Abstract
The rhizome of Atractylodes ovata De Candolle is rich in essential oils, which are usually removed by processing. In this study, anti-oxidative abilities of essential oils and aqueous extracts of A. ovata rhizome were explored, and the influence of processing on the anti-oxidative abilities was examined. Essential oils and aqueous extracts of A. ovata were extracted by boiling water and steam distillation, respectively. Quality of these two A. ovata samples was controlled by HPLC and GC-MS system, and anti-oxidative abilities were then evaluated. Results showed that surface color of A. ovata turned to brown and chemical components were changed by processing. Contents of both atractylon and atractylenolide II decreased in the essential oils, but only the contents of atractylon decreased by processing. Atractylenolide III increased in both A. ovata samples. However, A. ovata essential oils displayed stronger anti-oxidative abilities than aqueous extracts in DPPH-scavenging, TBH-induced lipid peroxidation and catalase activity assays. Moreover, the bioactivity of essential oils from raw A. ovata was stronger than oils from processed A. ovata. On the other hand, cytotoxicity of A. ovata essential oils was stronger than that of aqueous extracts, and was more sensitive on H9C2 cell than NIH-3T3 and WI-38 cells. In contrast, stir-frying processing method increased cytotoxicity of essential oils, but the cytotoxicity was ameliorated when processed with assistant substances. The results suggested that phytochemical components and bioactivity of A. ovata were changed after processing and the essential oils from raw A. ovata showed better anti-oxidative and fewer cytotoxicity effects.
1. Introduction
Traditional Chinese medicines (TCMs) are widely used to prevent and treat human diseases. However, the pharmacological functions of certain TCMs depend on free radical-scavenging activities [1]. Oxidative stress is associated with the pathogenesis of various diseases. Uncontrolled free radicals can damage myocardial cells, oxidize low-density lipoproteins and eventually result in cardiovascular diseases [2, 3]. In addition, excess reactive oxygen species (ROS) also induce hepatotoxicity and nephrotoxicity in mice [4]. ROS are generated through various pathways, for example, pollutants, UV light and other mechanisms [5].
Atractylodes ovata De Candolle is classified in TCMs as a tonic herb. Traditional applications of A. ovata were used to invigorate the stomach and spleen by eliminating dampness and to treat gastrointestinal diseases. Many pharmacological effects of the A. ovata aqueous extracts (ARE), including anti-inflammation, anti-tumor and immunoregulatory properties have been reported [6–8]. In regard to its chemical constituents, about 0.3–9% essential oils were found in A. ovata, including hinesol, β-eudesmol, palmitic acid and linoleic acid [9–11]. In addition, atractylon (AT), atractylenolides II (AT-II) and III (AT-III) were the major sesquiterpenoids in the A. ovata essential oils (ARO). In our previous study, AT was reported to have strong anti-oxidative abilities [12]. As also previously described, pharmacological effects of ARE were well studied, but the anti-oxidative abilities of the ARO were not very clear.
In TCMs, Chinese herbs are often processed before use. The chemical compositions, efficacy and cytotoxicity of herbs were changed after processing. Different processing methods were employed for TCMs, for example, stir-frying, soaking and carbonizing. A. ovata was usually heated and extra essential oils were removed for detoxification before use. Stir-frying with assistant substances (e.g., red soil or burnt clay) was the most popular processing method for A. ovata in TCM factories in Taiwan. Red soil and burnt clay are common assistant substances used in modern herbal processing. Red soil contains high concentrations of unhydrated iron oxides, aluminum oxide and heavy clay. Burnt clay is taken from the lining of kitchen stove [13]. Therefore, the anti-oxidative effects of ARE and ARO, as well as whether processing influences the anti-oxidative abilities and cytotoxicity of both samples were explored.
In this study, A. ovata was processed by different methods and the qualities of processed A. ovata were evaluated by colorimetric analysis. ARE and ARO were extracted by boiling water and steam distillation, respectively. The chemical compositions were analyzed by HPLC and GC-MS system. Cytotoxicity and anti-oxidative effects of raw and processed AREs and AROs were also measured by DPPH radical-scavenging, ESR-spin trapping, TBH-induced lipid peroxidation in heart tissue, catalase activity assays and MTT assay, respectively. Our experimental procedure is summarized in Figure 1.
Figure 1.
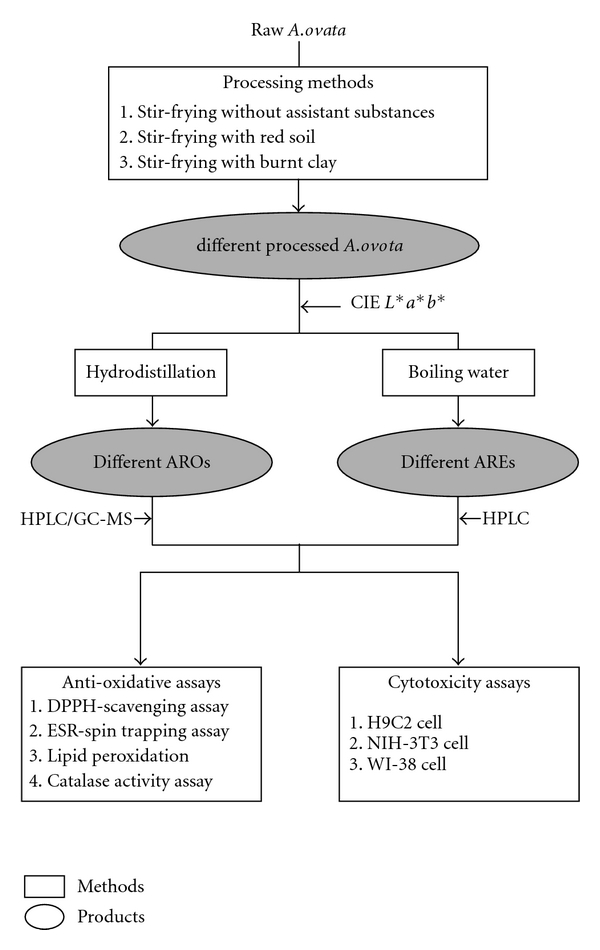
Summary of the experimental procedures of this study. Raw A. ovata was processed by different methods, and AREs and AROs were extracted by boiling water and steam distillation, respectively. AREs and AROs were quality controlled by colorimetry, HPLC and GC-MS system and were used to evaluate the anti-oxidative abilities and cytotoxicity effects of A. ovata.
