Abstract
With plant molecular biology entering the omics era, there is a need for simple cloning strategies that allow high throughput to systematically study the expression and function of large numbers of genes. Such strategies would facilitate the analysis of gene (sub)families and/or sets of coexpressed genes identified by transcriptomics. Here, we provide a set of 34 ligation-independent cloning (LIC) binary vectors for expression analysis, protein localization studies, and misexpression that will be made freely available. This set of plant LIC vectors offers a fast alternative to standard cloning strategies involving ligase or recombination enzyme technology. We demonstrate the use of this strategy and our new vectors by analyzing the expression domains of genes belonging to two subclades of the basic helix-loop-helix transcription factor family. We show that neither the closest homologs of TARGET OF MONOPTEROS7 (TMO7/ATBS1) nor the members of the ATBS1 INTERACTING FACTOR subclade of putative TMO7 interactors are expressed in the embryo and that there is very limited coexpression in the primary root meristem. This suggests that these basic helix-loop-helix transcription factors are most likely not involved in TMO7-dependent root meristem initiation.
Whole-genome analysis is becoming a standard analysis tool in reverse genetics plant research. Furthermore, there is often the need to study large gene families in Arabidopsis (Arabidopsis thaliana) due to redundancy. For these and other reasons, there is an increasing need in plant research for fast cloning strategies. Besides speed, these methods have to be characterized by easy handling in order to, for example, verify protein localizations with moderately high throughput. Unfortunately, most currently available cloning methods are not able to combine these characteristics. Current cloning procedures are either laborious and slow (such as classical cloning) or quick but expensive (such as the Gateway technology; Curtis and Grossniklaus, 2003; Karimi et al., 2007). Other, more recent advances, such as BAC recombineering (Zhou et al., 2011), while allowing precision cloning, have a clear disadvantage in that they introduce not only a gene of interest, but a complete genomic region. An emerging single-step method that is very suitable for moderate high-throughput cloning is ligation-independent cloning (LIC; Li and Elledge, 2007; Eschenfeldt et al., 2009). The LIC cloning system is characterized by a few simple steps, including linearization of the vector, amplification of the fragment of interest, the creation of sticky ends on the vector and insert by the 3′-5′ exonuclease activity of T4 DNA polymerase, and subsequent annealing of the fragment into the vector (Fig. 1). A related type of LIC cloning has been described, facilitating the assembly of multiple fragments in one reaction, called sequence and ligation-independent cloning, using in vitro homologous recombination and single-strand annealing (Li and Elledge, 2007). For most projects, however, single-purpose LIC cloning is sufficient.
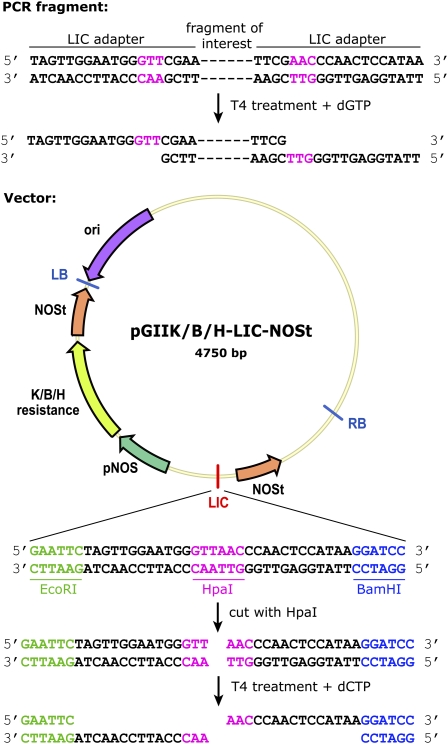
Figure 1.
LIC cloning procedure with modified LIC site. Vectors are first digested with HpaI restriction enzyme, and fragments are amplified by PCR with primers containing the LIC adapter sites. Overhangs are made by the 3′-5′ exonuclease activity of T4 DNA polymerase in excess of dCTP or dGTP for the vector and fragment, respectively. The sticky end overhangs that are created allow for easy annealing of vector and insert. LB, Left border; ori, origin of replication; RB, right border. [See online article for color version of this figure.]
