Abstract
β-adrenergic receptor blockers have demonstrated significant survival benefit and have become standard therapy for adults with dilated cardiomyopathy, although their efficacy in pediatric patients is still unproven. Recent data suggests that the two major cardiac β-adrenergic receptor subtypes (β1 and β2) couple differentially to intracellular signaling pathways regulating contractility and remodeling. This has led some to suggest that the β1 receptor is the “cardiotoxic subtype” whereas the β2 receptor is “cardioprotective.” Given this paradigm, there could be situations where subtype selective β-blockade or even subtype selective β-stimulation might be beneficial. However, since most of these studies have been performed in isolated cardiomyocytes, their application to clinical practice is unclear. To better understand the roles of β1- vs. β2-receptors in the pathogenesis of clinical cardiomyopathy, we and others have taken advantage of several well-characterized murine models of cardiovascular disease. These studies demonstrate that β-receptor regulation of the balance between cardioprotection and cardiotoxicity is even more complex than previously appreciated: the role of each β-receptor subtype may vary depending on the specific cardiac stressor involved (e.g. ischemia, pressure overload, genetic mutation, cardiotoxin). Furthermore, the remodeling effects of β-receptor signaling have a temporal component, depending on whether a cardiac stress is acute vs. chronic.
Keywords: Cardiomyopathy, adrenergic receptor, cell signaling, β-blocker, heart failure
The role of the sympathetic nervous system in causing or exacerbating cardiac disease has been recognized since the early 1900s, when a popular treatise on nervous conditions referred to the sympathetic nervous system as the “Mischief-Making Mechanism” of the body. In the 1990s, when initial studies were undertaken to find a biomarker for heart failure mortality, plasma norepinephrine was found to be one of the best predictors of one year survival (1, 2) and studies in cultured cardiomyocytes showed dramatic cell death after brief norepinephrine exposure. Over the past twenty years, armed with the tools of molecular cell biology, researchers began to unravel the mechanisms of catecholamine-mediated cell signaling. The α- and β-adrenergic receptors served as a model system for understanding how changes in extracellular hormones are transduced into intracellular signals (3–6).
At the same time as these advances were occurring in the laboratory, in the clinic the pendulum began to swing away from the use of β-agonists for patients with chronic heart failure towards the use of β-blockers. In the 1990s, large adult clinical trails such as CIBIS-II (bisoprolol) (7), MERIT-HF (metoprolol-XL) (8) and Copernicus (carvedilol) (9) showed 30–35% reductions in mortality risk for patients treated with these β-antagonists.
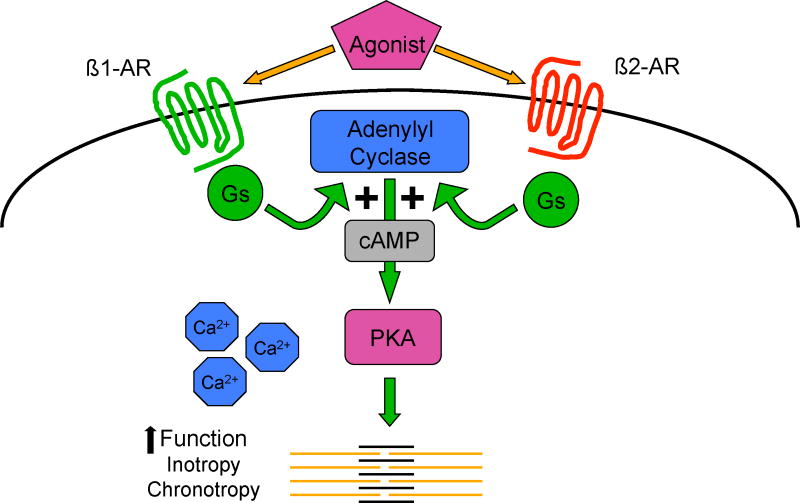
More recently, our understanding of β-AR signaling has changed radically. In the classical, linear model (Figure 1), β-adrenergic receptors were thought to primarily mediate cardiac function (inotropy, chronotropy, lusitropy) through activation of the stimulatory guanylyl nucleotide binding protein (Gs) pathway, adenylyl cyclase, and the second messenger cAMP. Increased cAMP activates protein kinase (PKA) which phosphorylates several downstream targets, including phospholamban (PLB) and the sarcoplasmic/endoplasmic reticulum calcium ATPase (SERCA) enhancing intracellular calcium dynamics. Under this linear model, coupling β-receptors to cardiac function, there was no explanation for how β-receptor stimulation resulted in cardiac cell injury.