2. Methods
2.1. Animals
Male Wistar rats weighing about 250 ± 10 g were purchased from the BioLASCO Taiwan Co., Ltd. and kept on a 12:12-h day: night cycle. Animals were maintained in polycarbonate cages at 21 ± 2°C and provided food and water ad libitum. All experimental procedures involving animals followed the ethical regulations of Taipei Medical University (Approval No. LAC-97-0122).
2.2. Quantitative Colorimetric Analysis of Processed A. ovata
The processing procedures of A. ovata were described in our previous study [13]. Processed samples were included raw materials, stir-frying without assistant substances for 5 min, stir-frying with red soil for 5 min, stir-frying with burnt clay for 5 min and stir-frying without assistant substances for 30 min. Color measurement of A. ovata was performed using the Konica Minolta Color Meter (Model CR-10, Konica Minolta Sensing, Osaka, Japan). The CR-10 was composed of 8 mm diameter measuring area with a diffuse illumination 8° viewing and the color measurements of A. ovata were detected on the surface. Each kind of processed A. ovata was measured in triplicate. Results were presented as the CIE L*a*b* color system. L* values indicated white or dark samples. A reduction in whiteness, as evidenced by a decrease in L* values, indicates darker samples. The highest a* values and b* values expressed redness and yellowness, respectively.
2.3. Preparation and Quality Control of the A. ovata Aqueous Extracts
Different A. ovata samples were immersed in purified deionized water and boiled for at least 30 min until half of the original amount was left. Aqueous solutions were then filtered, and vacuum freeze-dried. The above preparative procedures were stipulated by the Committee on Chinese Medicine and Pharmacy of Department of Health in Taiwan.
Amounts of AT, AT-II and AT-III were analyzed via HPLC system. HPLC apparatuses were composed of a SCL-10Avp System Controller, an LC-10ATvp Liquid Chromatograph Pump, an SPD-M10A Diode Array Detector, a SIL-10Avp Auto Injector, a CTO-10A Column Oven, FCV-10Avp Flow-Channel Selection Valves (Shimadzu, Tokyo, Japan) and an ERC-3415 Degasser (ERC, Altegolfsheim, Regensburg, Germany). The stationary phase consisted of a Purospher STAR RP-18e reversed-phase column (5 μm, 4 mm i.d × 250 mm, Merck) and an acetonitrile-water system was used as the mobile phase in the gradient mode as follows: acetonitrile: 0–10 min, 40–5%; 10–20 min, 45–55%; 20–30 min, 55–100% and 30–40 min, 100–55%. The flow rate was 1 ml min−1, and the oven temperature was maintained at 40°C. We used UV wavelengths of 220 nm to detect the amount of AT and 236 nm to detect the amounts of AT-II and AT-III.
2.4. Preparation and Quality Control of the A. ovata Essential Oils
A. ovata was crumbled and passed through a no. 20 mesh standard sieve. We weighed 70 g of powder samples and mixed them with 5 volumes purified deionized water. Steam distillation of the essential oil was performed in a Clevenger-type apparatus for 7 h according to the Pharmacopoeia of Traditional Medicine in Taiwan. The essential oils were stored in an anhydrous situation. Quality control of the essential oils was analyzed via HPLC and GC-MS system.
The HPLC analysis procedure was modified from our previous study [13]. Analytical instruments were the same as the previous described. The stationary phase consisted of a Purospher STAR RP-18e reversed-phase column (5 μm, 4 mm i.d × 250 mm, Merck) and mobile phase was CH3CN-H2O (80 : 20, v/v) for AT and CH3CN-H2O (45 : 55, v/v) for AT-II and AT-III. All mobile phases were degassed by ultrasonication and filtered through a 0.45-μm FP Vericel (PVDF) membrane (Pall Corporation, Ann Arbor, MI, USA). The flow rate was 1 ml min−1 and the oven temperature was maintained at 40°C. We used UV wavelengths of 220 nm to detect the amount of AT and 236 nm to detect the amounts of AT-II and AT-III.
The GC-MS system was composed of a gas chromatograph GC-2010 equipped with gas chromatography mass spectrometer GCMS-QP2010 (Shimadzu, Tokyo, Japan). A DB-5MS column (0.25 mm i.d × 30 m × 0.25 μm film, J&W Scientific, Folsom, CA, USA) was performed with Helium as the carrier gas at a constant pressure of 73.0 kPa. Injection temperature and ion source temperature were 150 and 200°C, respectively. The GC oven temperature was programmed to be maintained at 50°C for 5 min and then rise to 250°C at 20°C min−1 for 10 min. Results were obtained by collecting the scan mass spectra between the scan range 40 and 800 amu. The mass spectra of the six major peaks in GC-MS chromatogram were compared with the database of NIST/EPA/NIH Mass Spectral Library for identification of possible components.
2.5. Anti-Oxidative Assay
2.5.1. DPPH Scavenging Assay
The assay protocol of 1,1-diphenyl-2-picrylhydrazyl (DPPH)-scavenging activities was modified from the previous study [14]. Test samples were dissolved in 100% DMSO and serially diluted into different concentrations. Each sample was mixed with the DPPH ethanolic solution (100 μM) on a 96-well microplate at room temperature for 10 min. Ascorbic acid was used as the positive control. DPPH level of each well was evaluated by detecting the optical density of each well at 530 nm with ELISA spectrophotometer (μQuant, BioTek, USA). The DPPH scavenging rate (%) of each sample was calculated according the following equation: [1 − (S s/EC)] × 100, where S s and E C were the optical density value of the sample and control, respectively.
2.5.2. ESR-Spin Trapping Assay
A Bruker EMX electron spin resonance spectroscopy (ESR) spectrometer was used to detect the ESR trapping and the experimental protocol was modified from the previous report [15]. DPPH radical was used to generate the excess free radical. In sample preparation, DPPH (500 μM) and test samples were thoroughly mixed and reacted for 2 min. ESR spectra were recorded at room temperature using a quartz flat cell designed for aqueous solutions. Dead time of sample preparation and ESR analysis was exactly 30 s after the last addition. Conditions of ESR spectrometry were as follows: 20 mW power at 9.78 GHz, with a scan range of 100 G and a receiver gain of 5 × 104. The modulation amplitude, sweep time, and time constant are given in the figure captions.
