Abstract
We previously synthesized novel retinoid libraries, and after screening for bioactivity found one compound BT10 that functions as a specific agonist for retinoic acid receptors. This lead compound was further derivatized using SAR and LRD to obtain 3,5-disubstituted-1,2,4-oxadiazole-containing retinoids. The new oxadiazole (amide bioisosters)-containing retinoids (compounds 1, 2, 3, 4, 5, and 6) were synthesized in 42-65% yield by reacting with (E)-4-((3-ethyl,2-4,4,4-trimethylcyclohex-2-enylidene)methyl)benzoic acid and phenyl substituted amidoxime in DMF using CDI as the coupling reagent. The biological activities of the synthesized compounds are currently being evaluated.
Retinoids (retinol [vitamin A] and its biologically active metabolites) are essential signaling molecules that control various developmental pathways and influence the proliferation and differentiation of a variety of cell types in the adult.1,2 A number of synthetic retinoids have been synthesized that interact selectively with their receptors.3 Considering the importance of the retinoids, we were interested in synthesizing a small library of new retinoids.
In the context of our ongoing chemical biology project, studying the role of retinoic acid signaling pathways during zebrafish embryogenesis, we synthesized novel retinoid libraries.4a-f
This small library of compounds was screened for bioactivity in living zebrafish embryos. We found that several structurally related compounds significantly affect development. Distinct phenotypes are generated depending on time of exposure, and we characterized one compound BT10 (Figure 1) that produces specific cardiovascular defects when added 1 day post fertilization. When compared to all-trans retinoic acid (atRA), BT10 shows similar but not identical changes in the expression pattern of embryonic genes that are known targets of the retinoid pathway. Reporter assays determined that BT10 interacts with all three RAR receptor sub-types, but has no activity for RXR receptors, at all concentrations tested. 4g
Figure 1.
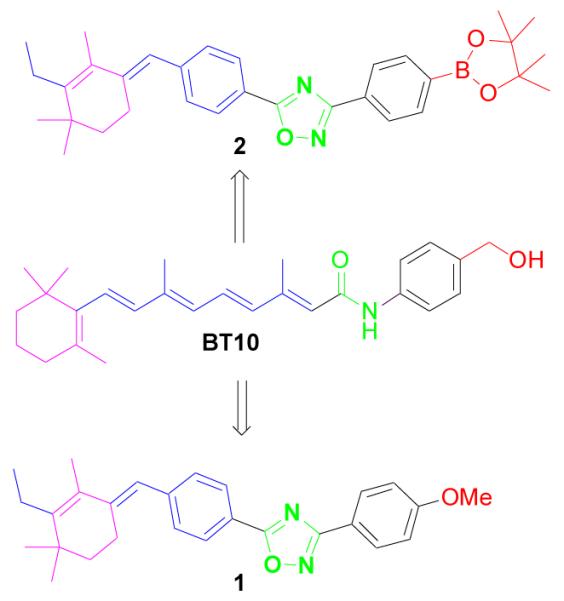
This lead compound may be useful for manipulating components of retinoid signaling networks, and may be further derivatized to enhance activity and selectivity. For that purpose we undertook this project to synthesize oxadiazole-containing novel retinoids as BT10 analogues.
Our lead molecule BT10 is an amide derivative of retinoic acid (containing polyene alkene spacers and amide linkage). Attempting to increase efficacy and receptor subtype specificity, we tried to synthesize new retinoic acid analogues by a) introducing a constrained phenyl ring system in place of the conjugated alkene backbone (spacers in atRA) to avoid the metabolism of atRA into its isomers, 9-cis-RA and 13-cis-RA, b) bio-isosterically replacing the amide linkage with oxadiazoles5 to prevent the in-vivo protease cleavage of the amide groups, and c) introducing the methoxy group by replacing the methyl alcohol group of BT10 to increase the efficacy. Here we report success at synthesizing novel oxadiazole-containing retinoids, which may provide new tools for probing retinoic acid signaling pathways.
