Abstract
Root cortical aerenchyma (RCA) is induced by hypoxia, drought, and several nutrient deficiencies. Previous research showed that RCA formation reduces the respiration and nutrient content of root tissue. We used SimRoot, a functional-structural model, to provide quantitative support for the hypothesis that RCA formation is a useful adaptation to suboptimal availability of phosphorus, nitrogen, and potassium by reducing the metabolic costs of soil exploration in maize (Zea mays). RCA increased the growth of simulated 40-d-old maize plants up to 55%, 54%, or 72% on low nitrogen, phosphorus, or potassium soil, respectively, and reduced critical fertility levels by 13%, 12%, or 7%, respectively. The greater utility of RCA on low-potassium soils is associated with the fact that root growth in potassium-deficient plants was more carbon limited than in phosphorus- and nitrogen-deficient plants. In contrast to potassium-deficient plants, phosphorus- and nitrogen-deficient plants allocate more carbon to the root system as the deficiency develops. The utility of RCA also depended on other root phenes and environmental factors. On low-phosphorus soils (7.5 μm), the utility of RCA was 2.9 times greater in plants with increased lateral branching density than in plants with normal branching. On low-nitrate soils, the utility of RCA formation was 56% greater in coarser soils with high nitrate leaching. Large genetic variation in RCA formation and the utility of RCA for a range of stresses position RCA as an interesting crop-breeding target for enhanced soil resource acquisition.
Root cortical aerenchyma (RCA), i.e. enlarged gas spaces in the root cortex that form through either cell death or cell separation (Evans, 2003), is commonly known to form in response to hypoxia (Jackson and Armstrong, 1999). Improved oxygen transport is an important function of RCA formation in flooded soils, where low oxygen availability may limit root respiration (Jackson and Armstrong, 1999). However, RCA also forms in response to a variety of other edaphic stresses, including phosphorus, nitrogen, and sulfur deficiency and drought (Konings and Verschuren, 1980; Drew et al., 1989; Bouranis et al., 2003, 2006; Fan et al., 2003; Zhu et al., 2010). Thus, it has been hypothesized that RCA formation has utility under a variety of edaphic stresses by reducing the metabolic costs of soil exploration (Lynch and Brown, 1998, 2008). RCA, formed in maize (Zea mays) by programmed cell death (Lenochová et al., 2009), reduces root nutrient content and respiration (Fan et al., 2003). Zhu et al. (2010) found that maize genotypes with high RCA formation under drought had 5 times greater biomass production and 8 times greater yield than closely related genotypes with less RCA. In a previous study (Postma and Lynch, 2010), we presented quantitative evidence that remobilization of phosphorus from the root cortex and a reduction in maintenance respiration may allow plants to maintain greater growth rates in soils with low phosphorus availability. We hypothesized that these two functions, remobilization of nutrients and reduced respiration, could be important functions of RCA under other nutrient deficiencies as well. In this paper, we evaluate the relative utility of RCA for the acquisition and utilization of nitrogen, phosphorus, and potassium. We present, to our knowledge, the first evidence for a growth benefit of RCA formation in nitrogen- and potassium-deficient maize.
Nitrate is a mobile resource that in agroecosystems often leaches into the subsoil during the growing season (Di and Cameron, 2002). The dynamics of nitrate leaching present challenges to root systems that may have to capture nitrate from increasing depths. In contrast to nitrate, phosphorus and potassium are often more available in surface soil horizons, and thus phenes that enhance “topsoil foraging” may be more useful for acquisition of these nutrients (Lynch and Brown, 2001). RCA may release resources that allow the plant to invest in new root growth. The soil depth at which these investments occur relative to the availability of the nutrients at that depth may affect the utility of RCA. As a consequence, the nitrate-leaching environment, as influenced by soil type and precipitation, may influence the utility of RCA in nitrogen-deficient plants.
Maize forms RCA in response to nitrogen and phosphorus deprivation (Konings and Verschuren, 1980; Drew et al., 1989; Fan et al., 2003). It is unknown if RCA is formed in response to low potassium availability, although circumstantial evidence suggests that it might be. Jung et al. (2009) show that ethylene mediates the response and tolerance to potassium deprivation in Arabidopsis (Arabidopsis thaliana), stimulating root hair formation and primary root growth. Ethylene can be considered a general stress hormone mediating responses to drought (Schachtman and Goodger, 2008) and a number of nutrient deficiencies (He et al., 1992; Borch et al., 1999; Brown et al., 2003). Since ethylene is involved in signaling RCA formation (Drew et al., 2000), a possible increase in ethylene production due to potassium deficiency (Jung et al., 2009) may also result in increased RCA formation. Since RCA can form constitutively in maize plants under optimal conditions (Fan et al., 2003; Lenochová et al., 2009; Burton, 2010; Zhu et al., 2010), it may have value for potassium acquisition even if it is not induced by low potassium availability.
The formation of RCA depends on many factors, including genetic, exogenous (environmental), and endogenous cues. As a result, RCA formation may differ among and within root classes of the same plant and may vary along the length of a root segment (Bouranis et al., 2006; Lenochová et al., 2009; Burton, 2010). Quantitative information on RCA distribution is sparse and difficult to quantitatively relate to exogenous or endogenous cues. In our previous simulation study on the benefit of RCA formation for plant growth on low-phosphorus soils (Postma and Lynch, 2010), we kept RCA formation equal for all root classes and only varied it depending on the age of the root segment. Currently, more information on local RCA formation has become available from a study by Burton (2010), which allows us to present, to our knowledge, the first spatiotemporal reconstruction of RCA formation in different genotypes.
The utility of a phene may depend on interactions with other phenes in integrated phenotypes. For example, long root hairs are more beneficial for phosphorus acquisition in roots with high root hair density (Ma et al., 2001) and in genotypes with shallow roots (Miguel, 2011). These phene synergisms may be important considerations in breeding crops with greater tolerance of edaphic stress. In this study, we evaluate a potential synergism between lateral branching density and RCA formation.
Quantitative information about the function of root phenes and how that function depends on the expression of the phene, other root phenes, and environmental factors is scarce but important for breeders and may aid in the understanding of phenotypic diversity. With our simulations, we provide quantitative estimates for the utility of RCA in different genotypes grown under different environmental conditions.
RESULTS
RCA Utility under Different Nutrient Deficiencies
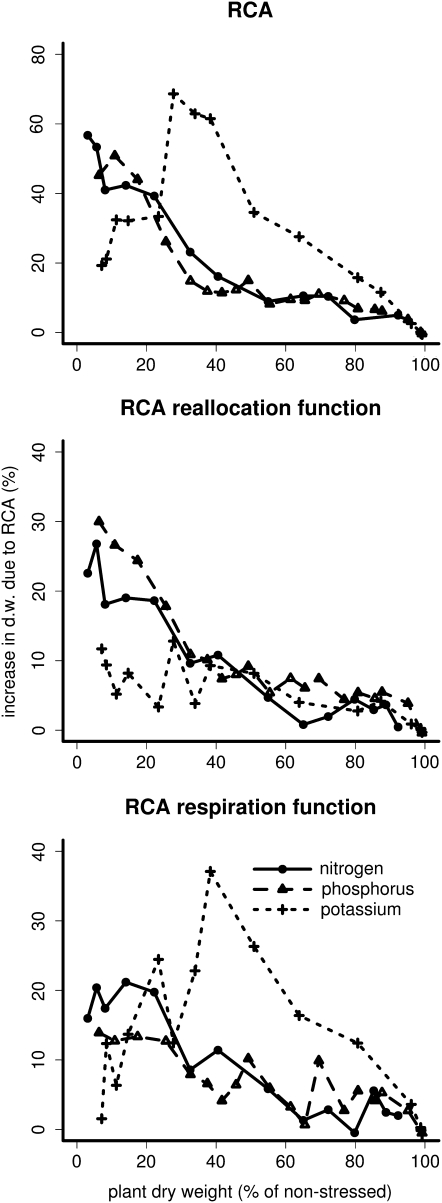
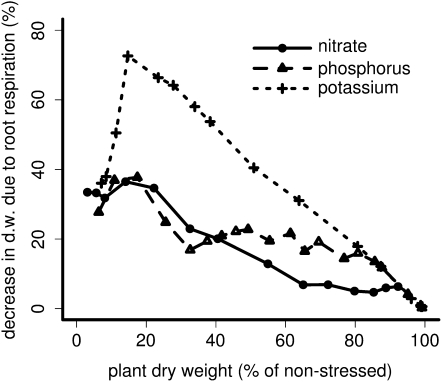
RCA had a positive effect on plant growth under suboptimal availability of nitrogen, phosphorus, and potassium (Fig. 1). The utility of RCA depended on the intensity of the nutrient deficiency and on the nutrient involved. At low to medium deficiencies (plant dry weight 30%–100% of nonstressed), RCA formation had the greatest utility when potassium was limiting compared with nitrogen and phosphorus, while in strongly deficient plants (plant dry weight 5%–30% of nonstressed), RCA formation had the greatest utility when phosphorus was limiting. The utility of RCA generally decreased with decreasing nutrient deficiency but peaked at medium deficiency levels when potassium was limiting. RCA reduced critical soil nutrient levels, defined as the nutrient level below which growth was reduced, by 13% for nitrogen, 12% for phosphorus, and 7% for potassium (data not shown). Plants benefited most from reallocating nutrients and to a lesser extent from a reduction in respiration. However, when potassium deficiency limited growth, reduced respiration was the most important benefit of RCA formation.