2.5.3. Lipid Peroxidation Assay
Heart tissue was obtained from Wistar rats and homogenized in PBS. This homogenized solution was centrifuged for 10 min at 1200 rpm. Protein level in the homogenized tissue was quantified with Bioquant (Merck, Germany). The homogenized tissue was treated with 125 mM tert-butylhydroperoxide (TBH) with or without test samples and eventually reacted with thiobarbituric acid (TBA) to form the pink adducts, malondialdehyde (MDA). The optical density was measured at 530 nm with μQuant spectrophotometer (BioTek, USA).
2.5.4. Catalase Activity Assay
The catalase assay protocol was modified from the previous study [16–18]. A discontinuous method for measuring catalase used the ferrous oxidation in xylenol orange solution and detected at 560 nm with μQuant spectrophotometer (BioTek, USA). Catalase activity was represented in units of μmol min−1 ml−1.
2.6. Cytotoxicity Assay
The H9C2 (normal rat cardiomyocytes) and NIH-3T3 (normal mouse embryonic fibroblast) cell lines were maintained in DMEM medium, supporting with 10% fetal bovine serum (FBS) and 10% fetal calf serum, respectively. WI-38 (normal human diploid cell line) cell line was maintained in MEM medium with 10% FBS. When confluent, cells were washed with phosphate-buffered saline (PBS), trypsinized with 0.25% trypsin-EDTA in PBS, washed with fresh culture medium and transferred to 96-well plates (1 × 105 cells ml−1) for the cytotoxicity assays. Different test samples were dissolved in DMSO at suitable concentrations and store at –20°C. Test samples were added to each cell line for 24 h without renewal of the medium. The number of surviving cells was then counted using the MTT assay. Finally, the products were evaluated by measuring the optical density of each well at 600 nm with μQuant spectrophotometer (BioTek, USA).
2.7. Statistical Analysis
Data are presented as the mean and standard deviation (SD). Significance was calculated using Student's t-test and one-way ANOVA test.
3. Results
3.1. Quality Control of Different Processed A. ovata
After being processed by different methods, CIE L*a*b* values of processed A. ovata were analyzed (Table 1). Raw A. ovata (A) expressed the highest L* values, indicating whitest color. Moreover, stir-frying with red soil for 5 min (C) displayed the highest a* values (redness) and b* values (yellowness), due to the assistant substances, red soil. Finally, we collected 200 pieces of processed A. ovata for each group whose CIE L*a*b* values were nearly the same for following assays.
Table 1.
Colorimetric values of the differently processed A. ovata samples.
| Colorimetric values | |||
|---|---|---|---|
| L* | a* | b* | |
| A | 62.3 ± 2.5 | 9.4 ± 1.1 | 26.0 ± 2.0 |
| B | 53.7 ± 1.7 | 12.5 ± 0.7 | 28.7 ± 0.9 |
| C | 55.4 ± 1.7 | 15.7 ± 0.8 | 31.1 ± 1.1 |
| D | 59.4 ± 2.2 | 7.8 ± 1.1 | 15.9 ± 1.4 |
| E | 24.3 ± 1.6 | 6.6 ± 1.2 | 6.7 ± 1.3 |
Two hundred pieces of A. ovata were collected and analyzed in triplicate. Raw materials, A; stir-frying without assistant substances for 5 min, B; with red soil for 5 min, C; with burnt clay for 5 min, D.; without assistant substances for 30 min, E.
First, the HPLC chromatogram of AT, AT-II and AT-III in AREs was shown in Figure 2(a) and their retention time were 35.5, 26.1 and 16.8 min, respectively. Low concentrations of AT, AT-II and AT-III were found in AREs. Raw A. ovata had the highest AT concentration (0.071 ± 0.003 μg mg−1) among the different AREs, but it was diminished by stir-frying. After processing, concentrations of AT-II and AT-III in the aqueous extracts from processed A. ovata were about 1.5–2.0-fold higher than those from the raw A. ovata (Table 2). Secondly, Figure 2(b) displayed the HPLC chromatograms of AT in AROs and its retention time was 12.5 min. Besides, the HPLC chromatogram of AT-II and AT-III in AROs was shown in Figure 2(c) and their retention time were 14.5 min and 32.1 min, respectively. Concentrations of the three sesquiterpenoids in the AROs were higher than those in AREs. The highest AT concentrations (266.70 ± 3.63 μg mg−1) were also found in raw ARO. AT and AT-II concentrations were also decreased by processing, but AT-III increased (Table 2) in the ARO. Moreover, the GC-MS fingerprint profile of raw ARO was shown in Figure 3(a). We chose six major components as standard peaks of the AROs. After comparing with the database, their retention time (min) and molecular weight (amu) were β-caryophyllene (10.6 and 204), β-selinene (11.0 and 204), γ-selinene (11.4 and 204), AT (12.3 and 216), AT-II (13.5 and 232) and stereoisomer of AT-II (15.2 and 232), respectively. Compositions of the processed AROs were totally different due to the different processing methods (Table 3). Likewise, concentrations of these six components in AROs were altered by the different processing methods (Figure 3(b)).
Figure 2.
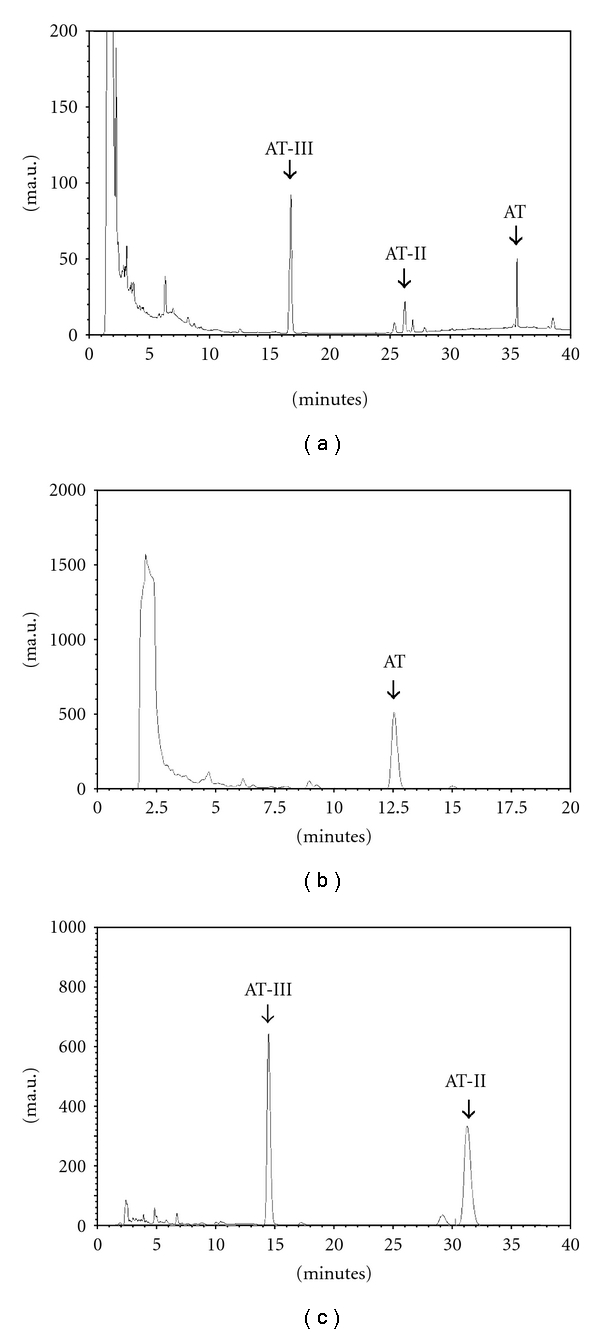
HPLC chromatograms of AT, AT-II and AT-III in AREs (a) and AT (b), AT-II and AT-III (c) in AROs.