Despite its potential to become a good alternative for current cloning strategies, LIC cloning has not been readily used in plant research so far, perhaps due to the absence of a comprehensive set of vectors. Over the years, only a small number of LIC-based vectors have been made available for protein production and purification (Doyle, 2005; Bardóczy et al., 2008), in planta expression (Oh et al., 2010), and construction of hairpin constructs (Hauge et al., 2009; Xu et al., 2010). Although these vectors are very useful for these purposes, a comprehensive collection of LIC-based vectors in plant research was missing.
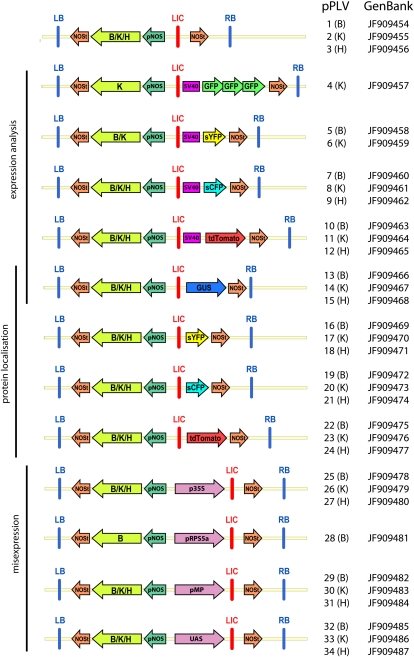
Here, we describe the creation of a multipurpose set of 34 LIC-compatible plant LIC vectors (pPLVs; Fig. 3; Table I) for expression analysis, protein localization studies, and various misexpression analyses in Arabidopsis and other plant species.
Figure 3.
Overview of LIC vectors. Indicated are left and right border (LB and RB), NOS promoter and terminator (pNOS and NOSt), resistance genes (B/K/H), nuclear localization signal (SV40), the LIC site (LIC), specific promoters for misexpression (p35S, pRPS5a, pMP, and upstream activating sequence), and respective fluorescent proteins (GFP, sCFP, sYFP, and tandemTomato). [See online article for color version of this figure.]
Table I. Overview of LIC-compatible vectors.
Respective use, names, antibiotic resistances, remarks, size in base pairs, and required LIC adapter sites for forward and reverse primers used to amplify the required fragment are indicated. ppt, Phosphinothricin; tdTomato, tandemTomato.
| Use | pPLV | Vector | Antibiotic Resistance | Adapter Forward Primer 5′-3′ | Adapter Reverse Primer 5′-3′ |
| Basic vector for custom use | pPLV01 | pGIIB-LIC-NOSt | Basta/ppt | – | – |
| pPLV02 | pGIIK-LIC-NOSt | Kanamycin | – | – | |
| pPLV03 | pGIIH-LIC-NOSt | Hygromycin | – | – | |
| Promoter analysis | pPLV04 | pGIIK-LIC-SV40-3xGFP-NOSt | Kanamycin | TAGTTGGAATGGGTTCGAA | TTATGGAGTTGGGTTCGAA |
| pPLV05 | pGIIB-LIC-SV40-sYFP-NOSt | Basta/ppt | TAGTTGGAATGGGTTCGAA | TTATGGAGTTGGGTTCGAA | |
| pPLV06 | pGIIK-LIC-SV40-sYFP-NOSt | Kanamycin | TAGTTGGAATGGGTTCGAA | TTATGGAGTTGGGTTCGAA | |
| pPLV07 | pGIIB-LIC-SV40-sCFP-NOSt | Basta/ppt | TAGTTGGAATGGGTTCGAA | TTATGGAGTTGGGTTCGAA | |
| pPLV08 | pGIIK-LIC-SV40-sCFP-NOSt | Kanamycin | TAGTTGGAATGGGTTCGAA | TTATGGAGTTGGGTTCGAA | |
| pPLV09 | pGIIH-LIC-SV40-sCFP-NOSt | Hygromycin | TAGTTGGAATGGGTTCGAA | TTATGGAGTTGGGTTCGAA | |
| pPLV10 | pGIIB-LIC-SV40-tdTomato-NOSt | Basta/ppt | TAGTTGGAATGGGTTCGAA | TTATGGAGTTGGGTTCGAA | |
| pPLV11 | pGIIK-LIC-SV40-tdTomato-NOSt | Kanamycin | TAGTTGGAATGGGTTCGAA | TTATGGAGTTGGGTTCGAA | |
| pPLV12 | pGIIH-LIC-SV40-tdTomato-NOSt | Hygromycin | TAGTTGGAATGGGTTCGAA | TTATGGAGTTGGGTTCGAA | |
| pPLV13 | pGIIB-LIC-GUS-NOSt | Basta/ppt | TAGTTGGAATGGGTTCGAA | TTATGGAGTTGGGTTCGAA | |
| pPLV14 | pGIIK-LIC-GUS-NOSt | Kanamycin | TAGTTGGAATGGGTTCGAA | TTATGGAGTTGGGTTCGAA | |
| pPLV15 | pGIIH-LIC-GUS-NOSt | Hygromycin | TAGTTGGAATGGGTTCGAA | TTATGGAGTTGGGTTCGAA | |