Figure 1.
The utility of RCA formation under different nutrient deficiencies. On the x axis, stress due to nutrient deficiency is expressed as the relative plant biomass at 40 d after germination compared with nonstressed plants. The RCA utility on the y axis is expressed as growth increase due to RCA formation (note the different scales). The top panel shows the overall benefit of RCA, and the following panels show the benefit of RCA due to reallocation of nutrients and the benefit of RCA due to reduction in respiration. Each data point is an average of two repetitions. d.w., Dry weight.
Utility of RCA Formation in Lateral Roots
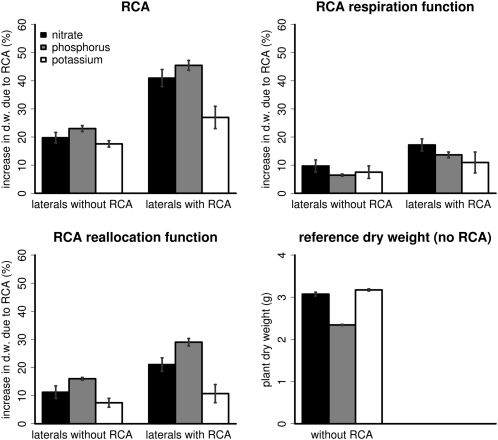
The model predicted larger benefits of RCA in plants that form RCA in lateral roots (Fig. 2). This utility of RCA formation in lateral roots was strongest on low-nitrogen and low-phosphorus soils. Both functions of RCA were equally affected by RCA formation in the lateral roots.
Figure 2.
The utility of RCA formation in roots when RCA only forms in the axial roots (laterals without RCA) or when RCA forms in all roots (laterals with RCA). The utility of RCA formation is given in percentage increase in plant dry weight (d.w.) at 40 d after germination relative to the dry weights of plants simulated without RCA given in the bottom right panel. Panels show utility on low-nitrogen, low-phosphorus, and low-potassium soils. Nitrogen, phosphorus, and potassium availability was such that yield reduction in plants without RCA was approximately 92%, corresponding to the typical yield reduction of small-scale subsistence farmers. Error bars present se for eight repeated runs. Variation is caused by simulated stochasticity in root growth rates, growth directions, and branching frequency.
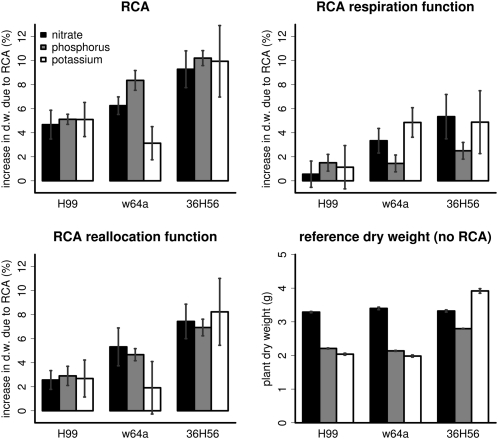
Utility of RCA Formation in Three Different Genotypes
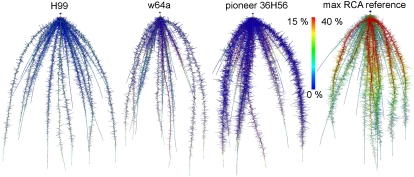
We simulated the root architecture of three maize genotypes and their RCA formation (Fig. 3; for animated movie, see Supplemental Appendix S2). The utility of RCA formation in these genotypes is less than the utility in our reference genotype (Figs. 2 and 4), which is understandable from the much reduced RCA formation in these genotypes (Fig. 3). RCA formation increased growth of the high-RCA genotypes, w64a and 36H56, more than the low-RCA genotype, H99, except on low-potassium soils, where H99 had a greater growth response to RCA than w64a. Although RCA had greater effects on the growth of w64a than on H99, the increase in RCA benefit was not proportional to the 3-fold increase in RCA in w64a compared with H99. RCA affected the growth of w64a most on low-nitrate soils, while RCA affected the growth of the other genotypes equally on low-nitrate, -phosphorus, or -potassium soils. These results cannot be totally explained by the simulation of the separate functions of RCA, suggesting that interactions between the functions exist. When simulating the separate functions of RCA in soils with greater resource availability, RCA sometimes had a small negative effect on growth (data not shown). The error bars show that stochasticity in root phenes other than RCA can cause large variation in the utility of RCA among individuals of a single genotype. Stochasticity was caused by variation in growth rates, growth directions, and branching frequencies of individual roots.
Figure 3.
Spatial map of RCA formation in simulated root systems at 40 d after germination. Colors show RCA formation as percentage of root cross-sectional area. The color range differed for the max RCA reference root system, which was rendered on a 0% to 40% scale instead of a 0% to 15% scale. See text for detailed description of the differences among these genotypes, which include variation in the steepness and number of major axes, lateral branching density, lateral root length, and RCA formation. Roots have been dilated (approximately two times) for better visibility and thus do not show true root thickness.
Figure 4.
Comparison of the utility of RCA for different genotypes. See Figure 2 for description of the panels and error bars. Utility of RCA is much less than in Figure 2, as RCA formation in these genotypes was much less (Fig. 3). d.w., Dry weight.
Interactions between RCA Formation and Lateral Root Formation
We used our high RCA reference plant, which forms equal amounts of RCA in all roots including lateral roots (Fig. 3), to simulate the utility of RCA formation under nitrate and phosphorus deficiency, given different lateral branching densities. The model predicted that RCA formation is more beneficial in plants with greater lateral branching densities when grown under low phosphorus but not when grown under low nitrate (Fig. 5). In soils with moderate nitrate availability, RCA benefited plants with normal lateral branching density the most. The RCA utility for plants with different branching densities was near equal in soils with low nitrate availability.
Figure 5.
Utility of RCA formation as affected by lateral root proliferation under nitrogen and phosphorus deficiency. Three levels of lateral root proliferation are shown: half, normal, and double, which correspond to 4, 8, and 16 lateral roots cm−1. This range represents the genotypic variation in lateral branching density measured by Trachsel et al. (2010). Low and medium nitrate availability correspond to residual nitrate in the top 60 cm (after fertilization), and low and medium phosphorus availability correspond to 5 and 7.5 μm in the buffered soil solution. d.w., Dry weight.