To synthesize our lead compound 1 (Fig.1), we first synthesized the acid; (E)-4-((3-ethyl,2-4,4,4-trimethylcyclohex-2-enylidene)methyl)benzoic acid 8. The acid 84d,4e was synthesized starting from β-cyclocitral in a manner reported by us previously3d. Next we synthesized different substituted amidoximes by refluxing an ethanolic solution of substituted phenyl nitriles and hydroxylamine hydrochloride using NaOH as base6. The oxadiazole-containing retinoid 1 was synthesized by an amide coupling strategy7 using methoxy amidoxime 7 and acid 8, which were readily available though simple transformations, as the substrates. The corresponding acid 8 was treated with 1.2 equiv of CDI in DMF for 30 min at room temperature, then the methoxy amidoxime 7 was added and the resulting reaction mixture was heated under reflux for about 12 h (or until the acid was consumed completely as monitored by TLC) (Scheme 1)8a. After purification by silica-gel chromatography (Hexanes: EtOAc: 3:1) compound 1 was obtained as a white solid melting point 133-135°C with 48 % yield.
Scheme 1.
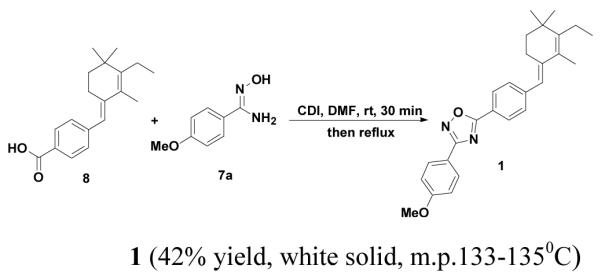
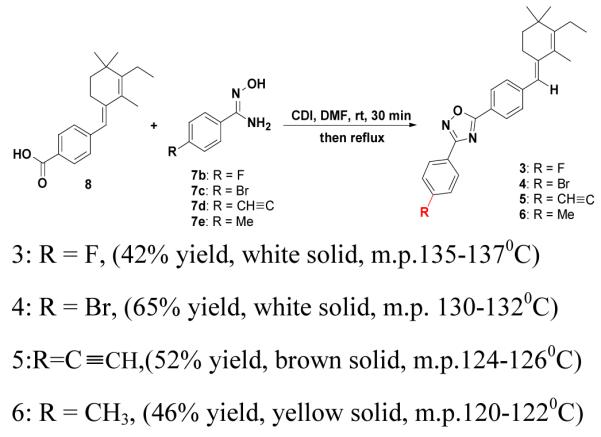
To validate the generality of the coupling reaction, we synthesized compounds 3,4,5, and 6 using the acid 8 and various substituted amidoximes (7b-7e) using CDI as coupling reagent in DMF (Scheme 2). Compounds 3,48b,5, and 6 were also successfully synthesized in 42-65% yield. Derivatives may have useful additional features. For example, compound 5 contains free acetylene, which could be further converted in vivo to a triazole derivative for target identification purposes. Compounds 3 and 6 could be further derivatized to 18F and 11C PET (Positron Emission Tomography) agents for noninvasive diagnostic agents, for diseases with over expressed RAR-alpha receptors.
Scheme 2.
We further envisioned developing a boron-based oxadiazole-containing small molecule retinoid library (Fig. 1), based on the hypothesis that introducing a boron atom in a biologically active framework might allow interaction with a target protein, not only through hydrogen bonds but also through covalent bonds. Such interactions could produce potent biological activity9. With this in mind, we synthesized compound 2.