Figure 1.
β-adrenergic receptor signaling: the classic (linear) model. Abbreviations: Gs=stimulatory guanylyl nucleotide binding protein; cAMP=cyclic adenosine monophosphate; PKA=protein kinase A.
Lefkowitz and colleagues, using predominantly in vitro systems, demonstrated that β-receptors were downregulated after exposure to an agonist such as epinephrine, i.e. the density of receptors on the cell surface decreased, leading to an attenuation of their activity (and thus explaining the clinical phenomenon known as tachyphylaxis) (10). In a pioneering study in humans, Bristow and colleagues demonstrated that β1-receptors were downregulated by 60% in failing human hearts explanted at the time of transplantation (11). This began the modern molecular era of β-receptor signaling research in heart failure and sparked a debate as to whether β-receptor downregulation was pathogenic in heart failure or whether it was part of a homeostatic process to protect the heart against catecholamine overload.
If β-receptor downregulation was a cause of cardiac dysfunction, then restoring receptor density to normal should rescue the failing heart. Initial studies using transgenic mice in which β-receptor expression was increased 50 to 200-fold seemed to support this hypothesis, as baseline contractility was enhanced dramatically compared to controls (12). However, as these mice aged, they developed progressive myocardial fibrosis and eventually a dilated cardiomyopathy (13). Further supporting the hypothesis that β-receptor downregulation was not the cause of heart failure, we totally deleted both β1 and β2-adrenergic receptors in the mouse using gene knockout technology (14–16). Despite the absence of both key cardiac β-adrenergic receptors, these mice developed normal hearts, had normal resting cardiac physiology and were even able to exercise as well as normal controls.
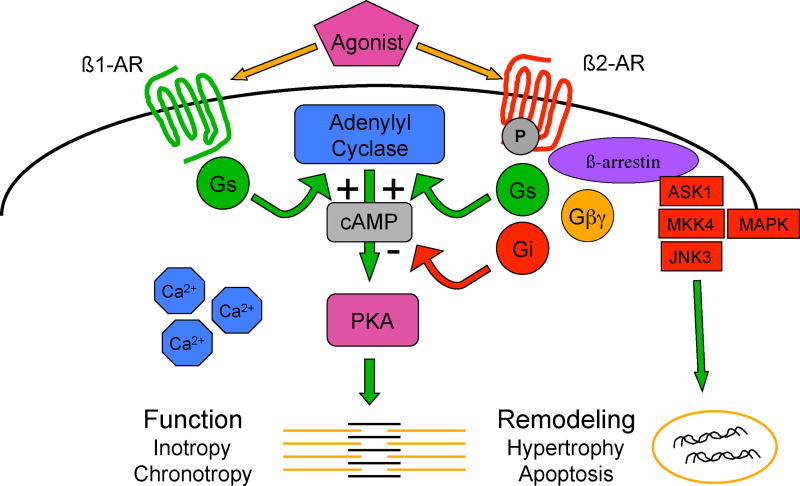
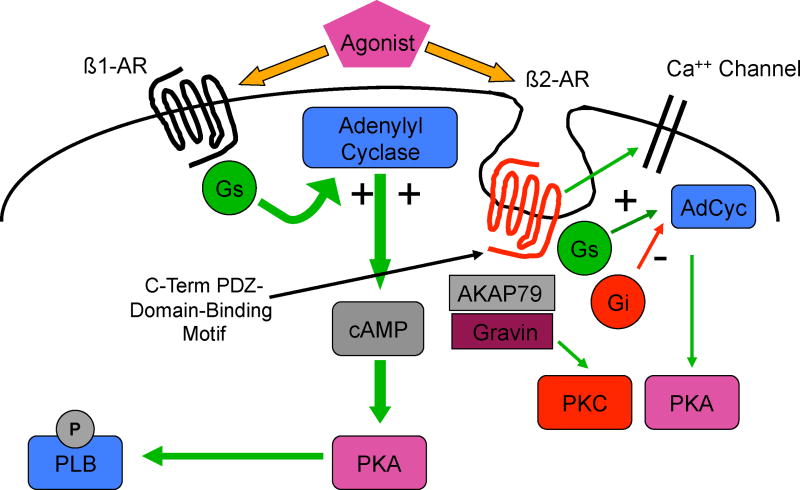
Our current appreciation of β-receptor signaling involves multiple pathways by which these receptors crosstalk with other signaling pathways (Figure 2). Recent studies suggest that β2-receptors can activate both cardiostimulatory (Gs) as well as cardioinhibitory (Gi) pathways (17), and crosstalk with pathways regulating gene transcription and cardiac remodeling (hypertrophy, apoptosis) (18, 19). Furthermore, the process of downregulation, initially thought to result only in removal of active receptor from the cell surface, is now understood to also be a mechanism for cell signaling (18, 20). Downregulation occurs due to the phosphorylation of serine and threonine residues on intracellular domains of the β-receptor by protein kinase A, the same key enzyme involved in enhancing