Influence of Soil Type and Precipitation on Nitrate Leaching and the Utility of RCA
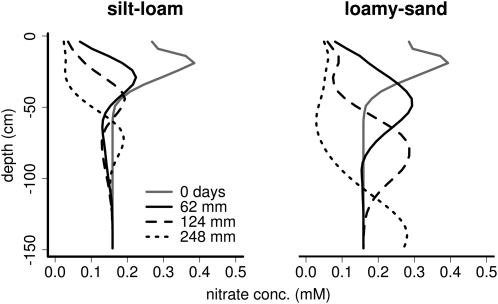
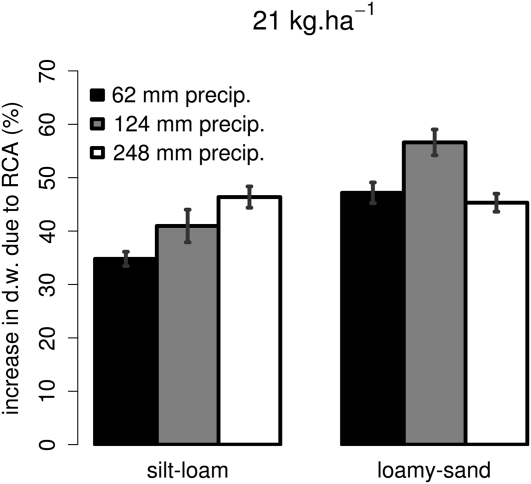
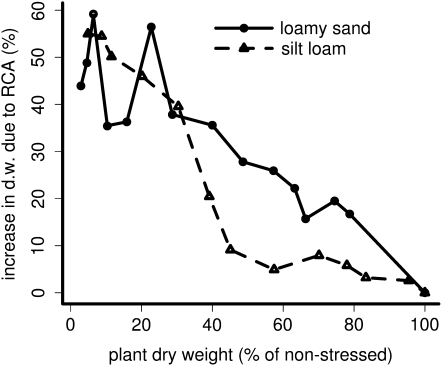
We simulated a loamy sand and a silt loam with three levels of precipitation to vary the intensity of nitrate leaching. As expected, nitrate leaching increased with increasing precipitation (Fig. 6). This increase was greater in the loamy sand than in the silt loam. Plants benefited more from RCA formation in high-leaching environments than in low-leaching environments (Fig. 7). This increase is not only caused by an increase in stress in these environments but also exists when comparing the RCA utility in different soils across a range of stress levels (Fig. 8).
Figure 6.
Nitrate leaching in a silt-loam and a loamy-sand soil given three different precipitation intensities. The total precipitation in mm over 40 d of growth is listed. The “0-d” gray line shows the nitrate profile at the start of growth, which had 21 kg ha−1 residual nitrogen in the top 60 cm. Data are from simulations of the maximum RCA reference genotype (Fig. 3).
Figure 7.
Utility of RCA formation over 40 d of growth on a silt-loam and a loamy-sand soil under different precipitation regimes. There was 21 kg ha−1 residual nitrogen in the top 60 cm (Fig. 6.) The simulated genotype was the maximum RCA reference genotype (Fig. 3). See Figure 2 for error bars. d.w., Dry weight.
Figure 8.
Utility of RCA in two different soils relative to the stress experienced due to nutrient deficiency. Axes are as in Figure 1. d.w., Dry weight.
Model Comparison
Both the Barber-Cushman and SWMS3D models simulated very similar amounts of potassium uptake under high potassium availability (see figure 1 in Supplemental Appendix S3). However, the spatiotemporal distribution of uptake differed strongly between the models (see figure 2 in Supplemental Appendix S3). The uptake per root class and over time varied more in the SWMS3D model. The Barber-Cushman model simulated greater total uptake than the SWMS3D model under low potassium availability, which resulted in significantly more growth (see figure 3 in Supplemental Appendix S3). The steeper response curve of the SWMS3D model to potassium availability (see figure 3 in Supplemental Appendix S3) caused RCA to be more beneficial in the SWMS3D model (see figure 3 in Supplemental Appendix S3). In accordance with our hypothesis, the SWMS3D model predicted greater uptake of phosphorus while the Barber-Cushman module simulated greater uptake of nitrate (see figure 4 in Supplemental Appendix S3).
Estimation of the Costs of Root Maintenance Respiration
Root maintenance respiration reduced plant growth on low-fertility soils up to 72% in comparison with plants with no root maintenance respiration (Fig. 9). The costs of root maintenance respiration were greatest for plants on low-potassium soils. Root maintenance respiration did not affect growth on high-fertility soils, as plant growth was sink limited, not source limited. Under conditions of high soil fertility, reduced respiration only increased the carbon storage in the plants without affecting growth (data not shown).
Figure 9.
Growth reduction (percentage plant dry weight [d.w.]) in 40-d-old maize plants due to root maintenance respiration. Simulations of the reference genotype with (w) and without (o) maintenance respiration were compared using the equation 100 × (o − w)/o. Plants did not form RCA. The x axis is as in Figure 1.
DISCUSSION
RCA Formation Is an Adaptation to Multiple Nutrient Deficiencies
RCA forms in response to suboptimal availability of nitrogen, phosphorus, sulfur, and water (Konings and Verschuren, 1980; Drew et al., 1989; Bouranis et al., 2003, 2006; Fan et al., 2003; Zhu et al., 2010). Our simulation results support the hypothesis that RCA formation may substantially benefit plants experiencing deficiencies of nitrogen and phosphorus and suggest that RCA could be beneficial under potassium deficiency as well (Fig. 1). These results indicate that RCA may have the greatest utility on low-fertility soils. However, RCA formation also decreased critical soil nutrient levels, defined as the soil fertility below which growth is reduced, by 13%, 12%, and 7% for nitrogen, phosphorus, and potassium, respectively. This suggests that cultivars with high RCA formation under nonstressed conditions may allow farmers to use substantially less fertilizer. Reduction in fertilizer use may be greater than the numbers presented here, as soil-available nutrients are only partly derived from fertilizers. We do not know if plants form RCA in response to potassium deficiency, but we propose that they may. It is common for stresses to occur simultaneously, although it is difficult to realistically simulate plant responses to simultaneous nutrient deficiencies (Dathe et al., 2011). In environments where multiple stresses may occur simultaneously (Rubio et al., 2003; Lynch and St Clair, 2004), tradeoffs for nutrient acquisition strategies often pose challenges to the plant. For example shallow rooting, which increases phosphorus acquisition, may reduce drought tolerance (Ho et al., 2005). Our results indicate that RCA may be a phene that is beneficial for several nutrient deficiencies.
The utility of RCA was greater in plants that were moderately potassium deficient than in plants that were severely (less than 10% potential growth) potassium deficient. This decline is caused by a reduction in the utility of the respiration function. Increased availability of carbon from reduced respiration causes the plant to grow more roots. Root growth, however, also requires the investment of nutrients. The time that it takes for nutrient acquisition to compensate for these investments increases with decreasing soil fertility (Postma and Lynch, 2010). The plant is more nutrient deficient during this period, which may have detrimental effects on shoot growth. The decline in RCA utility in severely potassium-deficient plants was less pronounced in phosphorus-deficient plants and not observed in nitrogen-deficient plants. In our previous simulation study of the utility of RCA in low-phosphorus soils, for which we used homogeneous soil profiles, this decline in the utility of RCA was more pronounced (Postma and Lynch, 2010). The heterogeneous soil profiles used in this study caused root growth, on average, to be more beneficial, as the opportunity to grow into soil domains with greater soil fertility existed. Actual plants may take advantage of soil heterogeneity by changing rooting depth (Zhu et al., 2005) and by root proliferation into areas with greater soil fertility (Borch et al., 1999; Hodge, 2004).
The Relative Importance of the Two Functions of RCA Depends on the Nutrient Deficiency Involved
Reallocation of nutrients is predicted by the model to be the more important function of RCA formation in nitrogen- and phosphorus-deficient plants (Fig. 1). The importance of reallocating nutrients agrees with the calculations of Robinson (1990), which show that reallocation of nutrients could be an important function of root cortical senescence in phosphorus-deficient plants. Cortical senescence, like lysigenous RCA, is a form of programmed cell death (Deacon et al., 1986; Liljeroth and Bryngelsson, 2001). A reduction in respiration is more important in potassium-deficient plants than in phosphorus- or nitrogen-deficient plants (Fig. 1) because root growth is strongly carbon limited in potassium-deficient plants, while this is not always the case for nitrogen- and phosphorus-deficient plants (Postma and Lynch, 2010). This carbon limitation of root growth in potassium-deficient plants is caused by (1) the strong reduction in photosynthesis caused by the deficiency and (2) the lack of an adaptive response in carbon allocation between roots and shoots. While in nitrogen- and phosphorus-deficient plants photosynthesis may be reduced as well, deficiency of nitrogen and phosphorus causes increased carbon allocation to roots at the expense of carbon allocation to shoots, which results in root growth being less carbon limited than it is under potassium deficiency. The strong carbon-limited root growth in potassium-deficient plants caused the opportunity costs of root maintenance respiration to be greater than the opportunity costs of root maintenance respiration of phosphorus- and nitrogen-deficient plants (Fig. 9).