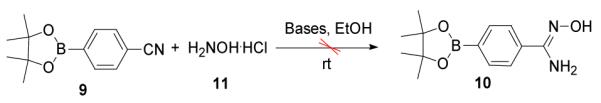
To synthesize compound 2 we first tried to synthesize 10 ((Z)-N’-hydroxy-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzimidamide) and planed to attach it with acid 8 using CDI as coupling reagent in DMF. To synthesize 10 we started with nitrile 9 (4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzonitrile) and hydroxylamine hydrochloride 11. But unfortunately, we failed to synthesize 10 in spite of using different bases [like NaOH/EtOH, K2CO3/ DMSO, Et3N/EtOH and (iPr)2NH/EtOH)] and reaction conditions (Scheme 3).10
Scheme 3.
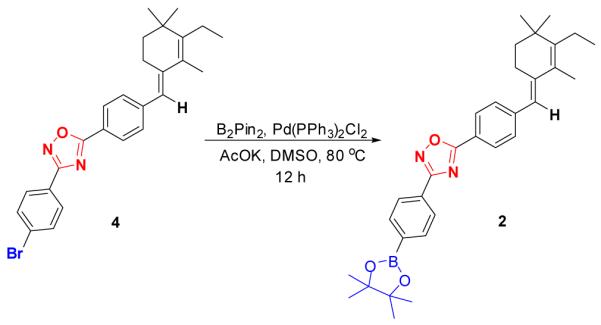
As we failed to synthesize 10, a separate reaction scheme was devised. A Suzuki coupling reaction was introduced using bromide compound 48b and B2Pin2 (Bis-pinocolatodiboron) as the substrates to give the boronic ester containing compound 2 in 45% yield as white solid (Scheme 4)11.
Scheme 4.
In conclusion, we report for the first time the synthesis of 3,5-disubstituted-1,2,4-oxadiazole-containing retinoids. The detailed biological evaluation of these compounds as possible RA pathway modulators is currently ongoing in our laboratory.
Supplementary Material
Acknowledgments
The author BCD is thankful to AECOM for start up funding. The instrumentation in the AECOM Structural NMR Resource is supported by the Albert Einstein College of Medicine and in part by Grants from the NSF (DBI9601607 and DBI0331934), the NIH (RR017998) and the HHMI Research Resources for Biomedical Sciences. Authors X-Y.T and SS are thankful to Dr.IlHwan An for synthesizing acid 8.
Footnotes
Supplementary Data The supplementary data (copies of 1H, 13C NMR and Mass spectra) are available online with this paper in Science Direct.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moon RC, Mehta RG, Rao KVN, Spron MB. In: The Retinoids: Biology, Chemistry and Medicine. 2nd edn Robert AB, Goodman DS, editors. Raven Press; New York: 1994. pp. 573–630. [Google Scholar]
- 2 (a).Loeliger P, Bollag W, Mayer H. Europ. J. Med. Chem. 1980;15:9–15. [Google Scholar]; (b) Dawson MI, Hobbs PD, Derdzinski K, Chan L-S, Gruber J, Chao W, Smith S, Thies RW, Schiff LJ. J. Med. Chem. 1984;27:1516–1531. doi: 10.1021/jm00377a022. [DOI] [PubMed] [Google Scholar]
- 3 (a).Nadzan AM. Annu. Rep. Med. Chem. 1995;30:119–128. [Google Scholar]; (b) Altucci LA, Leibowitz MD, Ogilvie KM, deLera AR, Gromemeyer H. Nature Review Drug Discovery. 2007;6:793. doi: 10.1038/nrd2397. [DOI] [PubMed] [Google Scholar]; (c) Kuendgen A, Schmid M, Schlenk R, Kinpp S, Hilderbrandt B, Steidi C. Cancer. 2006;19:1161. doi: 10.1002/cncr.21552. [DOI] [PubMed] [Google Scholar]