cardiac function. Another mechanism involved in receptor desensitization, and one that does not require agonist activation, is mediated by G-protein receptor kinase (GRK), which leads to the recruitment to the cell membrane of β-arrestin along with a group of signaling molecules including mitogen activated protein kinase (MAPK). These signaling pathways are well know mediators of the remodeling processes of both hypertrophy and programmed cell death (apoptosis). β-receptors also contain regions on their carboxyl terminus known as PDZ domain-binding motifs, which participate in the binding of additional signaling molecules, such as AKAP79, and lead to additional crosstalk, e.g. with protein kinase C, another important group of enzymes involved in control of both function and remodeling (21, 22).
Figure 2.
β-adrenergic receptor signaling crosstalk: the model undergoes revision. Abbreviations: Gs=stimulatory guanylyl nucleotide binding protein; Gi=inhibitory guanylyl nucleotide binding protein; Gβγ = a subunit of Gs which is dissociated from the α subunit after Gs is activated by the β-receptor; cAMP=cyclic adenosine monophosphate; PKA=protein kinase A; ASK1=apoptosis signal-regulating kinase 1; MKK4=mitogen activated protein kinase kinase 4; MAPK=mitogen activated protein kinase; JNK3= c-Jun N-terminal kinase.
There is also evidence that each β-receptor subtype signals within its own cellular microdomain: for example, β1-receptor-induced cAMP accumulation is cell-wide and activates both PKA as well as phosphorylates phospholamban (PLB). In contrast, β2-receptor-induced cAMP accumulation is localized, where it activates local pools of adenylyl cyclase as well as L-type calcium channels (23).
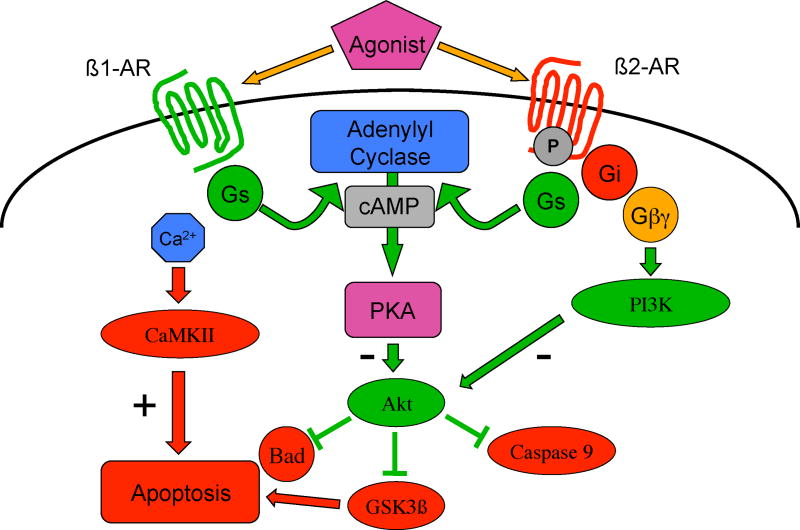
There is accumulating evidence, largely derived from studies in isolated cells, suggesting that β1-receptors are cardiotoxic and β2-receptors are cardioprotective (Figure 3) (24) This has led to the suggestion that there could be situations where subtype selective β1-blockade combined with subtype selective β2-stimulation might be beneficial (25). β1-receptors mediate pro-apoptotic signaling by activation of both PKA and calcium/calmodulin-dependent protein kinase (CaMK), acting through increased levels of intracellular calcium, alterations in several MAPKs, as well as by inhibiting the anti-apoptotic effects of protein kinase B (Akt) (26). In another example of the complexities of crosstalk, these cell remodeling pathways can also exert a negative influence on cardiac function. CaMK activation decreases contractility and increases arrhythmogenesis through its phosphorylation of the ryanodine receptor, increasing diastolic calcium leak from the sarcoplasmic reticulum (27).