RCA Formation in Lateral Roots Has Potential Utility
Burton (2010) comments on the lack of RCA formation in lateral roots of nonstressed maize plants. However, P. Saengwilai (unpublished data) did observe RCA formation in lateral roots of maize. Several scientists report RCA formation in lateral roots of other species in response to flooding (Laan et al., 1989; Gibberd et al., 2001; Thomas et al., 2005). According to our simulation results, RCA formation in lateral roots would benefit nutrient-deficient plants (Fig. 2). These results suggest that lateral roots, despite their fineness, should not be ignored in RCA research.
RCA Formation in Three Genotypes
Burton (2010) measured, in nonstressed plants, higher percentage RCA formation in thicker root classes. Brace roots were an exception to the rule, as they were thick but formed little RCA. RCA formation in these brace roots may still have been in progress, however, since the oldest brace roots were only 7 to 10 d old. The correlation between root thickness and RCA formation is less clear in data from Mano et al. (2006) and Jaramillo-Velastegui (2011). Fan et al. (2003) show that under stress conditions, relatively fine seminal roots can form high levels of RCA, up to 38% of the root cross-sectional area. Thicker root classes might not form more than 38% RCA under stressed conditions, as there is not much remaining living cortical area. Therefore, we kept RCA formation equal in our reference plant, which represents “potential” RCA formation, but simulated actual RCA formation as measured by Burton (2010) in three different genotypes grown at high nutrient supply (Fig. 3). We found that all three genotypes benefited from RCA formation when grown on low-nitrogen, -phosphorus, or -potassium soils (Fig. 4). As expected from the reduced RCA formation, this benefit was much less than the benefit simulated for our reference plant (Fig. 2). The difference in the utility of RCA formation between the reference plant and the simulated genotypes may indicate the potential of breeding for RCA formation in these genotypes. However, tradeoffs of RCA are currently not well understood (Postma and Lynch, 2010); furthermore, these genotypes may already form more RCA under stress. For example, low phosphorus availability increased RCA in seminal roots of w64a from 2% to 26% of the root cross-sectional area (Fan et al., 2003).
The reallocation and respiration functions of RCA in these genotypes depend on an interaction between the genotype and nutrient deficiency involved (Fig. 4). We observed slightly negative growth responses to RCA when the functions were simulated independently on soils with medium fertility (data not shown). These negative responses show that the two inbred lines did not allocate resources optimally under these specific conditions. In our simulation, nitrogen deficiency increased over time due to decreasing nitrate availability in the soil (caused by leaching and nitrate uptake by the plant). Adaptation to low nitrate availability by changing root-shoot ratios takes time; therefore, changes in carbon allocation must occur early if they are to result in growth benefits, especially considering that our simulations ended at 40 d after germination. Reallocation of nitrogen from lysed cells may cause the plant to be initially less deficient and thus respond with an attenuated adjustment of carbon partitioning between roots and shoots. However, the resulting greater shoot-root ratio may cause the plant to be more stressed during later growth stages. On the other hand, additional carbon for root growth requires an additional investment of nitrogen, which may cause the plant to be temporarily more stressed. Unless these investments in root growth pay off within a relatively short time, they may actually increase the negative effects of nutrient deficiency on growth (Postma and Lynch, 2010). Thus, both the reallocation and the respiration function have both positive and negative feedbacks in the model, and when the functions are considered independently, negative feedbacks may exceed positive feedbacks. However, when, as in actual plants, both functions occur together, negative feedbacks are attenuated and RCA has a net positive effect on plant growth. These negative feedbacks are caused by temporal disturbances in the nutrient homeostasis of the plant. Consequently, longer simulation times may change the negative responses to RCA into positive responses. Currently, our simulation times are restricted to 40 d, mainly because the data sets on which we based our parameterization do not extend beyond 40 d. We suggest that the study of older plants may be important in future research. Our results show that RCA is more beneficial if plants are able to invest their “saved” resources more optimally. However, the optimal investment of resources is inherently complex, as it depends on many factors and constraints, only a few of which are included in this study.
Synergism between RCA and Lateral Root Formation
H99, a low-RCA-forming genotype, benefits from RCA formation nearly as much as w64a, a high-RCA-forming genotype. H99 has a high lateral branching density of 15 lateral roots cm−1, in contrast to the other two genotypes, which have 10 lateral roots cm−1 on average. RCA is more beneficial in plants with greater lateral branching density (Fig. 5). Greater lateral branching density allows the plant to grow more root length, but only if it has the resources to do so. These resources can be made available through RCA formation. Greater lateral branching density is beneficial for phosphorus uptake, but it is less beneficial for nitrogen uptake, as it mostly leads to increased interroot competition. Thus, a positive interaction between lateral density and RCA formation for growth on low-phosphorus soils exists, but this interaction does not exist for growth on low-nitrogen soils (Fig. 5). Phene synergisms, like the synergism between RCA formation and lateral root formation presented here, are an important consideration for breeders, and it is important to note that these phene synergisms may exist in one environment but not in another.
RCA Is More Beneficial in Environments with Significant Leaching
RCA formation was more beneficial in environments with greater leaching (Fig. 7). In part, the benefit of RCA in leaching environments is caused by increased nutrient deficiency in such environments. However, when comparing equally stressed plants, the utility of RCA was still greater in the loamy sand than in the silt loam (Fig. 8). Therefore, new root growth, made possible by RCA formation, was more beneficial when nitrate had leached to greater depth. This positive interaction did not exist in severely deficient plants (growth less than 30% of potential), which had reduced rooting depth. These phene × environment interactions may partly explain the observed genetic variation in RCA formation and may be considered by breeders in targeting specific environments.
Model Choice Did Not Affect the Conclusions
We simulated nitrate and potassium uptake using the SWMS3D model and phosphorus uptake using the Barber-Cushman model, since we argued that SWMS3D is better for simulating mobile nutrients while the Barber-Cushman model is better for simulating immobile nutrients (see “Materials and Methods”). We predicted that SWMS3D would simulate greater uptake of phosphorus, an immobile nutrient, due to artificially increasing the depletion zones to 1 cm, and that Barber-Cushman would simulate greater nitrate uptake, a mobile nutrient, as it cannot simulate nitrate leaching and root competition in three dimensions. Our results confirm our predictions and support our choice to use different models for different nutrients (Supplemental Appendix S3). We also predicted that both models would simulate similar uptake of potassium, which has intermediate mobility in the soil. Both models simulated similar potassium uptake (see figures 2 and 4 in Supplemental Appendix S3); however, both models differed strongly in the spatiotemporal distribution of potassium uptake (see figure 3 in Supplemental Appendix S3). The potassium uptake rate was more constant over time and more equal among root classes when the Barber-Cushman model was used. These results suggest that new modeling approaches are needed to combine the strength of both models. Model choice affected our results on the utility of RCA formation in maize plants for a number of potassium runs (see figure 4 in Supplemental Appendix S3) but did not affect our conclusions that RCA is an adaptive phene to multiple nutrient deficiencies.
Model Development
Our results indicate several areas in which structural functional plant models could be improved. (1) Parameterization for root development of older plants, in order to simulate full plant life cycles, will be important for understanding the dynamics of physiological processes. (2) Root anatomy, which may affect whole plant physiology, as shown in this report, is currently not well understood in relation to root function and metabolic costs. It deserves more attention so that it might be incorporated into functional structural models in greater detail. (3) Interactions between plants and other organisms such as mycorrhizal fungi or neighboring plants are important for understanding ecosystem functioning and may have consequences for the utility of a root phene like RCA. It is important that the science community adopts an integrated and quantitative approach in studying these interactions by developing models that can explain their observations. The development of a functional quantitative plant-mycorrhizae model might be a priority. (4) We compared two nutrient models and showed that each has its strengths and weaknesses. A future challenge is to develop a model that combines the strengths of both nutrient models.