- 4 (a).Das BC, Smith ME, Kalpana GV. Bioorg. Med. Chem. Lett. 2008;18:4177. doi: 10.1016/j.bmcl.2008.05.097. [DOI] [PubMed] [Google Scholar]; (b) Das BC, Smith ME, Kalpana GV. Bioorg. Med. Chem. Lett. 2008;18:3805. doi: 10.1016/j.bmcl.2008.05.021. [DOI] [PubMed] [Google Scholar]; (c) Das BC, Evans T. Molecular Biosystem. (communicated) [Google Scholar]; (d) Das BC, Kabalka GW. Tetrahedron Lett. 2008;49:4695–4696. [Google Scholar]; (e) Das BC, Mahalingam SM, Evans T, Kabalka GW, Anguiano J, Hema K. Chem.Comm. 2009:2133. doi: 10.1039/b823063c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Das BC, Anguiano J, Mahalingam SM. Tetrahedron Lett. 2009;50:5670–5672. doi: 10.1016/j.tetlet.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Das BC, McCartin K, Liu T-C, Peterson RT, Evans T. PloS ONE. 2010;5(4) doi: 10.1371/journal.pone.0010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McBriar MD, Clader JW, Chu I, Del Vecchio RA, Favreau L, Greenlee WJ, Hyde LA, Nomeir AA, Parker EM, Pissarnitski DA, Song L, Zhang L, Zhao Z. Biorganic & Medicinal Chemistry Letters. 2008;18:215–219. doi: 10.1016/j.bmcl.2007.10.092. [DOI] [PubMed] [Google Scholar]
- 6.Vallin Karl S. A., Posaric David Wensbo, Hamersak Zdenko, Svensson Mats A., Minidis Alexander B. E Journal of Organic Chemistry. 2009;74:9328–9336. doi: 10.1021/jo901987z. [DOI] [PubMed] [Google Scholar]
- 7.Liang G,-B, Feng DD. Tetrahedron, Lett. 1996;37:6627–6630. [Google Scholar]
- 8 (a).General procedure for synthesis of compound 1: Acid 8 (0.5 mmol) and CDI (Carbonyl diimidazole) (0.6 mmol) were dissolved in 3 mL of DMF and stirred at room temperature. After 30 min, amidoxime 7a was added and the reaction mixture was heated under reflux for about 24 h (Monitored by TLC). Then the mixture was poured into water (20.0 mL), extracted by CHCl3 (3×15.0mL), and the combined organic solvent was dried over Na2SO4, filtered and concentrated in vacumn. The crude product was purified by silica-gel chromatography to give a white solid m.p 133-135°C with 48% yield.. 1H NMR (300 MHz, Acetone, TMS) δ 1.10 (t, J = 7.5 Hz, 3H, CH3), 1.13 (s, 6H, 2CH3), 1.52-1.58 (m, 2H, CH2), 1.96 (s, 3H, CH3), 2.25-2.33 (m, 2H, CH2), 2.65-2.62 (m, 2H, CH2), 2.83 (s, 3H, CH3), 6.59 (s, 1H, CH), 7.13-7.16 (m, 2H, Ar), 7.59 (d, J = 6.0 Hz, 2H, Ar), 8.10-8.13 (m, 2H, Ar), 8.18 (d, J = 6.0 Hz, 2H, Ar); 13C NMR (75 MHz, Acetone, TMS) 14.6, 15.0, 23.0, 24.6, 27.4, 36.0, 39.0, 55.3, 114.8, 119.7, 120.8, 121.7, 127.5, 128.0, 129.3, 130.4, 142.8, 144.3, 149.1, 162.7, 168.8, 175.8; HRMS (EI) Calcd. for C27H30N2O2 [M+H]+ requires 415.2386, found 415.2408.(8b) Analytical data’s of compound 4. Compound 4 (65%). A white solid. m.p. 130-132 °C. 1H NMR (300 MHz, CDCl3, TMS) δ 1.10 (t, J = 7.5 Hz, 3H, CH3), 1.12 (s, 6H, 2CH3), 1.52-1.58 (m, 2H, CH2), 1.96 (s, 3H, CH3), 2.26 (q, J = 7.5 Hz, 2H, CH2), 2.62-2.68 (m, 2H, CH2), 6.50 (s, 1H, CH), 7.46 (d, J = 8.1 Hz, 2H, Ar), 7.67 (d, J = 8.1 Hz, 2H, Ar), 8.08 (d, J = 8.1 Hz, 2H, Ar), 8.17 (d, J = 8.1 Hz, 2H, Ar); 13C NMR (75 MHz, CDCl3, TMS) ◻ 14.7, 15.2, 23.0, 24.4, 27.6, 35.9, 38.8, 120.3, 121.0, 125.6, 126.1, 127.0, 127.8, 129.0, 129.9, 132.1, 142.8, 144.0, 149.3, 168.2, 176.0. Anal. calcd. for C26H27BrN2O: C, 67.39%; H, 5.87%; N, 6.05%. Found: C, 67.13%; H, 6.08%; N, 5.68%..