| Protein localization | pPLV16 | pGIIB-LIC-sYFP-NOSt | Basta/ppt | TAGTTGGAATGGGTTCGAA | TTATGGAGTTGGGTTCGAAC |
| pPLV17 | pGIIK-LIC-sYFP-NOSt | Kanamycin | TAGTTGGAATGGGTTCGAA | TTATGGAGTTGGGTTCGAAC | |
| pPLV18 | pGIIH-LIC-sYFP-NOSt | Hygromycin | TAGTTGGAATGGGTTCGAA | TTATGGAGTTGGGTTCGAAC | |
| pPLV19 | pGIIB-LIC-sCFP-NOSt | Basta/ppt | TAGTTGGAATGGGTTCGAA | TTATGGAGTTGGGTTCGAAC | |
| pPLV20 | pGIIK-LIC-sCFP-NOSt | Kanamycin | TAGTTGGAATGGGTTCGAA | TTATGGAGTTGGGTTCGAAC | |
| pPLV21 | pGIIH-LIC-sCFP-NOSt | Hygromycin | TAGTTGGAATGGGTTCGAA | TTATGGAGTTGGGTTCGAAC | |
| pPLV22 | pGIIB-LIC-tdTomato-NOSt | Basta/ppt | TAGTTGGAATGGGTTCGAA | TTATGGAGTTGGGTTCGAAC | |
| pPLV23 | pGIIK-LIC-tdTomato-NOSt | Kanamycin | TAGTTGGAATGGGTTCGAA | TTATGGAGTTGGGTTCGAAC | |
| pPLV24 | pGIIH-LIC-tdTomato-NOSt | Hygromycin | TAGTTGGAATGGGTTCGAA | TTATGGAGTTGGGTTCGAAC | |
| pPLV13 | pGIIB-LIC-GUS-NOSt | Basta/ppt | TAGTTGGAATGGGTTCGAA | TTATGGAGTTGGGTTCGAAC | |
| pPLV14 | pGIIK-LIC-GUS-NOSt | Kanamycin | TAGTTGGAATGGGTTCGAA | TTATGGAGTTGGGTTCGAAC | |
| pPLV15 | pGIIH-LIC-GUS-NOSt | Hygromycin | TAGTTGGAATGGGTTCGAA | TTATGGAGTTGGGTTCGAAC | |
| Misexpression | pPLV25 | pGIIB-p35S-LIC-NOSt | Basta/ppt | TAGTTGGAATAGGTTC | AGTATGGAGTTGGGTTC |
| pPLV26 | pGIIK-p35S-LIC-NOSt | Kanamycin | TAGTTGGAATAGGTTC | AGTATGGAGTTGGGTTC | |
| pPLV27 | pGIIH-p35S-LIC-NOSt | Hygromycin | TAGTTGGAATAGGTTC | AGTATGGAGTTGGGTTC | |
| pPLV28 | pGIIB-pRPS5a-LIC-NOSt | Basta/ppt | TAGTTGGAATAGGTTC | AGTATGGAGTTGGGTTC | |
| pPLV29 | pGIIB-pMP-LIC-NOSt | Basta/ppt | TAGTTGGAATAGGTTC | AGTATGGAGTTGGGTTC | |
| pPLV30 | pGIIK-pMP-LIC-NOSt | Kanamycin | TAGTTGGAATAGGTTC | AGTATGGAGTTGGGTTC | |
| pPLV31 | pGIIH-pMP-LIC-NOSt | Hygromycin | TAGTTGGAATAGGTTC | AGTATGGAGTTGGGTTC | |
| pPLV32 | pGIIB-UAS-LIC-NOSt | Basta/ppt | TAGTTGGAATGGGTTCGAA | TTATGGAGTTGGGTTCGAAC | |
| pPLV33 | pGIIK-UAS-LIC-NOSt | Kanamycin | TAGTTGGAATGGGTTCGAA | TTATGGAGTTGGGTTCGAAC | |
| pPLV34 | pGIIH-UAS-LIC-NOSt | Hygromycin | TAGTTGGAATGGGTTCGAA | TTATGGAGTTGGGTTCGAAC |
RESULTS
Generating a Series of Vectors for LIC
We generated a versatile set of LIC vectors for general use in plant molecular biology. These include vectors for expression analysis of promoter fragments, protein localization studies, misexpression (using several different promoters), as well as standard empty vectors to generate custom LIC vectors for other purposes (Table I; Fig. 1). A range of fluorescent proteins, triple GFP, super CYAN FLUORESCENT PROTEIN (sCFP), super YELLOW FLUORESCENT PROTEIN (sYFP), and tandemTomato were selected and used due to their higher quantum yields compared to the original fluorescent proteins (Shaner et al., 2004; Kremers et al., 2006). Furthermore, the pGIIK/B/H-LIC-sYFP-NOSt and pGIIK/B/H-LIC-sCFP-NOSt vectors can be used for Förster resonance energy transfer as measured by fluorescence lifetime imaging analyses to detect protein-protein interactions in plants. Almost all of the vectors are available with different antibiotic resistances, increasing the versatility of this set of vectors through combinatorial use of multiple transgenes. All constructed vectors are based on a binary pGreenII (pGII) vector backbone with kanamycin (K), phosphinothricin/basta (B), or hygromycin (H) resistance (Hellens et al., 2000), in which a custom LIC site was introduced using EcoRI and BamHI restriction sites (Fig. 1).