Future Directions for Research
Our simulation results show that the formation of RCA can have utility for plant growth under multiple nutrient deficiencies. These results indicate that small-scale changes in root anatomy may impact whole plant physiology and growth. Root phenes, such as RCA, that influence the metabolic cost of soil exploration may thus be key to understanding agroecosystem functioning and could be targeted in breeding for nutrient-efficient plants. We have shown that the utility of RCA depends on other root phenes and on interactions with the environment. On soils with medium phosphorus availability, RCA was 2.9 times more beneficial in plants with high lateral branching density compared with plants with median branching density. Phene synergism was found for long and dense root hairs (Ma et al., 2001) and long root hairs with shallow root growth angles (Miguel, 2011), whereas phene antagonism was found between hypocotyl-borne and basal roots for phosphorus acquisition (Walk et al., 2006). We are aware of few other studies that have considered phene interactions, despite the possibility for strong effects such as those found here. We hypothesize that RCA may be synergistic with phenes that are beneficial for nutrient uptake but have high metabolic cost. For example, RCA may be synergistic with the number of axial roots. More axial roots allow the plant to grow a larger root system, but if the metabolic cost of the axial roots reduces lateral root growth, the phene may actually reduce plant growth on low-fertility soils. Thus, there is an optimal number of axial roots that may be greater for plants that form RCA. In addition to metabolically costly root phenes, RCA may be synergistic with root phenes that position additional root growth in soil domains with greater fertility. For example, RCA may be synergistic with shallow angles in low-phosphorus soils, where most of the phosphorus is available in the topsoil (Zhu et al., 2005). It may also be synergistic with root proliferation phenes in soils with low nitrogen. More research is needed on these phene interactions in integrated phenotypes. Integrated phenotypes need to be evaluated across a range of environments. RCA was more beneficial for maize growing on loamy sand than on silt loam and was more beneficial when precipitation was high on silt-loam soils. We hypothesized that RCA is more beneficial in environments with high nitrate leaching. Sulfur deprivation increases RCA formation (Bouranis et al., 2003, 2006). Low soil sulfur is usually the result of high sulfate leaching (McGrath and Zhao, 1995). RCA substantially increased the growth of maize under drought (Zhu et al., 2010). We hypothesize that RCA may have special utility for deep soil resources like nitrate, sulfate, and water. The utility of RCA for increasing the acquisition of these deep soil resources may depend on other root phenes such as steep branching angles and carbon allocation to growth at deep soil layers.
Figure 1 shows that a phene like RCA may have different utility at different fertility levels and that the relation between soil fertility and phene utility is not necessarily linear. As a consequence, the outcome of research in which high and low fertility levels are compared may depend on the actual fertility levels. Therefore, quantitative approaches are required to evaluate phenes and their interactions with the environment. Simulation models are especially suitable for such quantitative analyses (Vos et al., 2010). We expect that functional structural models such as SimRoot will continue to provide new insights into plant function for some time.
CONCLUSION
We have provided quantitative evidence that RCA is an adaptive phene for multiple nutrient deficiencies. The utility of RCA for maize plants growing on soils with suboptimal availability of nitrate, phosphorus, or potassium depends on multiple interactions between RCA formation, other root phenes, and environmental factors. We found RCA to be synergistic with lateral root formation on low-phosphorus soils. On low-nitrogen soils, RCA may be more beneficial in environments with substantial leaching. This variability in the utility of RCA may explain the large variation observed for RCA formation (Fan et al., 2003; Burton, 2010). Undoubtedly, tradeoffs for RCA formation, which are currently not well understood, contribute to this variability and therefore merit research. Functional structural models like SimRoot can be used to simulate this variation and thereby evaluate the utility of RCA in a specific genotype. We suggest that these models may become a valuable tool for evaluating breeding strategies that target a phene like RCA. Breeding for crop genotypes with enhanced soil resource acquisition will be an important strategy for reducing environmental pollution and decreasing agricultural reliance on fertilizer inputs (Lynch, 2007).
MATERIALS AND METHODS
We used SimRoot (Postma and Lynch, 2010), a functional-structural plant model (Vos et al., 2010), to simulate the utility of RCA formation in maize (Zea mays) growing in diverse environments. We simulated RCA formation in three different maize genotypes and varied the phosphorus, potassium, or nitrate availability in the soil. For the nitrate study, we simulated a loamy-sand and a silt-loam soil and varied precipitation to create six different leaching environments. We also evaluated a possible synergism between RCA formation and lateral branching density.
Model Description
SimRoot simulates the three-dimensional architecture and soil resource acquisition of a root system as it develops over time. The root system consists of roots of distinct root classes. Each root is represented by a growing number of root segments. This architectural component of SimRoot has been described by Lynch et al. (1997). SimRoot simulates shoot growth and photosynthesis nongeometrically (Postma and Lynch, 2010) using LINTUL (Spitters and Schapendonk, 1990). Growth in SimRoot is based on a source-sink model in which carbon is partitioned using a set of rules that have been described by Postma and Lynch (2010). SimRoot simulates the nutrient uptake for each root segment and compares the total uptake with the optimal and minimal nutrient requirements of the plant. A nutrient stress factor is calculated when nutrient uptake falls below the optimal nutrient requirements. This stress factor influences the leaf area expansion rate and photosynthetic efficiency of the shoot negatively in a nutrient-specific manner (see below). Phosphorus uptake is simulated using the Barber-Cushman model (Itoh and Barber, 1983; Postma and Lynch, 2010), while potassium and nitrate uptake are simulated by linking (see below) SimRoot to the three-dimensional hydrological model SWMS3D (Šimunek et al., 1995). We considered the Barber-Cushman model inadequate for simulating nitrate uptake, as this model does not simulate leaching of nitrate and cannot simulate root competition in three dimensions. On the other hand, SWMS3D is not able to simulate the narrow phosphorus depletion zones at submillimeter resolution, as does the Barber-Cushman model (Postma et al., 2008; Postma and Lynch, 2010), as the resulting large number of finite element (FEM) nodes would require excessive computation (Hardelauf et al., 2007). The coarse resolution of the SWMS3D grid may cause the narrow phosphorus depletion zones to be artificially enlarged. Since we used two different models for simulating nutrient uptake, each with its strengths and weaknesses, we include a comparison of the two simulation modules and determined the effect of model choice on our results. We hypothesized that the Barber-Cushman model in comparison with SWMS3D would predict greater uptake of mobile nutrients (nitrate) but less uptake of immobile nutrients (phosphate and potassium).
RCA formation in maize is simulated for each root segment using empirical data from Burton (2010), who determined the percentage RCA for different root classes and at different locations along the root. The model interpolated RCA formation between these locations. RCA formation is allowed to either reduce the nutrient content of the root segments (reallocation function) or the respiration of the root segments (respiration function) or both, as is the case in actual plants (Fan et al., 2003). Reductions in nutrient content and respiration of the root segment are based on a regression between the amount of RCA and nutrient content and root respiration of live plants, as presented by Fan et al. (2003).
Effects of Nutrient Deficiency on Plant Growth
The nutrient stress factor was allowed to affect the potential leaf area expansion rate and light use efficiency independently. A negative impact of the nutrient stress factor on light use efficiency resulted in reduced carbon availability for growth. A negative impact on the potential leaf area expansion rate resulted in reduced sink strength of the shoot and consequently greater carbon availability for root growth. In this way, the nutrient stress factor functioned as a growth regulator altering root-shoot ratios. A nutrient-specific stress response curve was used to determine the effect of internal nutrient concentrations (internal nitrogen, potassium, and phosphate) on light use efficiency and potential leaf area expansion rate (Supplemental Appendix S1). Suboptimal inorganic potassium strongly reduces light use efficiency (Terry and Ulrich, 1973; Stamp and Geisler, 1980; Zhao et al., 2001) but does not affect the potential leaf area expansion rate (Cakmak et al., 1994). In contrast, suboptimal inorganic phosphate strongly affects potential leaf area expansion rate but has minor effects on light use efficiency of the leaves (Lynch et al., 1991). The inorganic nitrogen strongly affects both the potential leaf area expansion rate and light use efficiency (Sinclair and Horie, 1989; Uhart and Andrade, 1995).