Table 2.
Sesquiterpenoids concentrations of essential oils and aqueous extracts from different processed A. ovata.
| Sesquiterpenoids in essential oils (μg mg−1) | Sesquiterpenoids in aqueous extracts (μg mg−1) | |||||
|---|---|---|---|---|---|---|
| AT | AT-II | AT-III | AT | AT-II | AT-III | |
| A | 266.70 ± 3.63 | 113.24 ± 3.01 | 74.05 ± 1.06 | 0.071 ± 0.003 | 0.039 ± 0.001 | 0.239 ± 0.020 |
| B | 203.33 ± 6.64 | 41.74 ± 1.05 | 98.70 ± 0.41 | 0.046 ± 0.004 | 0.058 ± 0.001 | 0.297 ± 0.006 |
| C | 145.07 ± 3.04 | 78.43 ± 4.12 | 92.82 ± 2.72 | 0.057 ± 0.003 | 0.061 ± 0.001 | 0.290 ± 0.006 |
| D | 147.51 ± 3.40 | 49.21 ± 4.64 | 81.28 ± 1.90 | 0.008 ± 0.001 | 0.060 ± 0.001 | 0.301 ± 0.004 |
| E | 95.81 ± 0.77 | 13.84 ± 0.84 | 25.98 ± 0.51 | 0.000 ± 0.000 | 0.027 ± 0.001 | 0.053 ± 0.001 |
Raw materials, A; stir-frying without assistant substances for 5 min, B; with red soil for 5 min, C; with burnt clay for 5 min, D; without assistant substances for 30 min, E.
Figure 3.
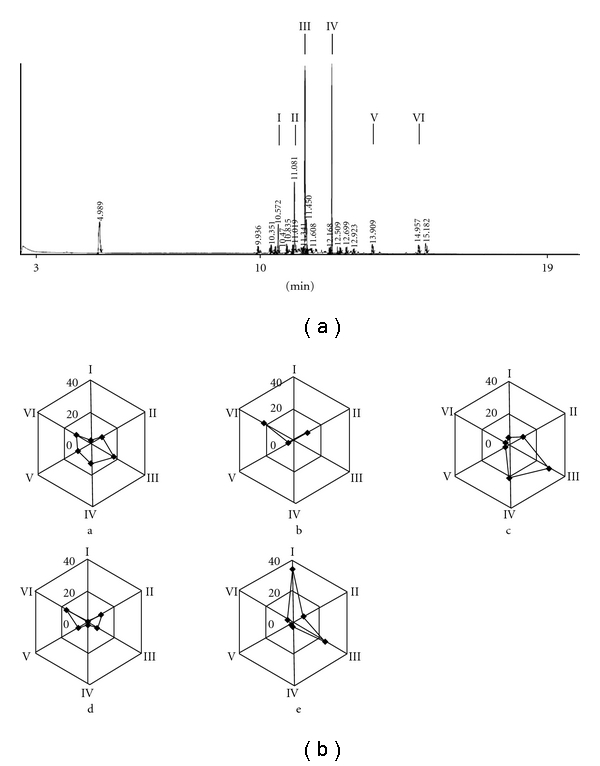
GC-MS fingerprint profile of raw ARO. The arrow indicated the six major components (β-caryophyllene, I; β-selinene, II; γ-selinene, III; AT, IV; AT-II, V; stereoisomer of AT-II, VI) of raw ARO (a). Radar chart analysis of the essential oils from differently processed A. ovata (b). a–e indicated the essential oils from raw materials, stir-frying without assistant substances for 5 min, stir-frying with red soil for 5 min, stir-frying with burnt clay for 5 min and stir-frying without assistant substances for 30 min, respectively.
Table 3.
Peak area percentage of essential oils from differently processed A. ovata samples analyzed by GC-MS.
| Peak area (%) | ||||||
|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | |
| A | 3.53 | 13.69 | 28.16 | 20.76 | 15.87 | 17.99 |
| B | 1.72 | 25.29 | 4.37 | 2.06 | 9.69 | 56.87 |
| C | 6.90 | 14.94 | 41.99 | 29.42 | 3.10 | 3.64 |
| D | 1.70 | 23.33 | 16.89 | 3.80 | 16.43 | 37.84 |
| E | 46.36 | 11.21 | 32.54 | 3.00 | 2.00 | 4.88 |
Raw materials, A; stir-frying without assistant substances for 5 min, B; with red soil for 5 min, C; with burnt clay for 5 min, D.; without assistant substances for 30 min, E. The six major components were β-caryophyllene (I), β-selinene (II), γ-selinene (III), AT (IV), AT-II (V), stereoisomer of AT-II (VI).
3.2. DPPH-Scavenging Effects of the A. ovata Aqueous Extracts and Essential Oils
DPPH-radical scavenging activities were employed to evaluate the chemically anti-oxidative effects of different AREs and AROs. Firstly, no significant DPPH-scavenging abilities were found in AREs at 5 mg ml−1 (data not shown). However, as shown in Table 4, essential oils from raw A. ovata (A, 5 mg ml−1) displayed the same DPPH-scavenging abilities as ascorbic acid (2.5 mM) and with the IC50 value of 2.71 mg ml−1. On the other hand, the other samples expressed insignificant DPPH-scavenging effects, and the inhibitory effects were in the following order of essential oils from stir-frying with red soil for 5 min (C, 47.08%), stir-frying with burnt clay for 5 min (D, 38.63%), stir-frying without assistant substances for 5 min (B, 36.87%) and stir-frying without assistant substances for 30 min (E, 33.19%). According to the above results, AROs were evaluated in further anti-oxidative assays.
