D. Volaufova J. Matthews DE. Cefalu WT. Chromium picolinate supplementation attenuates body weight gain and increase insulin senistivity in subjects with type 2 diabetes. Diabetes Care. 2006;29:1826–1832. doi: 10.2337/dc06-0254. [DOI] [PubMed] [Google Scholar]
- 10.Althuis MD. Jordan NE. Ludington EA. Wittes JT. Glucose and insulin responses to dietary chromium supplements: a meta-analysis. Am J Clin Nutr. 2002;76:148–155. doi: 10.1093/ajcn/76.1.148. [DOI] [PubMed] [Google Scholar]
- 11.Ainsworth BE. Haskell WL. Whitt MI. Irwin ML. Swartz AM. Strath SJ. O'Brien WL. Bassett DR., Jr Schmitz KH, et al. Compendium of physical activities: and update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:s498–516. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 12.Bernstein M. Sloutskis D. Kumanyika S. Sparti A. Schutz Y. Morabia A. Data-based approach for developing a physical activity frequency questionnaire. Am J Epidemiol. 1998;147:147–154. doi: 10.1093/oxfordjournals.aje.a009427. [DOI] [PubMed] [Google Scholar]
- 13.Bergman R. Ider Y. Bowden C. Cobelli C. Quantitative estimation of insulin sensitivity. Am J Physiol. 1979;236:E667–E677. doi: 10.1152/ajpendo.1979.236.6.E667. [DOI] [PubMed] [Google Scholar]
- 14.Boston R. Stefanovski D. Moate P. Sumner AE. Watanabe RM. Bergman R. MINMOD Millenium: a computer program to calculate glucose effectiveness and insulin sensitivity from the frequently sampled intravenous glucose tolerance test. Diabetes Technol Therapeut. 2003;5:1003–1015. doi: 10.1089/152091503322641060. [DOI] [PubMed] [Google Scholar]
- 15.Lawson J. Li H. Rokach J. Adiyaman M. Hwang SW. Khanapure SP. FitzGerald GA. Identification of two major F2 isoprostanes 8, 12-iso-and 5-epi-8, 12-iso-isoprostane F2a-VI in human urine. J Biol Chem. 1998;273:29295–29301. doi: 10.1074/jbc.273.45.29295. [DOI] [PubMed] [Google Scholar]
- 16.Anderson R. Polansky MM. Bryden NA. Patterson KY. Veillon C. Glinsmann WH. Effects of chromium supplementation on urinary Cr excretion of human subjects and correlation of Cr excretion with selected clinical parameters. J Nutrit. 1983;113:276–281. doi: 10.1093/jn/113.2.276. [DOI] [PubMed] [Google Scholar]
- 17.Potter J. Levin P. Anderson R. Freiberg J. Andres R. Elahi D. Glucose metabolism in glucose-intolerant older people during chromium supplementation. Metabolism. 1985;34:199–204. doi: 10.1016/0026-0495(85)90001-0. [DOI] [PubMed] [Google Scholar]
- 18.Cefalu WT. Bell-Farrow AD. Stegner J. Wang ZQ. King T. Terry JG. Effect of chromium picolinate on insulin sensitivity in vivo. J Trace Elements Exp Med. 1999;12:71–83. [Google Scholar]
- 19.Amato P. Morales AJ. Yen SSC. Effects of chromium picolinate xupplementation on insulin sensitivity, serum lipids, and body composition in healthy, nonobese, older men and women. J Gerontol A Biol Sci Med Sci. 2000;55:M260–M263. doi: 10.1093/gerona/55.5.m260. [DOI] [PubMed] [Google Scholar]
- 20.Gunton JE. Cheung NW. Hitchman R. Hams G. O'Sullivan C. Foster-Powell K. McElduff A. Chromium supplementation does not improve glucose tolerance, insulin sensitivity, or lipid profile: a randomized, placebo-controlled, double-blind trial of supplementation in subjects with impaired glucose tolerance. Diabetes Care. 2005;28:712–713. doi: 10.2337/diacare.28.3.712. [DOI] [PubMed] [Google Scholar]