Linking SWMS3D to SimRoot
We linked SWMS3D (Šimunek et al., 1995) to SimRoot (Postma and Lynch, 2010) in order to simulate nitrate uptake by the plant. SWMS3D is a three-dimensional hydraulic simulation model that includes a solute transport model. It simulates water transport in the soil by solving the Richards equation and solute transport by solving the convection-dispersion equation. SWMS3D includes a water extraction term in the Richards equation (Richards, 1931) that can be used to simulate water uptake by roots. The solute transport model also includes an extraction term for nutrient uptake by roots. We calculated the water uptake by roots by dividing the potential transpiration equally over the root length of the root system, following Somma et al. (1998). Although this is a simplification, we consider this a valid approximation in wet soils with a relatively uniform water distribution, the environment that we simulated. We calculated nutrient uptake by the roots using Michaelis-Menten kinetics (Claassen and Barber, 1974). Thus, nutrient uptake becomes a function of the nutrient concentration in the profile, while nutrient concentrations in the profile depend on the uptake. We used a two-step method to solve this mutual dependency, where the initial prediction was calculated using forward Euler and the final result was calculated using backward Euler (Šimunek et al., 1995). Stability of the results was verified by checking the nutrient balance of the whole system, which remained within 1% accuracy.
In order to link SimRoot to SWMS3D, we matched the root nodes in SimRoot, spaced 0.5 to 1 cm apart, to the nodes of the 1-cm3 FEM grid used by SWMS3D. To do so, we matched all the FEM nodes within a distance of 3 (i.e. the diagonal length of one FEM) to the root nodes. We distributed nutrient uptake of the root nodes over the nearby FEM nodes using the weighting factor (1/di)3/(∑(1/d)3), where d is the distance between the nearby FEM nodes and the root node. We calculated the average nutrient concentration at the root node surface by averaging the nutrient concentrations of the nearby nodes using the same weighting factor. The weighting factor gives a greater weight to the nearest node in an effort to avoid artificially increasing the domain of soil exploration.
Mineralization
We added a simple, one-pool mineralization model as described by Yang and Janssen (2000) to SWMS3D and ran it for each FEM node. We varied mineralization parameters along the vertical dimension only but allowed three-dimensional variation in soil water content to influence mineralization. Indirect interactions between the plant model and the mineralization model occurred, as local drying of the rhizosphere was allowed to affect mineralization, and changes in mineral nitrogen content due to mineralization were allowed to affect nutrient uptake rates.
Distribution of RCA Formation within the Root System
We assumed that RCA formation starts behind the cell elongation zone of a root and increases over time until a maximum is reached. Thus, the greatest percentage of RCA per cross-sectional area can be found close to the base of the root. This is in accordance with observations by Fan et al. (2003) but not with those of Bouranis et al. (2006), Lenochová et al. (2009), and Burton (2010), who observed lower RCA formation at the base of the roots and greater RCA formation in the middle root sections. We did not simulate low RCA formation in the basal parts of the root system, as RCA formation is only reduced in the first 5 cm of the root (Bouranis et al., 2006; Mano et al., 2006). This is a very small part of the total root length and forms a negligible effect (less than 2%) on the total RCA formation.
Within maize, there is significant genotypic variation in RCA formation and distribution of RCA in the root system (Fan et al., 2003; Burton, 2010). We used data from Burton (2010) for high-RCA (w64a) and low-RCA (H99) inbred genotypes and a high-RCA hybrid genotype (Pioneer 35H56) and developmental data from Mano et al. (2006) to simulate the temporal and spatial dynamics of RCA formation. We also simulated a high-RCA reference phenotype in which a high percentage (39%) of the root cross-sectional area of all root classes is RCA. This value of 39% RCA is the greatest amount of RCA formation reported in the literature (Fan et al., 2003).
Description of the Genotypes
w64a and H99 are two inbred lines of maize differing in RCA production, with w64a forming approximately 3 times more RCA than H99 (Burton, 2010). We assumed that both genotypes had comparable vigor, as the literature reports both greater and lesser biomass production for w64a (Silva and Gabelman, 1992; Kaeppler et al., 2000; Mickelson and Kaeppler, 2005). H99 has 15° steeper nodal roots than w64a, thicker major axes, and 1.5 times greater lateral branching frequency. Compared with hybrids, lateral extension is reduced in both inbred lines (Picard and Bosco, 2006). H99 has larger seed (0.28 g) than w64a (0.2 g) but lower seed phosphorus concentration, 0.36% instead of 0.48% (Pletch-Rivera and Kaeppler, 2007).
The hybrid (Pioneer 36H56) that we simulated forms a similar amount of RCA as w64a (Burton, 2010). This hybrid is more vigorous than the inbred lines, has longer lateral roots than inbred lines, and has more nodal roots. The thickness of the roots is intermediate to that of H99 and w64a. We did not have data on the steepness of the root system of this hybrid and assumed that the crown roots had an angle of 70° from horizontal, which is the median angle for crown roots in the NAM populations (S. Trachsel, unpublished data). The NAM populations are a set of 5,000 recombinant inbred lines that were obtained by crossing 25 very diverse inbred lines with one common parent, B73 (http://www.panzea.org/lit/germplasm.html).
We simulated four genotypes: a reference hybrid (Pioneer 36H56) and two inbred lines, w64a and H99. Since our reference genotype forms RCA in lateral roots, and since RCA formation in lateral roots is under discussion, we also simulated the reference genotype without RCA formation in lateral roots. In these simulations, we kept nutrient availability low, such that the approximate growth reduction of non-RCA plants was about 92%. This is a realistic level of stress on small-scale subsistence farms in many parts of the world (Lynch, 2007) and is, according to our previous results (Postma and Lynch, 2010), the stress level at which RCA formation has the greatest metabolic utility.
Phene Synergism between Lateral Branching Density and RCA Formation
Trachsel et al. (2010) found significant genetic variation in branching density and length of lateral roots among maize genotypes. We simulated the extremes of this variation to study any possible synergisms between RCA formation and lateral branching density under low nitrogen and phosphorus availability.
Heterogeneity in Resource Availability
We varied nutrient availability with depth, with greater nutrient availability in surface strata (Fig. 6; Supplemental Appendix S1). Nitrate leaching was varied by simulating two different soils, a loamy sand and a silt loam, and three different precipitation regimes, 62, 124, and 248 mm in 40 d. A rainfall of 124 mm in 40 d corresponds to the rainfall in Rock Springs, (PA) during the first 40 d of the 2009 growth season, while 62 and 248 mm are half and double this rainfall.
Metabolic Costs of Root Maintenance Respiration
Nielsen et al. (2001) estimated that the carbon costs of root maintenance respiration in phosphorus-deficient bean (Phaseolus vulgaris) plants could be as high as 39% of daily photosynthesis. In our previous simulation study (Postma and Lynch, 2010), root maintenance respiration varied between 16% and 37% of the cumulative photosynthesis. These direct metabolic costs of root respiration are substantial on low-fertility soils; however, the opportunity costs of root maintenance respiration might be even greater. The opportunity costs of root respiration are the costs of the missed opportunity to invest carbohydrates in root growth rather than respiring them (Lynch and Ho, 2005). Additional root growth would have increased nutrient uptake and thereby growth. We simulated maize plants with and without root maintenance respiration (without altering respiration caused by growth and nutrient assimilation) in order to estimate the opportunity costs of root maintenance respiration.
System Description
We simulated the first 40 d of growth of a single plant, which was a representative individual of a uniform, monoculture plant community with a between-row spacing of 60 cm and a within-row spacing of 26 cm. Aboveground competition was simulated by including a shading function (Postma and Lynch, 2010). Belowground, realistic root density was simulated by mirroring the roots at mid distance between the simulated and the hypothetical neighboring plants (Fig. 10). Root competition in SimRoot was a result of the depletion of nutrients of neighboring roots. Root competition in the Barber-Cushman model was implemented as described by Postma and Lynch (2010).