Table 4.
DPPH scavenging activities of essential oils from differently processed A. ovata samples.
| DPPH inhibitory effects | ||
|---|---|---|
| Sample (mg ml−1) | Inhibitiona (%) | IC50 value |
| A | 63.53 ± 0.25 | 2.71 |
| B | 36.87 ± 0.23 | >5 |
| C | 47.08 ± 2.88 | >5 |
| D | 38.63 ± 3.34 | >5 |
| E | 33.19 ± 0.99 | >5 |
| Ascorbic acid (mM) | 67.94 ± 0.96 | 1.22 |
Concentration of each sample was 5 mg ml−1, except ascorbic acid (2.5 mM). Raw materials, A; stir-frying without assistant substances for 5 min, B; with red soil for 5 min, C; with burnt clay for 5 min, D.; without assistant substances for 30 min, E.
3.3. ESR-Spin Trapping Assay of the A. ovata Essential Oils
In the ESR-spin trapping assay, DPPH radical was used as the free radical generator. As shown in Figure 4, five major free radical signals were found in the DPPH group. All five different AROs showed inhibitory activity against DPPH free radical generation compared to the DPPH group. Among them, raw ARO (A, 5 mg ml−1) displayed the strongest anti-oxidative effect.
Figure 4.
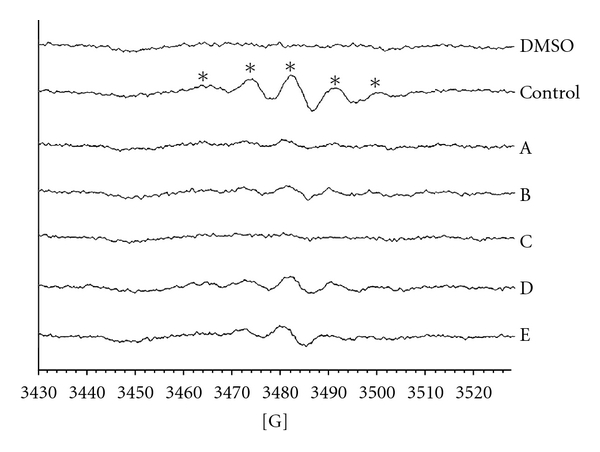
ESR spectra scanning of the anti-oxidative effects of essentials oils from differently processed A. ovata samples. The reaction mixture contained 100 mM DMPO and 100% DMSO was added as the solvent control. DPPH (500 μM) was used as the free radical generator, and then the essential oil from raw materials was added, A; stir-frying without assistant substances for 5 min, B; stir-frying with red soil for 5 min, C; stir-frying with burnt clay for 5 min, D; or stir-frying without assistant substances for 30 min, E. Sample concentration was 5 mg ml−1. Instrument parameters were as follows: modulation amplitude, 1 G; time constant, 164 ms; scanning for 40 s with three scans accumulated. Asterisk indicate DPPH free radical adduct.
3.4. Lipid Peroxidative Inhibitory Effects and Catalase Activity of the A. ovata Essential Oils
According to many etiological studies, oxidative stress played an important role in causing many diseases, that is, inflammation and malignant tumor. Thus, the effects of different AROs on TBH-induced lipid peroxidation were evaluated. Essential oils from raw (A, 500 μg ml−1) and stir-frying with red soil for 5 min (C, 500 μg ml−1) A. ovata displayed significant anti-lipid peroxidative activities compared to the other AROs (Figure 5(a)), suggesting that it has stronger MDA inhibitory effects. In addition, all AROs exhibited increased catalase activity. Essential oils from raw A. ovata (A, 500 μg ml−1) significantly displayed stronger catalase-increasing activity compared to the other AROs (Figure 5(b)).
Figure 5.
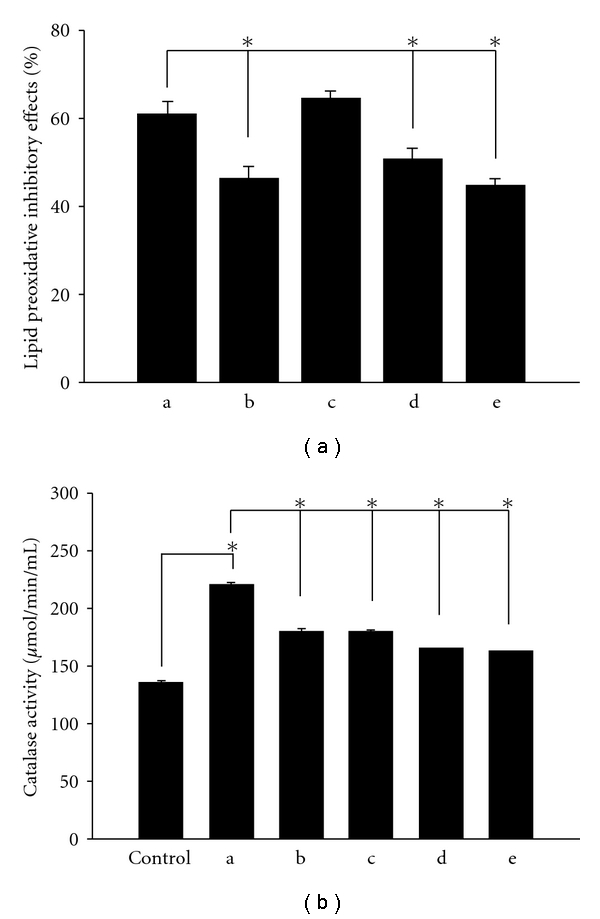
MDA inhibitory effects (a) and catalase activity (b) of essential oils from differently processed A. ovata samples. Results are presented as the mean ± SD in triplicate. a–e indicated as the essential oils from raw materials, stir-frying without assistant substances for 5 min, stir-frying with red soil for 5 min, stir-frying with burnt clay for 5 min and stir-frying without assistant substances for 30 min, respectively. Sample concentration was 500 μg ml−1. *P < .05.