Figure 3.
β-adrenergic receptor signaling: differential regulation of apoptosis. Gs=stimulatory guanylyl nucleotide binding protein; cAMP=cyclic adenosine monophosphate; PKA=protein kinase A; CaMKII=calcium/calmodulin-dependent protein kinase II; PI3K=phosphoinositide 3-kinase; Akt=protein kinase B; GSK3β=glycogen synthase kinase 3β; Bad=Bcl-2-associated death promoter.
In contrast, β2-ARs appear to mediate anti-apoptotic signaling through activation of Gi, PI3K and Akt (28–30) (28–30). Interestingly, when Gi signaling is blocked, β2-receptor signaling is able to switch from being anti-apoptotic to pro-apoptotic, mediated through p38 MAPK (30). β2-agonists can prevent apoptosis induced in vitro by catecholamines, hypoxia or reactive oxygen species (ROS) (28, 29, 31), and in vivo, by coronary ligation (32, 33). Genetic deletion of the β2-receptor increases susceptibility to isoproterenol-induced apoptosis (34). We have also shown that β2-receptor signaling is involved in the protective effects of some forms of preconditioning (35). However, not all in vivo data suggests that β2-receptors are cardioprotective. As mentioned earlier, although transgenic overexpression of the β2-receptor initially increases contractility, these mice develop cardiomyopathy as they age, with the severity related to the “dose” of β2-receptor (how many fold over baseline the protein is expressed) (13). β2-receptor overexpression fails to rescue the genetic cardiomyopathy induced by the knockout of the sarcomeric structural protein muscle LIM protein (MLP) although interestingly, overexpression of an inhibitor of GRK (the kinase which mediates β-receptor downregulation) does rescue these mice (36). Finally, β2-receptor overexpression increases susceptibility to ischemia reperfusion injury, although only in male mice (37). The fact that overexpression of the β1-receptor results in a more severe cardiomyopathy at lower receptor expression levels than the β2-receptor (38), has been cited as further evidence suggesting that β1-receptors are more closely coupled to cardiotoxic pathways.
The majority of data on the cardiotoxic/cardioprotective role of β-receptor subtypes has been obtained using isolated cardiomyocytes in culture. In most of these experiments, the receptor or signaling protein of interest is often overexpressed to supraphysiologic levels to increase readout. Whether these in vitro systems represent accurate models of β-receptor subtype signaling in vivo at physiologic levels of receptor expression is a critical question (39).
To address this issue, we utilized β1- and β2-receptor knockout mice to examine the role of each subtype in mediating cardiotoxicity and cardioprotection using in vivo models of cardiovascular disease. One such model is the toxic cardiomyopathy secondary to the chemotherapeutic anthracycline doxorubicin (Adriamycin). In this model, mice are administered a single dose of 15 mg/kg, equivalent to the therapeutic-range dose of 40 mg/m2 in humans. Wildtype control mice show no acute effects, but gradually develop a dilated cardiomyopathy over a period of several weeks. When doxorubicin was given to β1-receptor knockout mice, no acute effects were observed, similar to wildtype. However, when given to β2-receptor knockout mice, 100% died within 30 minutes (40). Blood pressure and fractional shortening dropped precipitously within the first several minutes of drug administration. The pro-death MAPK p38 was activated 20-fold over baseline compared to wildtype, and there was evidence of disruption of mitochondrial integrity. Signaling pathways, such as Akt and PKC, which regulate mitochondrial cell death signaling, and which have been previously shown to crosstalk with β2-receptors, were altered by the deletion of the β2-receptor. As further proof of this subtype-specific effect, we were able to recapitulate these effects in cardiomyocytes isolated from each of the β-receptor knockout mice (41).