The pGIIK/B/H-LIC-NOSt vectors served as the base for the LIC vectors that were generated, except for the pPLV04 vector, which was modified from a previously described pGIIB-SV40-3GFP-NOSt vector (Takada and Jürgens, 2007). The custom LIC site that was introduced contains a unique HpaI restriction site, which is used for linearizing vectors (Fig. 1). The vectors used for expression analysis and protein localization were created by introducing the respective fluorescent protein or GUS fragment in the BamHI restriction site at the 3′ flank of the LIC site. In protein localization vectors, the resulting linker between the genomic fragment (without stop codon) and the fluorescent protein is illustrated in Figure 2. The forward primer used to amplify the fluorescent proteins also created a SpeI restriction site, which was used to introduce the SV40 nuclear localization signal in the vectors used for expression analysis of promoter fragments. To allow cloning of the tandemTomato vectors, a remnant 424-bp Lac promoter fragment, which caused a reverse reading frame in combination with the tandem Tomato, was removed. This Lac promoter fragment at the 3′ end of the construct was removed by cutting with NotI and StuI restriction enzymes and reintroducing an MluI restriction site. Standard analyses of protein localization are done using the endogenous promoter by cloning the full genomic fragment in the protein localization vectors. This has the advantage of capturing all potential regulatory sequences both in the promoter region and in intronic regions. Nevertheless, these vectors could also be easily adapted to drive expression from different promoters by inserting these into the multiple cloning site upstream of the LIC site by standard cloning strategies or by cloning a chimeric construct (created using standard overlap extension PCR) into the existing vectors. For the misexpression vectors, the p35S and pRPS5a promoters (Odell et al., 1985; Weijers et al., 2001) were introduced using KpnI and ApaI restriction enzymes. The pRPS5a promoter fragment contained an HpaI restriction site, which had to be mutated in order to enable linearization of the vector. For this, we mutated the GTTAAC sequence of the HpaI site into GTAAAC using site-directed mutagenesis (Sawano and Miyawaki, 2000) without affecting the promoter activity. The pMP promoter (Schlereth et al., 2010) was introduced using KpnI and XhoI restriction enzymes, and the GAL4-dependent pUAS (upstream activating sequence; West et al., 1984; Weijers et al., 2003) fragment was cloned using PstI and BamHI restriction sites. An overview of the set of LIC vectors is given in Figure 3 and Table I.