- 21.Wilson B. Gondy A. Effects of chromium supplementation on fasting insulin levels and lipid parameters in healthy, nonobese young subjects. Diabetes Res Clin Pract. 1995;28:179–184. doi: 10.1016/0168-8227(95)01097-w. [DOI] [PubMed] [Google Scholar]
- 22.Anderson RA. Cheng N. Bryden NA. Polansky MM. Cheng N. Chi J. Feng J. Elevated intakes of supplemental chromium improve glucose and insulin variables in individuals with type 2 diabetes. Diabetes. 1997;46:1786–1791. doi: 10.2337/diab.46.11.1786. [DOI] [PubMed] [Google Scholar]
- 23.Joseph LJO. Farrell PA. Davey SL. Evans WJ. Campbell WW. Effect of resistance training with or without chromium picolinate supplementation on glucose metabolism in older men and women. Metabolism. 1999;48:546–553. doi: 10.1016/s0026-0495(99)90048-3. [DOI] [PubMed] [Google Scholar]
- 24.Boston R. Moate P. Stefanovski D. Sumner AE. Bergman RN. AKA-Glucose: a program for kinetic and epidemiological analysis of frequently sampled intravenous glucose tolerance test data using database technology. Diabetes Technol Therapeut. 2005;7:298–307. doi: 10.1089/dia.2005.7.298. [DOI] [PubMed] [Google Scholar]
- 25.Hanley AJG. D'Agostino R., Jr Wagenknecht LE. Saad MF. Savage PJ. Bergman R. Haffner SM. Increased proinsulin levels and decreased acute insulin response independently predict the incidence of type 2 diabetes in the insulin resistance atherosclerosis study. Diabetes. 2002;51:1263–1270. doi: 10.2337/diabetes.51.4.1263. [DOI] [PubMed] [Google Scholar]
- 26.Novartis announces largest diabetes cardiovascular disease prevention trial with Starlix Diovan. Novartis Pharmaceuticals USA. 2006. http://www.pharma.us.novartis.com/newsroom/pressReleases/releaseDetail.jsp?PRID=141/ http://www.pharma.us.novartis.com/newsroom/pressReleases/releaseDetail.jsp?PRID=141/ 2-22-2006.
- 27.Pittler M. Stevinson C. Ernst E. Chromium picolinate for reducing body weight: Meta-analysis of randomized trials. Int J Obesity. 2003;27:522–529. doi: 10.1038/sj.ijo.0802262. [DOI] [PubMed] [Google Scholar]
- 28.Press R. Geller J. Evans G. The effect of chromium picolinate on serum cholesterol and apolipoprotein fractions in human subjects. West J Med. 1990;152:41–45. [PMC free article] [PubMed] [Google Scholar]
- 29.Vincent JB. Recent advances in the nutritional biochemistry of trivalent chromium. Proc Nutr Soc. 2004;63:41–47. doi: 10.1079/PNS2003315. [DOI] [PubMed] [Google Scholar]
- 30.Cerulli J. Grabe D. Gauthier I. Malone M. McGoldrick MD. Chromium picolinate toxicity. Ann Pharmacother. 1998;32:428–431. doi: 10.1345/aph.17327. [DOI] [PubMed] [Google Scholar]
- 31.Wasser WG. Feldman NS. D'Agati VD. Chronic renal failure after ingestion of over-the-counter chromium picolinate. Ann Intern Med. 1997;126:410. doi: 10.7326/0003-4819-126-5-199703010-00019. [DOI] [PubMed] [Google Scholar]
- 32.Wilson B. Gondy A. Effects of chromium supplementation on fasting insulin levels and lipid parameters in healthy, nonobese young subjects. Diabetes Res Clin Pract. 1995;28:179–184. doi: 10.1016/0168-8227(95)01097-w. [DOI] [PubMed] [Google Scholar]