These data would seem to confirm in vitro data that the β2-receptor is primarily cardioprotective. However, when we treated mice with a lower dose regimen of doxorubicin (2 mg/kg weekly for 8 weeks), resulting in a more gradually developing cardiomyopathy, the role of the β2-receptor switched from being cardioprotective to cardiotoxic: the β2-receptor knockout mice survived longer than the wildtype controls (42).
Finally, using the same genetic model of cardiomyopathy (MLP knockout) that Rockman et al. had failed to rescue with β2-receptor overexpression, we have found a similar switching of the roles of the two β-receptor subtypes. For these studies, we crossbred β-receptor knockouts with the MLP knockouts, producing mice lacking both the β1-receptor and MLP or both the β2-receptor and MLP. In contrast to our findings with acute doxorubicin toxicity, the β2-receptor knockout rescued the MLP mice from cardiomyopathy, suggesting that in this genetic cardiomyopathy, the β2-receptor was acting in a cardiotoxic fashion (43). The β1-receptor knockout so dramatically enhanced the toxicity of the MLP model such that no β1/MLP double knockout mice survived to birth. As has been described in vitro, β-receptor mediation of intracellular calcium transients appears to play an important role in rescuing/worsening this genetic cardiomyopathy.
These data show that in vivo, β-receptor subtypes do regulate cardiotoxicity and cardioprotection. As in vitro, they do so through crosstalk with other signaling pathways (CaMK, PI3K, the MAPKs, several isozymes of PKC, and Akt), through alterations in intracellular calcium transients, and through the regulation of the intrinsic mitochondrial cell death pathway. However, the assignment of one β-receptor subtype to the classification “cardiotoxic” and the other to “cardioprotective” is probably overly simplistic. This dichotomy between cell survival and cell death appears to be regulated by β-receptor subtypes in a manner dependent not only on the type of cardiac stressor but also by the duration of the cardiac stress.
Figure 4.
Scaffolding proteins mediate β-receptor crosstalk. Abbrevations: Gs=stimulatory guanylyl nucleotide binding protein; Gi=inhibitory guanylyl nucleotide binding protein; PLB=phospholamban; cAMP=cyclic adenosine monophosphate; PKC=protein kinase C; PKA=protein kinase A; AdCyc=adenylyl cyclase; AKAP79=A-kinase anchor protein 79.
Acknowledgments
This work was supported by a grant (HL061535) from the NIH/NHLBI to Dr. Bernstein.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Benedict C, Shelton B, Johnstone D, Francis G, Greenberg B, Konstam M, et al. Prognostic significance of plasma norepinephrine in patients with asymptomatic left ventricular dysfunction. SOLVD Investigators. Circulation. 1996;94:690–7. doi: 10.1161/01.cir.94.4.690. [DOI] [PubMed] [Google Scholar]
- 2.Francis G, Cohn J, Johnson G, Rector T, Goldman S, Simon A. Plasma norepinephrine, plasma renin activity, and congestive heart failure: relations to survival and the effects of therapy in V-HeFT II. Circulation. 1993;87(Suppl VI):VI-40–8. [PubMed] [Google Scholar]
- 3.Lefkowitz RJ, Michel T. Plasma membrane receptors. J Clin Invest. 1983 Oct;72(4):1185–9. doi: 10.1172/JCI111073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffman BB, Lefkowitz RJ. Adrenergic receptors in the heart. Annu Rev Physiol. 1982;44:475–84. doi: 10.1146/annurev.ph.44.030182.002355. [DOI] [PubMed] [Google Scholar]