3.5. Cytotoxicity Effects of the Essential Oils and Aqueous Extracts from A. ovata
As shown in Table 5, the cytotoxicity effects of AT were stronger than those of AT-II and AT-III, and AT was more sensitive in the H9C2 cell line. In addition, cytotoxicity of AROs on H9C2 cell was also more sensitive than the other cell lines. Firstly, AROs resulting from stir-frying without assistant substances for 5 min (B) and for 30 min (E) displayed stronger cytotoxicity on H9C2 cell line than did raw AROs. Once A. ovata was stir-fried with assistant substances, for example, red soil and burnt clay, cytotoxicity effects were ameliorated on H9C2 cell. On NIH-3T3 and WI-38 cell lines, raw ARO showed less cytotoxicity than other AROs. However, none of the aqueous extracts from A. ovata showed cytotoxicity effects at 1000 μg ml−1 for 24 h on these three normal cell lines.
Table 5.
Cytotoxicity effects of sesquiterpenoids and essential oils from differently processed A. ovata samples.
| IC50 values | |||
|---|---|---|---|
| H9C2 | NIH-3T3 | WI-38 | |
| Sesquiterpenoids | |||
| ATa | 40.82 ± 4.96 | 46.71 ± 4.69 | 62.29 ± 10.11 |
| AT-II | >400 | >400 | >400 |
| AT-III | >400 | >400 | >400 |
|
| |||
| Essential oils | |||
| Ab | 200.27 ± 1.11 | 283.30 ± 9.77 | 252.73 ± 16.79 |
| B | 163.03 ± 2.83c | 244.35 ± 11.94 | 218.15 ± 9.29 |
| C | 217.92 ± 2.67d | 241.49 ± 20.69 | 271.67 ± 14.75 |
| D | 266.14 ± 2.65d | 225.19 ± 3.18 | 214.21 ± 17.25 |
| E | 170.68 ± 1.86c | 219.96 ± 6.43 | 247.81 ± 1.05 |
aUnits of AT to AT-III and A to E were μM and μg ml−1, respectively.
bConcentrations of A–E were 1000 μg ml−1.
cCompared with A, P < .05.
dCompared with A, P < .05.
Raw materials, A; stir-frying without assistant substances for 5 min, B; with red soil for 5 min, C; with burnt clay for 5 min, D.; without assistant substances for 30 min, E. Each test was in triplicate.
4. Discussion
Essential oils from herbs possessed different bioactivities. For example, essential oils from Vaccinium corymbosum, Garcinia brasiliensis, Cyperus rotundus and Thymus vulgaris exhibited effective anti-oxidative activities [19–22]. In addition, the vasorelaxive effect of Croton nepetaefolius and anti-fungal activities of Chenopodium ambrosioides were due to the essential oils [23, 24]. A. ovata was rich in essential oils, but few researches had examined the pharmacological functions of the A. ovata essential oils. Only in Zhang's study, essential oils from Atractylodes lancea had regulatory effects on delayed gastric emptying in stress-induced rats [25]. Pharmacological characteristics of A. ovata were warm, bitter and sweet and commonly used for tonifying and regulating Qi. Doctors usually used A. ovata to supple the spleen, boost the stomach, dry dampness and harmonize the center. A. ovata could be processed using different methods to meet different clinical needs. Raw A. ovata displayed stronger tonifying effects than processed A. ovata and was usually used to treat appetite loss, indigestion and tiredness. However, processing enhanced the drying dampness effects of A. ovata.
Processing is an important part of preparing Chinese herbs. Characteristics of Chinese herbs are changed through different processing methods. Concentrations of glucosinolates, the active components of Brassica vegetables, decreased after being heated [26]. Variations in chemical composition after processing were correlated to alterations in cytotoxicity and efficacy of Chinese herbs. In Li's study, processing reduced the acute diarrhea effects of Rheum palmatum due to a decrease in the anthraquinone contents [27]. However, maintaining an equal processing level was an important issue. Many reactions occurred during thermal processing of Chinese herbs, like the changes in flavor and color seen after heat treatment. The browning reaction that caused the color changes of Chinese herbs was formed by variations of phytochemical components during heating, resulting in the plant color changes [28]. Prodelphinidin D3 and procyanidin B3, the phenolic compounds of barley grain, were easily auto-oxidized during heating and contributed to browning of the heated barley products [29]. Thus, we controlled the processed level through colorimetric values in this study (Table 1). The different processing techniques changed the various AREs and AROs compositions. In our previous study, we described that AT, AT-II and AT-III were the major A. ovata components. Hence, the amounts of AT, AT-II and AT-III in raw and processed A. ovata were firstly quantified by HPLC analysis system. In addition, because of the evaporative tendency of the ARO sesquiterpenoids, we used GC-MS system to evaluate the ARO component variations after heat-processing.
In the anti-oxidative assays, AROs displayed stronger DPPH-scavenging effects than AREs (Table 4). Our results showed that raw ARO displayed stronger anti-oxidative abilities than processed AROs in ESR-spin trapping, DPPH-scavenging, TBH-induced lipid peroxidation in heart tissue and catalase activity assays. Changes in the ARO chemical compositions were correlated with their respective anti-oxidative activities. AT was the most potent anti-oxidative components in ROS-induced lipid peroxidation [30]. In stir-frying processing, AT was converted to the non-anti-oxidative components, AT-II and AT-III, and the A. ovata essential oils had largely evaporated because of the heating. These phenomenons were possibly the reason that processed A. ovata lost its anti-oxidative abilities.
Cytotoxicity effects of AROs and AREs on H9C2, NIH-3T3 and WI-38 cells were also evaluated. The normal rat cardiomyocytes (H9C2) cell line was chosen because of the TBH-induced heart lipid peroxidation assay. In addition, based on the European Centre for the Validation of Alternative Methods (ECVAM, ISO 9001), NIH-3T3 and WI-38 cell lines were used to evaluate the alternative non-animal cytotoxicity tests [31]. As shown in Table 5, AT displayed the strongest cytotoxicity effects among the three sesquiterpenoids. In our previous study, we discussed the variations in chemical components and cytotoxicity between raw and processed A. ovata [13]. AT showed the strongest cytotoxicity on different tumor cell line, but AT-II and AT-III did not. However, cytotoxicity effects of the essential oils from raw A. ovata which contained the greatest amounts of AT were not stronger than those from processed A. ovata on these three normal cell lines. We suggested that although AT was the stronger compound, amounts of AT in the essential oils of raw A. ovata (266.70 ± 3.63 μg mg−1) was insufficient to cause cell death.