Figure 2.
The linker introduced by LIC cloning. As an example, the linker is shown between a genomic fusion without stop codon and a fluorescent protein of choice (sYFP) by LIC cloning. AA, Amino acid. [See online article for color version of this figure.]
The overall efficiency of the LIC-based vector cloning described here is very high, although it is important to note that, in our hands, LIC cloning works best when high concentrations (about 1.5 μg for one T4 treatment) of very pure vector and PCR fragment are used for the T4 treatment. Although there can be a large variability in the number of colonies after transformation, the rate of positive colonies is usually high (between 60% and 100%). Due to the reduced stability of the triple GFP construct in Escherichia coli, cloning with the pPLV04 vector as well as cloning larger fragments (>5 kb) into any of the vectors can be less efficient. Nonetheless, we routinely clone fragments of up to 5 kb in several of these LIC vectors.
Examples of Constructs Generated by LIC
To provide an example of the speed and efficiency of our LIC-based vectors, we analyzed the promoter expression domains of TARGET OF MONOPTEROS7 (TMO7/ATBS1; Wang et al., 2009; Schlereth et al., 2010) and its three closest homologs, namely, PRE1/BNQ1, PRE2/BNQ2, and PRE4/BNQ3 (Lee et al., 2006; Mara et al., 2010).
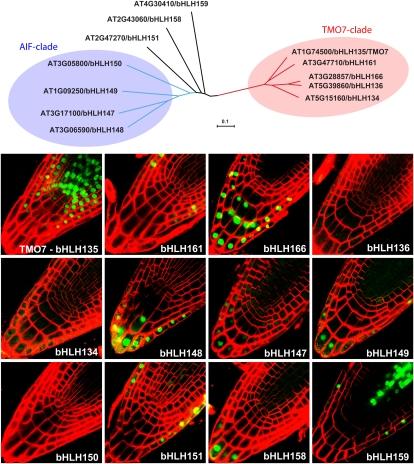
TMO7 was first identified as Activation-Tagged Bri1-Suppressor1 (ATBS1) in a screen for genes that could rescue the dwarfed bri1 phenotype when overexpressed (Wang et al., 2009). More recently, TMO7/ATBS1 (from now on referred to as TMO7) was found to be a direct target of MP and is required during embryogenesis (Schlereth et al., 2010). Specifically, TMO7 was shown to move from the proembryo toward the uppermost suspensor cell to specify this cell as hypophysis, which is required for establishing the primary root meristem (Schlereth et al., 2010). Although RNA interference suppression of TMO7 led to embryo defects and a low rate of rootless seedlings (Schlereth et al., 2010), it is still possible that other closely related genes act redundantly with TMO7. As a first step in addressing this issue, we tested the expression of TMO7 and its four closest homologs by fusing their promoters to SV40-3xGFP in the pPLV4 vector (Fig. 3). None of the TMO7 homologs showed any expression during embryo development (data not shown); therefore, we analyzed the expression patterns in the postembryonic root. Consistent with its role in the establishment of the primary root meristem, TMO7 was strongly expressed in the quiescent center and surrounding proximal stem cells, but absent from the columella root cap cells (Fig. 4). The TMO7 homolog bHLH161/PRE4/BNQ3 only expressed in the lateral root cap, while bHLH166, bHLH136/PRE1/BNQ1, and bHLH134/PRE2/BNQ2 are weakly expressed in the root cap and columella cells (Fig. 4). Hence, it appears that none of the TMO7 relatives shows a strong coexpression with TMO7 during embryogenesis or in the primary root meristem. In the mature root, however, there is an overlap in expression domains for TMO7, bHLH161, and bHLH166, but not for bHLH136 or bHLH134 (Supplemental Fig. S1).
Figure 4.