- 9 (a).Groziak MP. In: Progress in Heterocyclic Chemistry. Gribble GC, Gilchrist TL, editors. Vol. 12. Pergamon; Oxford: 2000. pp. 1–21. [Google Scholar]; (b) Morin C. Tetrahedron. 1994;50:12521–12569. [Google Scholar]; (c) Yang W, Gao X, Wang B. Med. Res. Rev. 2003;23:346. doi: 10.1002/med.10043. [DOI] [PubMed] [Google Scholar]; (d) Matterson DS. Tetrahedron. 1989;45:1859. [Google Scholar]; (e) Tian Z-Q, Brown BB, Mack DP, Hutton CA, Bartlett PA. J. Org. Chem. 1997;62:514. doi: 10.1021/jo9615007. [DOI] [PubMed] [Google Scholar]; (f) Leung D, Abbenante G, Fairlie DP. J. Med. Chem. 2003;63:1144. doi: 10.1021/jm990412m. [DOI] [PubMed] [Google Scholar]; (g) Kabalka GW, Das BC, Das S. Tetrahedron Lett. 2001;42:7145–7146. [Google Scholar]
- 10.Kianmehr E, Yahyaee M, Tabatabai K. Tetrahedron Lett. 2007;48:2713–2715. [Google Scholar]
- 11.General procedure for synthesis of compound 2: The desired bromide compound (0.2 mmol) together with B2Pin2 (0.44 mmol, 111.7 mg), AcOK (1.0 mmol, 98.1 mg), Pd(PPh3)2Cl2 (0.02 mmol, 14.0 mg) and DMSO (3mL) was added into a 15.0 mL three-necked RBF under N2. The resulting mixture was stirred at rt for 10 min then heat at 80 °C for about 12 h under N2. After the reaction complete (Monitored by TLC), the reaction mixture was poured into 10 mL of water and extracted by DCM (3×10.0 mL). The combined organic solvent was dried over Na2SO4, filtered and concentrated in vacumn. The crude product was purified by silica-gel chromatography to give the boron-containing compound 2.Compound 2 (45%). A white solid. m.p. 139-141 °C. 1H NMR (300 MHz, CDCl3, TMS) δ 1.11 (t, J = 7.5 Hz, 3H, CH3), 1.12 (s, 6H, 2CH3), 1.53-1.57 (m, 2H, CH2), 1.96 (s, 3H, CH3), 2.25 (q, J = 7.5 Hz, 2H, CH2), 2.67 (t, J = 5.7 Hz, 2H, CH2), 6.50 (s, 1H, CH), 7.46 (d, J = 8.4 Hz, 2H, Ar), 7.97 (d, J = 8.4 Hz, 2H, Ar), 8.19 (dd, J = 8.4 Hz, 4H, Ar); 13C NMR (75 MHz, CDCl3, TMS) δ 14.7, 15.2, 22.9, 24.4, 24.9, 27.6, 35.9, 38.8, 84.1, 120.3, 121.1, 126.6, 127.0, 127.8, 129.4, 129.8, 135.1, 142.7, 143.9, 149.2, 168.9, 175.8. HRMS (EI) Calcd. for C32H40BN2O3 [M+H]+ requires 511.3132, found 511.3131.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