In TCM, the general therapeutical rule of Qi-tonifying herbs was reported to promote blood circulation, activate vital energy circulation and to strengthen the body [32]. Previous study had shown that essential oil played an important role in promoting blood circulation and flow [33, 34]. According to Lou's study, circulatory function correlated to the anti-oxidative activities [35]. In this study, heat processing caused a large loss of A. ovata essential oils. We found that loss of essential oils resulted in a reduction of A. ovata anti-oxidative effects. Taken together, the cytotoxicity effects of A. ovata essential oils were slightly influenced by processing. On the other hand, processing resulted in a large loss of the anti-oxidative abilities of the A. ovata essential oils.
In conclusion, A. ovata was a potent anti-oxidative Chinese herb whose anti-oxidative effects were decreased by processing. As shown in Figure 6, we suggested that raw A. ovata essential oil was a more useful anti-oxidant for its tonifying effects than was processed A.ovata essential oil.
Figure 6.
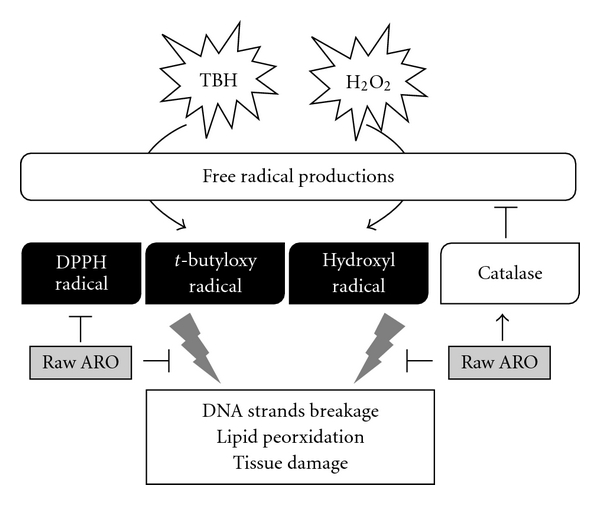
Main effects of raw ARO on oxidative injuries.
Funding
Committee on Chinese Medicine and Pharmacy, Department of Health, Executive Yuan, Taiwan grants (CCMP96-CP-009).
References
- 1.Stevenson DE, Hurst RD. Polyphenolic phytochemicals—just antioxidants or much more? Cellular and Molecular Life Sciences. 2007;64(22):2900–2916. doi: 10.1007/s00018-007-7237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Veerapur VP, Prabhakar KR, Parihar VK, et al. Ficus racemosa stem bark extract: a potent antioxidant and a probable natural radioprotector. Evidence-Based Complementary and Alternative Medicine. 2009;6(3):317–324. doi: 10.1093/ecam/nem119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen CN, Weng MS, Wu CL, Lin JK. Comparison of radical scavenging activity, cytotoxic effects and apoptosis induction in human melanoma cells by Taiwanese Propolis from different sources. Evidence-Based Complementary and Alternative Medicine. 2004;1:175–185. doi: 10.1093/ecam/neh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weyers A, Ugnia LI, Ovando HG, Gorla NB. Antioxidant capacity of vitamin C in mouse liver and kidney tissues. Biocell. 2008;32(1):27–31. [PubMed] [Google Scholar]
- 5.Cadenas E. Biochemistry of oxygen toxicity. Annual Review of Biochemistry. 1989;58:79–110. doi: 10.1146/annurev.bi.58.070189.000455. [DOI] [PubMed] [Google Scholar]
- 6.Lee J-C, Lee K-Y, Son Y-O, et al. Stimulating effects on mouse splenocytes of glycoproteins from the herbal medicine Atractylodes macrocephala Koidz . Phytomedicine. 2007;14(6):390–395. doi: 10.1016/j.phymed.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Huang H-L, Chen C-C, Yeh C-Y, Huang R-L. Reactive oxygen species mediation of Baizhu-induced apoptosis in human leukemia cells. Journal of Ethnopharmacology. 2005;97(1):21–29. doi: 10.1016/j.jep.2004.09.058. [DOI] [PubMed] [Google Scholar]
- 8.Prieto JM, Recio MC, Giner RM, Máñez S, Giner-Larza EM, Ríos JL. Influence of traditional Chinese anti-inflammatory medicinal plants on leukocyte and platelet functions. Journal of Pharmacy and Pharmacology. 2003;55(9):1275–1282. doi: 10.1211/0022357021620. [DOI] [PubMed] [Google Scholar]
- 9.Zhou RB, Wu J, Tong QZ, Liu YM, Liu XD. Studies on volatile oil from Atractylodes macrocephala with different distill methods. Zhong Yao Cai. 2008;31(2):229–232. [PubMed] [Google Scholar]
- 10.Satoh K, Nagai F, Kano I. Inhibition of H+,K+-ATPase by hinesol, a major component of So-jutsu, by interaction with enzyme in the E1 state. Biochemical Pharmacology. 2000;59(7):881–886. doi: 10.1016/s0006-2952(99)00399-8. [DOI] [PubMed] [Google Scholar]
- 11.Satoh K, Nagai F, Kano I. Interaction of β-eudesmol with Na+ K+-ATPase inhibition of K+-pNPPase activity. Biochemical Pharmacology. 1992;44(5):993–995. doi: 10.1016/0006-2952(92)90134-5. [DOI] [PubMed] [Google Scholar]
- 12.Wang C-C, Lin S-Y, Cheng H-C, Hou W-C. Pro-oxidant and cytotoxic activities of atractylenolide I in human promyeloleukemic HL-60 cells. Food and Chemical Toxicology. 2006;44(8):1308–1315. doi: 10.1016/j.fct.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Wang K-T, Chen L-G, Yang L-L, Ke W-M, Chang H-C, Wang C-C. Analysis of the sesquiterpenoids in processed Atractylodis Rhizoma. Chemical and Pharmaceutical Bulletin. 2007;55(1):50–56. doi: 10.1248/cpb.55.50. [DOI] [PubMed] [Google Scholar]
- 14.Tseng S-H, Chien T-Y, Tzeng C-F, Lin Y-H, Wu C-H, Wang C-C. Prevention of hepatic oxidative injury by Xiao-Chen-Chi-Tang in mice. Journal of Ethnopharmacology. 2007;111(2):232–239. doi: 10.1016/j.jep.2006.11.030. [DOI] [PubMed] [Google Scholar]
- 15.Chou D-S, Lee J-J, Hsiao G, et al. Baicalein induction of hydroxyl radical formation via 12-lipoxygenase in human platelets: an ESR study. Journal of Agricultural and Food Chemistry. 2007;55(3):649–655. doi: 10.1021/jf062584f. [DOI] [PubMed] [Google Scholar]
