Abstract
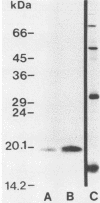
Recently, the complete amino acid sequence of a protein expressed in Escherichia coli from cloned Brucella abortus DNA was reported. On the basis of amino acid homology, this protein was identified as a copper-zinc superoxide dismutase (Cu-Zn SOD) (B. L. Beck, L. B. Tabatabai, and J. E. Mayfield, Biochemistry 29:372-376, 1990). We demonstrate in this paper that the sequenced protein is the same as the previously studied salt-extractable protein BCSP20. The plasmid-encoded protein expressed from recombinant E. coli is identical to the Brucella-derived BCSP20 in molecular mass, N-terminal amino acid sequence, and cross-reactivity with homologous and heterologous rabbit sera against either the recombinant gene product or the Brucella-derived protein. A survey of the expression of the Cu-Zn SOD protein in Brucella biovars representing all species was done by Western blotting (immunoblotting) using antisera raised against the recombinant E. coli-derived protein. With the exception of B. neotomae and B. suis biovar 2, the Cu-Zn SOD protein was detectable in all Brucella species and biovars tested, including eight biovars of B. abortus.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck B. L., Tabatabai L. B., Mayfield J. E. A protein isolated from Brucella abortus is a Cu-Zn superoxide dismutase. Biochemistry. 1990 Jan 16;29(2):372–376. doi: 10.1021/bi00454a010. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricker B. J., Tabatabai L. B., Deyoe B. L., Mayfield J. E. Conservation of antigenicity in a 31-kDa Brucella protein. Vet Microbiol. 1988 Dec;18(3-4):313–325. doi: 10.1016/0378-1135(88)90096-x. [DOI] [PubMed] [Google Scholar]
- Corbel M. J., Cockrem D. S., Brewer R. A. The interaction of Brucella cell surface components with plant agglutinins. Dev Biol Stand. 1984;56:169–175. [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mayfield J. E., Bricker B. J., Godfrey H., Crosby R. M., Knight D. J., Halling S. M., Balinsky D., Tabatabai L. B. The cloning, expression, and nucleotide sequence of a gene coding for an immunogenic Brucella abortus protein. Gene. 1988;63(1):1–9. doi: 10.1016/0378-1119(88)90540-9. [DOI] [PubMed] [Google Scholar]
- Steffens G. J., Michelson A. M., Otting F., Puget K., Strassburger W., Flohé L. Primary structure of Cu-Zn superoxide dismutase of Brassica oleracea proves homology with corresponding enzymes of animals, fungi and prokaryotes. Biol Chem Hoppe Seyler. 1986 Oct;367(10):1007–1016. doi: 10.1515/bchm3.1986.367.2.1007. [DOI] [PubMed] [Google Scholar]
- Tabatabai L. B., Deyoe B. L. Characterization of salt-extractable protein antigens from Brucella abortus by crossed immunoelectrophoresis and isoelectricfocusing. Vet Microbiol. 1984 Oct;9(6):549–560. doi: 10.1016/0378-1135(84)90017-8. [DOI] [PubMed] [Google Scholar]
- Tabatabai L. B., Deyoe B. L., Patterson J. M. Immunogenicity of Brucella abortus salt-extractable proteins. Vet Microbiol. 1989 May;20(1):49–58. doi: 10.1016/0378-1135(89)90006-0. [DOI] [PubMed] [Google Scholar]
- Tabatabai L. B., Deyoe B. L., Ritchie A. E. Isolation and characterization of toxic fractions from Brucella abortus. Infect Immun. 1979 Nov;26(2):668–679. doi: 10.1128/iai.26.2.668-679.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabatabai L. B., Deyoe B. L. Specific enzyme-linked immunosorbent assay for detection of bovine antibody to Brucella abortus. J Clin Microbiol. 1984 Aug;20(2):209–213. doi: 10.1128/jcm.20.2.209-213.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]