- 5.Hausdorff WP, Caron MG, Lefkowitz RJ. Turning off the signal: desensitization of beta-adrenergic receptor function. FASEB J. 1990 Aug;4(11):2881–9. [PubMed] [Google Scholar]
- 6.Raymond JR, Hnatowich M, Lefkowitz RJ, Caron MG. Adrenergic receptors. Models for regulation of signal transduction processes. Hypertension. 1990 Feb;15(2):119–31. doi: 10.1161/01.hyp.15.2.119. [DOI] [PubMed] [Google Scholar]
- 7.CIBIS-II Investigators and Committees. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999 Jan 2;353(9146):9–13. [PubMed] [Google Scholar]
- 8.MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF) Lancet. 1999 Jun 12;353(9169):2001–7. [PubMed] [Google Scholar]
- 9.Eichhorn EJ, Bristow MR. The Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) trial. Curr Control Trials Cardiovasc Med. 2001;2(1):20–3. doi: 10.1186/cvm-2-1-020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sibley DR, Lefkowitz RJ. Molecular mechanisms of receptor desensitization using the beta-adrenergic receptor-coupled adenylate cyclase system as a model. Nature. 1985 Sep 12–18;317(6033):124–9. doi: 10.1038/317124a0. [DOI] [PubMed] [Google Scholar]
- 11.Bristow M, Ginsburg R, Umans V, Fowler M, Minobe W, Rasmussen R, et al. β1- and β2-adrenergic-receptor subpopulations in nonfailing and failing human ventricular myocardium: coupling of both receptor subtypes to muscle contraction and selective down-regulation in heart failure. Circ Res. 1986;59:297–309. doi: 10.1161/01.res.59.3.297. [DOI] [PubMed] [Google Scholar]
- 12.Milano C, Allen L, Rockman H, Dolber P, McMinn T, Chien K, et al. Enhanced myocardial function in transgenic mice overexpressing the β2-adrenergic receptor. Science. 1994;264:582–6. doi: 10.1126/science.8160017. [DOI] [PubMed] [Google Scholar]
- 13.Liggett S, Tepe N, Lorenz J, Canning A, Jantz T, Mitarai S, et al. Early and delayed consequences of β2-adrenergic receptor overexpression in mouse hearts. Critical role for expression level. Circulation. 2000;101:1707–14. doi: 10.1161/01.cir.101.14.1707. [DOI] [PubMed] [Google Scholar]
- 14.Rohrer D, Desai K, Jasper J, Stevens M, Regula D, Barsh G, et al. Targeted disruption of the mouse β1-adrenergic receptor gene: developmental and cardiovascular effects. Proc Natl Acad Sci. 1996;93:7375–80. doi: 10.1073/pnas.93.14.7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rohrer DK, Bernstein D, Chruscinski A, Desai KH, Schauble E, Kobilka BK. The developmental and physiological consequences of disrupting genes encoding beta 1 and beta 2 adrenoceptors. Adv Pharmacol. 1998;42:499–501. doi: 10.1016/s1054-3589(08)60798-x. [DOI] [PubMed] [Google Scholar]
- 16.Chruscinski AJ, Rohrer DK, Schauble E, Desai KH, Bernstein D, Kobilka BK. Targeted disruption of the beta2 adrenergic receptor gene. J Biol Chem. 1999;274(24):16694–700. doi: 10.1074/jbc.274.24.16694. [DOI] [PubMed] [Google Scholar]
- 17.Xiao R-P, Ji X, Lakatta E. Functional coupling of the β2-adrenoceptor to a pertussis toxin-senstive G protein in cardiac myocytes. Mol Pharmacol. 1995;47:322–9. [PubMed] [Google Scholar]
- 18.Daaka Y, Luttrell LM, Lefkowitz RJ. Switching of the coupling of the β2-adrenergic receptor to different G proteins by protein kinase A. Nature. 1997;390(6):88–91. doi: 10.1038/36362. [DOI] [PubMed] [Google Scholar]