Figure 10.
Side view of a simulated maize root system and its mirror image. The image shows how the model simulates a realistic root density by mirroring the roots back into the column.
Parameterization
Parameterization was based on empirical data only; we did not calibrate the model. Parameters used in this study, with the references for these parameters, can be found in Supplemental Appendix S1.
Random Number Generator
SimRoot uses a random number generator to simulate natural variation in growth rates, direction, and branching frequency. The model results are different when we simulate this variation than if we use average values because of the nonlinearity of many processes (data not shown). The random number generator causes some variation in the results from different runs, which is multiplied when calculating the RCA benefit, because this calculation involves data of two simulation runs. We repeated our runs eight times to estimate this variation and show se bars for this variation in the figures.
Runs
In total, we performed 2,200 runs on the PennState clusters lionxi and lionxj (http://rcc.its.psu.edu/hpc/systems). We varied the following: (1) percentage RCA formation; (2) the functional utility of RCA, either a relocation or a respiration benefit, or both; (3) the availability of nitrate, phosphorus, and potassium in the soil, from low to sufficient; (4) soil texture: loamy sand and silt loam; (5) precipitation: 62, 124, and 248 mm over 40 d of growth; (6) genotypes: inbred genotypes H99 and w64a and hybrid genotype 36H56; (7) our “max RCA” reference genotype with and without RCA formation in the lateral roots; and (8) our reference genotype with and without root maintenance respiration.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Appendix S1. SimRoot parameterization.
Supplemental Appendix S2. Animated movie of root growth and RCA distribution.
Supplemental Appendix S3. Figures for comparing the Barber-Cushman model with SWMS3D.
Supplementary Material
Acknowledgments
We acknowledge the important contributions of Dr. Annette Dathe to the development of both the Simunek- and Barber-Cushman-based modules that were used in this article. We are grateful for her assistance in solving the underlying mathematics of the Barber-Cushman model and her intense involvement in the strategic planning, implementation, and testing of the link between SimRoot and SWMS3D. We thank Dr. Maria Bredina Postma-Blaauw and Dr. Eric Nord for their helpful review of the manuscript.
References
- Borch K, Bouma TJ, Lynch JP, Brown KM. (1999) Ethylene: a regulator of root architectural responses to soil phosphorus availability. Plant Cell Environ 22: 425–431 [Google Scholar]
- Bouranis DL, Chorianopoulou SN, Kollias C, Maniou P, Protonotarios VE, Siyiannis VF, Hawkesford MJ. (2006) Dynamics of aerenchyma distribution in the cortex of sulfate-deprived adventitious roots of maize. Ann Bot (Lond) 97: 695–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouranis DL, Chorianopoulou SN, Siyiannis VF, Protonotarios VE, Hawkesford MJ. (2003) Aerenchyma formation in roots of maize during sulphate starvation. Planta 217: 382–391 [DOI] [PubMed] [Google Scholar]
- Brown KM, Zhang YJ, Kim HJ, Lynch JP. (2003) The ethylene underground. Acta Hortic 618: 193–198 [Google Scholar]
- Burton AL. (2010) Phenotypic evaluation and genetic basis of anatomical and architectural root traits in the genus Zea. PhD thesis. The Pennsylvania State University, University Park, PA [Google Scholar]
- Cakmak I, Hengeler C, Marschner H. (1994) Partitioning of shoot and root dry matter and carbohydrates in bean plants suffering from phosphorus, potassium and magnesium deficiency. J Exp Bot 45: 1245–1250 [Google Scholar]
- Claassen N, Barber SA. (1974) A method for characterizing the relation between nutrient concentration and flux into roots of intact plants. Plant Physiol 54: 564–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dathe A, Postma JA, Lynch JP. (2011) Modeling resource interactions under multiple edaphic stresses. Ahuja L, Reddy V, Saseendran S, Yu Q, , Advances in Agricultural Systems Modeling II. ASA-CSSA-SSSA, Madison, WI: (in press) [Google Scholar]
- Deacon JW, Drew MC, Darling A. (1986) Progressive cortical senescence and formation of lysigenous gas space (aerenchyma) distinguished by nuclear staining in adventitious roots of Zea mays. Ann Bot (Lond) 58: 719–727 [Google Scholar]
- Di H, Cameron K. (2002) Nitrate leaching in temperate agroecosystems: sources, factors and mitigating strategies. Nutr Cycl Agroecosyst 64: 237–256 [Google Scholar]
- Drew MC, He CJ, Morgan PW. (1989) Decreased ethylene biosynthesis, and induction of aerenchyma, by nitrogen- or phosphate-starvation in adventitious roots of Zea mays L. Plant Physiol 91: 266–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew MC, He CJ, Morgan PW. (2000) Programmed cell death and aerenchyma formation in roots. Trends Plant Sci 5: 123–127 [DOI] [PubMed] [Google Scholar]
- Evans DE. (2003) Aerenchyma formation. New Phytol 161: 35–49 [Google Scholar]
- Fan M, Zhu J, Richards C, Brown KM, Lynch JP. (2003) Physiological roles for aerenchyma in phosphorus-stressed roots. Funct Plant Biol 30: 493–506 [DOI] [PubMed] [Google Scholar]
- Gibberd MR, Gray JD, Cocks PS, Colmer TD. (2001) Waterlogging tolerance among a diverse range of Trifolium accessions is related to root porosity, lateral root formation and ‘aerotropic rooting’. Ann Bot (Lond) 88: 579–589 [Google Scholar]
- Hardelauf H, Javaux M, Herbst M, Gottschalk S, Kasteel R, Vanderborght J, Vereecken H. (2007) PARSWMS: a parallelized model for simulating three-dimensional water flow and solute transport in variably saturated soils. Vadose Zone J 6: 255–259 [Google Scholar]
- He CJ, Morgan PW, Drew MC. (1992) Enhanced sensitivity to ethylene in nitrogen- or phosphate-starved roots of Zea mays L. during aerenchyma formation. Plant Physiol 98: 137–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho MD, Rosas JC, Brown KM, Lynch JP. (2005) Root architectural trade-offs for water and phosphorus acquisition. Funct Plant Biol 32: 737–748 [DOI] [PubMed] [Google Scholar]
- Hodge A. (2004) The plastic plant: root responses to heterogeneous supplies of nutrients. New Phytol 162: 9–24 [Google Scholar]
- Itoh S, Barber SA. (1983) A numerical solution of whole plant nutrient uptake for soil-root systems with root hairs. Plant Soil 70: 403–413 [Google Scholar]
- Jackson MB, Armstrong W. (1999) Formation of aerenchyma and the processes of plant ventilation in relation to soil flooding and submergence. Plant Biol 1: 274–287 [Google Scholar]
- Jaramillo-Velastegui RE. (2011) The edaphic control of plant response to climate change: extent, interactions and mechanisms of plant adaptation. PhD thesis. The Pennsylvania State University, University Park, PA [Google Scholar]
- Jung JY, Shin R, Schachtman DP. (2009) Ethylene mediates response and tolerance to potassium deprivation in Arabidopsis. Plant Cell 21: 607–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeppler SM, Parke JL, Mueller SM, Senior L, Stuber C, Tracy WF. (2000) Variation among maize inbred lines and detection of quantitative trait loci for growth at low phosphorus and responsiveness to arbuscular mycorrhizal fungi. Crop Sci 40: 358–364 [Google Scholar]
- Konings H, Verschuren G. (1980) Formation of aerenchyma in roots of Zea mays in aerated solutions, and its relation to nutrient supply. Physiol Plant 49: 265–270 [Google Scholar]
- Laan P, Berrevoets MJ, Lythe S, Armstrong W, Blom CWPM. (1989) Root morphology and aerenchyma formation as indicators of the flood-tolerance of Rumex species. J Ecol 77: 693–703 [Google Scholar]