Overview of promoter expression patterns of a subclade of TMO7-related bHLH transcription factors using the pGIIK-LIC-VP40-3GFP-NOSt vector. The phylogenetic tree shows a subclade of the bHLH transcription factors based on full-length protein sequences. Branch lengths indicate phylogenetic distances (see scale bar: fraction of deviations). Confocal images of primary root meristems were counterstained using FM4-64 dye (red). [See online article for color version of this figure.]
In a yeast two-hybrid screen, four closely related basic helix-loop-helix (bHLH) transcription factors were shown to be able to interact with TMO7 and were named ATBS1 interacting factors (AIF1-4; Wang et al., 2009). Although TMO7 was shown to interact with AIF1-4 in vitro and in vivo (in seedlings overexpressing both AIF1 and TMO7), it is not clear whether these genes are actually expressed in the same tissues and, thus, if their interaction is biologically meaningful.
To address this question, we analyzed the expression of the four AIF1-4 genes as well as that of the related bHLH151/UPB (Tsukagoshi et al., 2010), bHLH158, and bHLH159 genes. Again, none of these genes appears to be expressed during embryo development (data not shown). In the primary root meristem, bHLH150/AIF1 expression cannot be detected, while bHLH148/AIF2, bHLH147/AIF3, and bHLH149/AIF4 are all expressed in the root cap and lower columella cells (Fig. 4). A similar expression pattern was observed for bHLH158, while bHLH159 is expressed in the lateral root cap and vascular tissues. Similar to published data (Tsukagoshi et al., 2010), bHLH151/UPB is expressed in the lateral root cap and in the vascular tissues (Fig. 4). In the mature root, bHLH149/AIF4, bHLH150/AIF1, bHLH151/UPB, and bHLH159 are expressed in all cell types, while bHLH148/AIF2 and bHLH147/AIF3 appear to be more specific for vascular tissues (Supplemental Fig. S1).
Additionally, we analyzed the protein localization (pPLV16 or pGIIB-LIC-sYFP-tNOS vector) for some of these bHLH transcription factors to support the expression domains found using the pPLV04 (pGIIK-LIC-SV40-3GFP-tNOS) vector (Supplemental Fig. S2). All analyzed protein localization domains fully overlapped with the promoter expression domains and with available data for bHLH151/UPB (Tsukagoshi et al., 2010), supporting the validity of this set of vectors.
DISCUSSION
We created a set of LIC-compatible vectors for diverse purposes in plant molecular biology research, including expression analysis of promoter fragments, protein localization studies, and misexpression. LIC cloning allows for a single-step way to clone in a moderately high-throughput fashion. Despite the obvious advantages of LIC cloning, there are some drawbacks using this system. The most obvious downside is that every fragment needs to be sequenced when used in different vectors, which is not the case for Gateway cloning. This highlights the need for a high-quality proofreading polymerase enzyme for amplification. In our hands, however, we only very rarely encounter erroneous base pairs (e.g. one mistake in 10 to 20 kb sequenced; in line with the error rate of the proofreading polymerase used). Another disadvantage is that cloned fragments cannot be recombined as with Gateway cloning. However, in our experience, this is not a problem for most purposes since the majority of cloning reactions are single-purpose projects.
The set of vectors presented here allowed us to misexpress all members of a gene family of over 20 members from four different promoters and to determine/validate transcription patterns for up to 150 genes identified in microarray experiments (data will be published elsewhere). Here, as an example, we show the use of one of our LIC-compatible vectors to examine the promoter expression domains of the TMO7 subclade of bHLH transcription factors and its putative AIF interactors. Interestingly, none of the examined homologs showed expression or overlap in expression with TMO7 in the embryo or in the hypophysis descendants in the primary root meristem, further supporting the proposed single gene function for TMO7 in root meristem establishment (Schlereth et al., 2010). Furthermore, there is no expression of the putative AIF1-4 interactors in the embryo nor is there a coexpression with the TMO7 expression domain in the primary root meristem. Notably, bHLH150/AIF1 does not appear to be expressed in the embryo nor the root meristem, precluding the possibility of an interaction with TMO7 in these tissues. In conclusion, our analysis has shown that none of the TMO7 homologs or its putative interactors is likely to be involved in the process of TMO7-dependent root meristem initiation.