- 16.Ou P, Wolff SP. A discontinuous method for catalase determination at ’near physiological’ concentrations of H2O2 and its application to the study of H2O2 fluxes within cells. Journal of Biochemical and Biophysical Methods. 1996;31(1-2):59–67. doi: 10.1016/0165-022x(95)00039-t. [DOI] [PubMed] [Google Scholar]
- 17.Jiang Z-Y, Woollard ACS, Wolff SP. Hydrogen peroxide production during experimental protein glycation. FEBS Letters. 1990;268(1):69–71. doi: 10.1016/0014-5793(90)80974-n. [DOI] [PubMed] [Google Scholar]
- 18.Kataya HAH, Hamza AA. Red cabbage (Brassica oleracea) ameliorates diabetic nephropathy in rats. Evidence-Based Complementary and Alternative Medicine. 2008;5(3):281–287. doi: 10.1093/ecam/nem029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang CY, Wang SY, Chen C. Increasing antioxidant activity and reducing decay of blueberries by essential oils. Journal of Agricultural and Food Chemistry. 2008;56:3587–3592. doi: 10.1021/jf7037696. [DOI] [PubMed] [Google Scholar]
- 20.Martins FT, Doriguetto AC, de Souza TC, et al. Composition, and anti-inflammatory and antioxidant activities of the volatile oil from the fruit peel of Garcinia brasiliensis. Chemistry and Biodiversity. 2008;5(2):251–258. doi: 10.1002/cbdv.200890022. [DOI] [PubMed] [Google Scholar]
- 21.Kilani S, Ledauphin J, Bouhlel I, et al. Comparative study of Cyperus rotundus essential oil by a modified GC/MS analysis method. Evaluation of its antioxidant, cytotoxic, and apoptotic effects. Chemistry and Biodiversity. 2008;5(5):729–742. doi: 10.1002/cbdv.200890069. [DOI] [PubMed] [Google Scholar]
- 22.Chizzola R, Michitsch H, Franz C. Antioxidative properties of Thymus vulgaris leaves: comparison of different extracts and essential oil chemotypes. Journal of Agricultural and Food Chemistry. 2008;56(16):6897–6904. doi: 10.1021/jf800617g. [DOI] [PubMed] [Google Scholar]
- 23.Magalhães PJC, Lahlou S, Jucá DM, et al. Vasorelaxation induced by the essential oil of Croton nepetaefolius and its constituents in rat aorta are partially mediated by the endothelium. Fundamental and Clinical Pharmacology. 2008;22(2):169–177. doi: 10.1111/j.1472-8206.2008.00571.x. [DOI] [PubMed] [Google Scholar]
- 24.Jardim CM, Jham GN, Dhingra OD, Freire MM. Composition and antifungal activity of the essential oil of the brazilian Chenopodium ambrosioides L. Journal of Chemical Ecology. 2008;34(9):1213–1218. doi: 10.1007/s10886-008-9526-z. [DOI] [PubMed] [Google Scholar]
- 25.Zhang H, Han T, Sun L-N, et al. Regulative effects of essential oil from Atractylodes lancea on delayed gastric emptying in stress-induced rats. Phytomedicine. 2008;15(8):602–611. doi: 10.1016/j.phymed.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 26.Song L, Thornalley PJ. Effect of storage, processing and cooking on glucosinolate content of Brassica vegetables. Food and Chemical Toxicology. 2007;45(2):216–224. doi: 10.1016/j.fct.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 27.Li X-D, Huang L-Q. Influence of processing rhubarb on it’s anthraquinone contents. Zhongguo Zhong Yao Za Zhi. 2005;30(12):904–943. [PubMed] [Google Scholar]
- 28.Azaizeh H, Saad B, Khalil K, Said O. The state of the art of traditional Arab herbal medicine in the Eastern region of the Mediterranean: a review. Evidence-Based Complementary and Alternative Medicine. 2006;3(2):229–235. doi: 10.1093/ecam/nel034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kohyama N, Fujita M, Ono H, et al. Effects of phenolic compounds on the browning of cooked barley. Journal of Agricultural and Food Chemistry. 2009;57:6402–6407. doi: 10.1021/jf901944m. [DOI] [PubMed] [Google Scholar]
- 30.Kiso Y, Tohkin M, Hikino H. Mechanism of antihepatotoxic activity of atractylon, I: effect on free radical generation and lipid peroxidation. Planta Medica. 1985;2:97–100. doi: 10.1055/s-2007-969416. [DOI] [PubMed] [Google Scholar]
- 31.Combes R, Grindon C, Cronin MTD, Roberts DW, Garrod JF. Integrated decision-tree testing strategies for acute systemic toxicity and toxicokinetics with respect to the requirements of the EU REACH legislation. Alternatives to Laboratory Animals. 2008;36(1):45–63. doi: 10.1177/026119290803600107. [DOI] [PubMed] [Google Scholar]
- 32.Kim JY, Pham DD. Sasang constitutional medicine as a holistic tailored medicine. Evidence-Based Complementary and Alternative Medicine. 2009;6(1):11–19. doi: 10.1093/ecam/nep100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cotter MA, Cameron NE. Effects of dietary supplementation with arachidonic acid rich oils on nerve conduction and blood flow in streptozotocin-diabetic rats. Prostaglandins Leukotrienes and Essential Fatty Acids. 1997;56(5):337–343. doi: 10.1016/s0952-3278(97)90581-0. [DOI] [PubMed] [Google Scholar]
- 34.Cameron NE, Cotter MA, Hohman TC. Interactions between essential fatty acid, prostanoid, polyol pathway and nitric oxide mechanisms in the neurovascular deficit of diabetic rats. Diabetologia. 1996;39(2):172–182. doi: 10.1007/BF00403960. [DOI] [PubMed] [Google Scholar]
- 35.Luo XM, Hu YH, Yu J, Wang H, Xu QY, Zhan H. Effects of tea polyphenols on microcirculation and antioxidation in aircrew. Space Medicine & Medical Engineering. 1999;12(5):338–341. [PubMed] [Google Scholar]