- Lenochová Z, Soukup A, Votrubová O. (2009) Aerenchyma formation in maize roots. Biol Plant 53: 263–270 [Google Scholar]
- Liljeroth E, Bryngelsson T. (2001) DNA fragmentation in cereal roots indicative of programmed root cortical cell death. Physiol Plant 111: 365–372 [DOI] [PubMed] [Google Scholar]
- Lynch JP. (2007) Roots of the second Green Revolution. Aust J Bot 55: 493–512 [Google Scholar]
- Lynch JP, Brown KM. (1998) Regulation of root architecture by phosphorus availability. Lynch JP, Deikman J, , Phosphorus in Plant Biology: Regulatory Roles in Molecular, Cellular, Organismic, and Ecosystem Processes. Current Topics in Plant Physiology. American Society of Plant Physiologists, Rockville, MD, pp 148–156 [Google Scholar]
- Lynch JP, Brown KM. (2001) Topsoil foraging: an architectural adaptation of plants to low phosphorus availability. Plant Soil 237: 225–237 [Google Scholar]
- Lynch JP, Brown KM. (2008) Root strategies for phosphorus acquisition. White PJ, Hammond JP, , The Ecophysiology of Plant-Phosphorus Interactions. Springer, Dordrecht, The Netherlands, pp 83–116 [Google Scholar]
- Lynch JP, Ho MD. (2005) Rhizoeconomics: carbon costs of phosphorus acquisition. Plant Soil 269: 45–56 [Google Scholar]
- Lynch JP, Lauchli A, Epstein E. (1991) Vegetative growth of the common bean in response to phosphorus nutrition. Crop Sci 31: 380–387 [Google Scholar]
- Lynch JP, Nielsen KL, Davis RD, Jablokow AG. (1997) SimRoot: modelling and visualization of root systems. Plant Soil 188: 139–151 [Google Scholar]
- Lynch JP, St Clair SB. (2004) Mineral stress: the missing link in understanding how global climate change will affect plants in real world soils. Field Crops Res 90: 101–115 [Google Scholar]
- Ma Z, Walk TC, Marcus A, Lynch JP. (2001) Morphological synergism in root hair length, density, initiation and geometry for phosphorus acquisition in Arabidopsis thaliana: a modeling approach. Plant Soil 236: 221–235 [Google Scholar]
- Mano Y, Omori F, Takamizo T, Kindiger B, Bird RM, Loaisiga CH. (2006) Variation for root aerenchyma formation in flooded and non-flooded maize and teosinte seedlings. Plant Soil 281: 269–279 [Google Scholar]
- McGrath S, Zhao F. (1995) A risk assessment of sulphur deficiency in cereals using soil and atmospheric deposition data. Soil Use Manage 11: 110–114 [Google Scholar]
- Mickelson SM, Kaeppler SM. (2005) Evaluation of six mycorrhizal isolates for their ability to promote growth of maize genotypes under phosphorus deficiency. Maydica 50: 137–146 [Google Scholar]
- Miguel MA. (2011) Functional role and synergistic effect of root traits for phosphorus acquisition efficiency and their genetic basis in common bean (Phaseolus vulgaris L.). PhD thesis. The Pennsylvania State University, University Park, PA [Google Scholar]
- Nielsen KL, Eshel A, Lynch JP. (2001) The effect of phosphorus availability on the carbon economy of contrasting common bean (Phaseolus vulgaris L.) genotypes. J Exp Bot 355: 329–339 [PubMed] [Google Scholar]
- Picard C, Bosco M. (2006) Heterozygosis drives maize hybrids to select elite 2,4-diacethylphloroglucinol-producing Pseudomonas strains among resident soil populations. FEMS Microbiol Ecol 58: 193–204 [DOI] [PubMed] [Google Scholar]
- Pletch-Rivera LA, Kaeppler SM. (2007) Phosphorus accumulation in maize grain is not influenced by xenia (Zea mays L.). Maydica 52: 151–157 [Google Scholar]
- Postma JA, Jaramillo RE, Lynch JP. (2008) Towards modeling the function of root traits for enhancing water acquisition by crops. Ahuja L, Reddy V, Saseendran S, Yu Q, , Response of Crops to Limited Water: Understanding and Modeling Water Stress Effects on Plant Growth Processes. Advances in Agricultural Systems Modeling. ASA-CSSA-SSSA, Madison, WI, pp 251–276 [Google Scholar]
- Postma JA, Lynch JP. (2010) Theoretical evidence for the functional benefit of root cortical aerenchyma in soils with low phosphorus availability. Ann Bot 107: 829–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards LA. (1931) Capillary conduction of liquids through porous mediums. Physics 1: 318–333 [Google Scholar]
- Robinson D. (1990) Phosphorus availability and cortical senescence in cereal roots. J Theor Biol 145: 257–265 [Google Scholar]
- Rubio G, Zhu JM, Lynch JP. (2003) A critical test of the two prevailing theories of plant response to nutrient availability. Am J Bot 90: 143–152 [DOI] [PubMed] [Google Scholar]
- Schachtman DP, Goodger JQ. (2008) Chemical root to shoot signaling under drought. Trends Plant Sci 13: 281–287 [DOI] [PubMed] [Google Scholar]
- Silva ÁE, Gabelman WH. (1992) Screening maize inbred lines for tolerance to low-P stress condition. Plant Soil 146: 181–187 [Google Scholar]
- Šimunek J, Huang K, van Genuchten MT. (1995) The SWMS 3D Code for Simulating Water Flow and Solute Transport in Three-Dimensional Variably-Saturated Media. Salinity Laboratory, U.S. Department of Agriculture, Riverside, CA [Google Scholar]
- Sinclair TR, Horie T. (1989) Leaf nitrogen, photosynthesis, and crop radiation use efficiency: a review. Crop Sci 29: 90–98 [Google Scholar]
- Somma F, Hopmans JW, Clausnitzer V. (1998) Transient three-dimensional modeling of soil water and solute transport with simultaneous root growth, root water and nutrient uptake. Plant Soil 202: 281–293 [Google Scholar]
- Spitters CJT, Schapendonk AHCM. (1990) Evaluation of breeding strategies for drought tolerance in potato by means of crop growth simulation. Plant Soil 123: 193–203 [Google Scholar]
- Stamp P, Geisler G. (1980) Effect of potassium deficiency on C3 and C4 cereals. J Exp Bot 31: 371–377 [Google Scholar]
- Terry N, Ulrich A. (1973) Effects of potassium deficiency on the photosynthesis and respiration of leaves of sugar beet. Plant Physiol 51: 783–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AL, Guerreiro SMC, Sodek L. (2005) Aerenchyma formation and recovery from hypoxia of the flooded root system of nodulated soybean. Ann Bot (Lond) 96: 1191–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachsel S, Kaeppler SM, Brown KM, Lynch JP. (2010) Shovelomics: high throughput phenotyping of maize (Zea mays L.) root architecture in the field. Plant Soil 341: 75–87 [Google Scholar]
- Uhart SA, Andrade FH. (1995) Nitrogen deficiency in maize. I. Effects on crop growth, development, dry matter partitioning, and kernel set. Crop Sci 35: 1376–1383 [Google Scholar]
- Vos J, Evers JB, Buck-Sorlin GH, Andrieu B, Chelle M, de Visser PHB. (2010) Functional-structural plant modelling: a new versatile tool in crop science. J Exp Bot 61: 2101–2115 [DOI] [PubMed] [Google Scholar]
- Walk TC, Jaramillo R, Lynch JP. (2006) Architectural tradeoffs between adventitious and basal roots for phosphorus acquisition. Plant Soil 279: 347–366 [Google Scholar]
- Yang HS, Janssen BH. (2000) A mono-component model of carbon mineralization with a dynamic rate constant. Eur J Soil Sci 51: 517–529 [Google Scholar]
- Zhao D, Oosterhuis D, Bednarz C. (2001) Influence of potassium deficiency on photosynthesis, chlorophyll content, and chloroplast ultrastructure of cotton plants. Photosynthetica 39: 103–109 [Google Scholar]
- Zhu J, Brown KM, Lynch JP. (2010) Root cortical aerenchyma improves the drought tolerance of maize (Zea mays L.). Plant Cell Environ 33: 740–749 [DOI] [PubMed] [Google Scholar]
- Zhu J, Kaeppler SM, Lynch JP. (2005) Topsoil foraging and phosphorus acquisition efficiency in maize (Zea mays L.). Funct Plant Biol 32: 749–762 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.