In the primary root, however, several of the analyzed bHLH transcription factors have overlapping expression domains with TMO7, allowing potential interactions in these tissues. Furthermore, as the TMO7 protein was shown to move from the proembryo to the future hypophysis during embryonic root development (Schlereth et al., 2010), we cannot exclude a similar movement to the columella cells in the primary root meristem. Therefore, an overlap in the protein expression domains (and potential for interaction) between TMO7 and bHLH148/AIF2, bHLH147/AIF3, and bHLH149/AIF4 in the columella region remains possible. In any case, further research is required to investigate the biological significance of these interactions. Furthermore, it is important to note that the expression domains of previously published genes, such as bHLH151/UPB (Tsukagoshi et al., 2010), are identical to what we have found using our LIC-compatible vectors, supporting the quality of our set of vectors.
In conclusion, we believe that this set of LIC-compatible vectors will provide a useful resource for researchers in plant biology that depend highly on cloning of large numbers of constructs. Therefore, the availability of this quick and versatile cloning system may aid progress in the current omics era.
MATERIALS AND METHODS
The pPLV vectors are all available from the Nottingham Arabidopsis Stock Centre for distribution. The sequences of all vectors have been added to the article as a supplemental text file (Supplemental Data Set S1) and are accessible at GenBank (http://www.ncbi.nlm.nih.gov/genbank/). Accession numbers are provided in Figure 3.
To introduce a fragment of interest into a LIC-based vector, extensions are added to the fragment during amplification by PCR using primers with LIC adapter sites (Table I). This LIC-compatible fragment is next treated with T4 DNA polymerase and an excess of dGTP. The 3′-5′ exonuclease activity of the T4 DNA polymerase creates 15-bp single-strand overhangs (Fig. 1). LIC vectors are prepared by linearizing using a unique restriction site in the LIC site of the vector. Subsequent T4 DNA polymerase treatment with excess of dCTP then creates 15-bp single-strand overhangs, complementary to those available on the T4-treated fragment (Fig. 1). Vector and insert are then combined, allowed to anneal, and transformed into a bacterial host, which will repair the introduced nicks. The sequence of the LIC site itself ensures correct orientation of the inserted fragment. A detailed protocol can be found below in this section and a quick lab protocol in the supplemental data online.
Preparation of Vectors
For a standard preparation, 2 to 4 μg of vector is cut with 1 μL HpaI fast cut restriction enzyme (Fermentas) in duplicate for 2 h at 37°C. Linearized vector is next purified from agarose gel using the QIAEXII gel extraction kit (Qiagen), and duplicates are pooled. Linearized vectors are then precipitated overnight (or minimum 2 h) using 0.5 volumes ammonium acetate (7.5 m) and 2.5 volumes of 100% ethanol at −20°C. The precipitated vector is pelleted by centrifugation for 30 min at maximum speed. The supernatant is removed, and the pellet is washed with 100 μL of 70% ethanol followed by a 100% ethanol wash. The pellet is next dried and resuspended in 50 μL of water (at 50°C for 5 to 10 min). For T4 treatment (New England Biolabs), 200 to 400 ng of linearized vector, 4 μL 10× T4 buffer, 4 μL 100 mm dCTP, 2 μL 100 mm dithiothreitol, 0.4 μL bovine serum albumin, 0.8 μL T4 DNA polymerase (New England Biolabs), and water to 40 μL total volume are mixed. The mixture is centrifuged at maximum speed for 1 min, incubated at 22°C for at least 30 min (up to 2 h), inactivated at 75°C for 20 min, and centrifuged again at maximum speed for 1 min. T4 treated vectors can be stored at 4°C until further use.
Preparation of Fragments
The DNA fragment of choice is first amplified by PCR using primers with respective LIC adapter sites (dependent on destination vector; Table I). PCR is performed in 50-μL volume in duplicate using Phusion Flash polymerase (Finnzymes; or another high-quality polymerase enzyme with proofreading) using the amplification protocol provided by the supplier. Fragments are next purified from agarose gel using the QIAEXII gel extraction kit, and duplicates are pooled. For T4 treatment (New England Biolabs), 200 to 400 ng of purified fragment, 2 μL 10× T4 buffer, 2 μL 100 mm dGTP, 1 μL 100 mm dithiothreitol, 0.2 μL bovine serum albumin, 0.4 μL T4 DNA polymerase (New England Biolabs), and water to 20 μL total volume are mixed. The mixture is centrifuged at maximum speed for 1 min, incubated at 22°C for at least 30 min (up to 2 h), inactivated at 75°C for 20 min, and centrifuged again at maximum speed for 1 min. T4 treated fragments can be stored at 4°C until further use.