- 19.Post G, Brown J. G protein-coupled receptors and signalling pathways regulating growth responses. FASEB J. 1996;10:741–9. doi: 10.1096/fasebj.10.7.8635691. [DOI] [PubMed] [Google Scholar]
- 20.Zhu H, Qi M, McElwee-Witmer S, Merkel-Jordan L, Perrone M, Clark K, et al. The relationship between hypertrophy and apoptosis in cultured neonatal ventricular myocytes. Circulation. 1998;98:I-345. [Google Scholar]
- 21.Xiang Y, Kobilka B. The PDZ-binding motif of the beta2-adrenoceptor is essential for physiologic signaling and trafficking in cardiac myocytes. Proc Natl Acad Sci U S A. 2003 Sep 16;100(19):10776–81. doi: 10.1073/pnas.1831718100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiang Y, Devic E, Kobilka B. The PDZ binding motif of the beta 1 adrenergic receptor modulates receptor trafficking and signaling in cardiac myocytes. J Biol Chem. 2002 Sep 13;277(37):33783–90. doi: 10.1074/jbc.M204136200. [DOI] [PubMed] [Google Scholar]
- 23.Xiao RP, Cheng H, Zhou YY, Kuschel M, Lakatta EG. Recent advances in cardiac beta(2)-adrenergic signal transduction. Circ Res. 1999 Nov 26;85(11):1092–100. doi: 10.1161/01.res.85.11.1092. [DOI] [PubMed] [Google Scholar]
- 24.Zheng M, Zhu W, Han Q, Xiao RP. Emerging concepts and therapeutic implications of beta-adrenergic receptor subtype signaling. Pharmacol Ther. 2005 Dec;108(3):257–68. doi: 10.1016/j.pharmthera.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 25.Xiao RP, Zhu W, Zheng M, Chakir K, Bond R, Lakatta EG, et al. Subtype-specific beta-adrenoceptor signaling pathways in the heart and their potential clinical implications. Trends Pharmacol Sci. 2004 Jul;25(7):358–65. doi: 10.1016/j.tips.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 26.Zhu WZ, Wang SQ, Chakir K, Yang D, Zhang T, Brown JH, et al. Linkage of beta1-adrenergic stimulation to apoptotic heart cell death through protein kinase A-independent activation of Ca2+/calmodulin kinase II. J Clin Invest. 2003 Mar;111(5):617–25. doi: 10.1172/JCI16326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kohlhaas M, Zhang T, Seidler T, Zibrova D, Dybkova N, Steen A, et al. Increased sarcoplasmic reticulum calcium leak but unaltered contractility by acute CaMKII overexpression in isolated rabbit cardiac myocytes. Circ Res. 2006 Feb 3;98(2):235–44. doi: 10.1161/01.RES.0000200739.90811.9f. [DOI] [PubMed] [Google Scholar]
- 28.Communal C, Singh K, Sawyer DB, Colucci WS. Opposing effects of beta(1)- and beta(2)-adrenergic receptors on cardiac myocyte apoptosis: role of a pertussis toxin-sensitive G protein. Circulation. 1999;100(22):2210–2. doi: 10.1161/01.cir.100.22.2210. [DOI] [PubMed] [Google Scholar]
- 29.Chesley A, Lundberg MS, Asai T, Xiao RP, Ohtani S, Lakatta EG, et al. The beta(2)-adrenergic receptor delivers an antiapoptotic signal to cardiac myocytes through G(i)-dependent coupling to phosphatidylinositol 3′-kinase. Circ Res. 2000 Dec 8;87(12):1172–9. doi: 10.1161/01.res.87.12.1172. [DOI] [PubMed] [Google Scholar]
- 30.Zhu WZ, Zheng M, Koch WJ, Lefkowitz RJ, Kobilka BK, Xiao RP. Dual modulation of cell survival and cell death by beta(2)-adrenergic signaling in adult mouse cardiac myocytes. Proc Natl Acad Sci U S A. 2001;98(4):1607–12. doi: 10.1073/pnas.98.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zaugg M, Xu W, Lucchinetti E, Shafiq SA, Jamali NZ, Siddiqui MA. Beta-adrenergic receptor subtypes differentially affect apoptosis in adult rat ventricular myocytes. Circulation. 2000 Jul 18;102(3):344–50. doi: 10.1161/01.cir.102.3.344. [DOI] [PubMed] [Google Scholar]
- 32.Ahmet I, Krawczyk M, Heller P, Moon C, Lakatta EG, Talan MI. Beneficial effects of chronic pharmacological manipulation of beta-adrenoreceptor subtype signaling in rodent dilated ischemic cardiomyopathy. Circulation. 2004 Aug 31;110(9):1083–90. doi: 10.1161/01.CIR.0000139844.15045.F9. [DOI] [PubMed] [Google Scholar]
- 33.Xydas S, Kherani AR, Chang JS, Klotz S, Hay I, Mutrie CJ, et al. beta(2)-Adrenergic stimulation attenuates left ventricular remodeling, decreases apoptosis, and improves calcium homeostasis in a rodent model of ischemic cardiomyopathy. J Pharmacol Exp Ther. 2006 May;317(2):553–61. doi: 10.1124/jpet.105.099432. [DOI] [PubMed] [Google Scholar]
- 34.Patterson AJ, Zhu W, Chow A, Agrawal R, Kosek J, Xiao RP, et al. Protecting the myocardium: a role for the beta2 adrenergic receptor in the heart. Crit Care Med. 2004 Apr;32(4):1041–8. doi: 10.1097/01.ccm.0000120049.43113.90. [DOI] [PubMed] [Google Scholar]
- 35.Tong H, Bernstein D, Murphy E, Steenbergen C. The role of beta-adrenergic receptor signaling in cardioprotection. FASEB J. 2005;19:983–5. doi: 10.1096/fj.04-3067fje. [DOI] [PubMed] [Google Scholar]
- 36.Rockman HA, Chien KR, Choi DJ, Iaccarino G, Hunter JJ, Ross J, Jr, et al. Expression of a beta-adrenergic receptor kinase 1 inhibitor prevents the development of myocardial failure in gene-targeted mice. Proc Natl Acad Sci U S A. 1998;95(12):7000–5. doi: 10.1073/pnas.95.12.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cross HR, Murphy E, Koch WJ, Steenbergen C. Male and female mice overexpressing the beta(2)-adrenergic receptor exhibit differences in ischemia/reperfusion injury: role of nitric oxide. Cardiovasc Res. 2002 Feb 15;53(3):662–71. doi: 10.1016/s0008-6363(01)00528-4. [DOI] [PubMed] [Google Scholar]
- 38.Bisognano JD, Weinberger HD, Bohlmeyer TJ, Pende A, Raynolds MV, Sastravaha A, et al. Myocardial-directed overexpression of the human β1-adrenergic receptor in transgenic mice. J Mol Cell Cardiol. 2000;32:817–30. doi: 10.1006/jmcc.2000.1123. [DOI] [PubMed] [Google Scholar]
- 39.Dumont JE, Pecasse F, Maenhaut C. Crosstalk and specificity in signalling. Are we crosstalking ourselves into general confusion? Cell Signal. 2001 Jul;13(7):457–63. doi: 10.1016/s0898-6568(01)00168-1. [DOI] [PubMed] [Google Scholar]
- 40.Bernstein D, Fajardo G, Zhao M, Urashima T, Powers J, Berry G, et al. Differential cardioprotective/cardiotoxic effects mediated by {beta}-adrenergic receptor subtypes. Am J Physiol Heart Circ Physiol. 2005 Dec;289(6):H2441–9. doi: 10.1152/ajpheart.00005.2005. [DOI] [PubMed] [Google Scholar]
- 41.Fajardo G, Zhao M, Powers J, Bernstein D. Differential cardiotoxic/cardioprotective effects of beta-adrenergic receptor subtypes in myocytes and fibroblasts in doxorubicin cardiomyopathy. J Mol Cell Cardiol. 2006 Mar;40(3):375–83. doi: 10.1016/j.yjmcc.2005.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fajardo G, Zhao M, Urashima T, Kobilka B, Bernstein D. β-adrenergic receptor subtypes mediate a temporal switch between cardiotoxic and cardioprotective signaling in doxorubicin cardiomyopathy. Pediatr Res. 2007 abstract. [Google Scholar]
- 43.Fajardo G, Zhao M, Urashima T, Kobilka B, Bernstein D. Opposing roles of β-adrenergic receptor subtypes on myocyte contractile function and calcium transients in muscle LIM protein cardiomyopathy. Pediatr Res. 2007 abstract. [Google Scholar]