Annealing, Transformation in Escherichia coli, and Sequence Verification
To anneal the linearized, T4-treated vector and the T4-treated PCR fragment, 10 to 40 ng vector and insert are combined in a 1:3 mratio for 30 min to 2 h at 22°C (usually about 1 to 3 μL each) or overnight at 4°C. The whole mixture is then transformed into electrocompetent DH5α E. coli cells (transformation efficiency >107 colony forming units/μg), plated on Luria-Bertani (LB)-agar plates with 25 mg/L kanamycin as antibiotic (Table I), and incubated at 37°C overnight. A T4-treated vector without added insert can be used to analyze the amount of background colonies. The next day, colonies are verified for inserts using colony PCR and positives grown overnight in 6 mL LB with 25 mg/L kanamycin. Plasmids are extracted (GeneJET plasmid mini prep kit from Fermentas) and checked by restriction digest before sequencing. Because of the proofreading DNA polymerase, point mutations are very uncommon.
A Simplified Plant Transformation Procedure Allowing Moderate Throughput
Plasmids are transformed into electrocompetent Agrobacterium tumefaciens GV3101 containing the pGreen helper plasmid pSOUP (Hellens et al., 2000) using standard protocols and plated on LB plates with the appropriate antibiotics (Table I). Following 2 d of growth at 28°C, a smear of multiple colonies is inoculated into 20 mL of liquid LB medium with the appropriate antibiotics and grown overnight at 28°C in a shaker. The next day, the volume of the culture is increased to 50 mL LB with antibiotics and grown, again at 28°C, to an OD600 of around 0.7 (0.5 to 0.9 is acceptable). If the optical density is too high, the cultures can be diluted to the correct OD600 using LB. Next, 2.5 g Suc and 10 to 20 μL Silwet is added to 50 mL of culture and shaken until the Suc is dissolved. Five to ten plants are then floral dipped in this mixture, placed in a box, and covered with cling film for 1 d before growing them in the growth room until seeds can be harvested.
Plant Growth and Selection
Plants (Columbia-0 ecotype) were grown under standard conditions at 23°C in a 16-h-light/8-h-dark cycle. Selection for transgenes was performed on solid Murashige and Skoog medium supplemented with 25 mg/L kanamycin (pPLV04) or 15 mg/L phosphinothricin (pPLV16).
Microscopy
Gene expression or protein accumulation was analyzed in roots of homozygous T3 lines carrying a single T-DNA insert as determined by segregation of kanamycin or phosphinothricin resistance. Four- to five-day-old vertically grown seedlings were incubated in water containing 1 μm FM4-64 (Invitrogen) for 1 min and subsequently imaged on a Zeiss LSM510 confocal laser scanning microscope.
All vector sequences have been deposited at GenBank and can be found using the following accession numbers: JF909454 (pPLV01), JF909455 (pPLV02), JF909456 (pPLV03), JF909457 (pPLV04), JF909458 (pPLV05), JF909459 (pPLV06), JF909460 (pPLV07), JF909461 (pPLV08), JF909462 (pPLV09), JF909463 (pPLV10), JF909464 (pPLV11), JF909465 (pPLV12), JF909466 (pPLV13), JF909467 (pPLV14), JF909468 (pPLV15), JF909469 (pPLV16), JF909470 (pPLV17), JF909471 (pPLV18), JF909472 (pPLV19), JF909473 (pPLV20), JF909474 (pPLV21), JF909475 (pPLV22), JF909476 (pPLV23), JF909477 (pPLV24), JF909478 (pPLV25), JF909479 (pPLV26), JF909480 (pPLV27), JF909481 (pPLV28), JF909482 (pPLV29), JF909483 (pPLV30), JF909484 (pPLV31), JF909485 (pPLV32), JF909486 (pPLV33), and JF909487 (pPLV34).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Overview of promoter expression patterns of a subclade of TMO7-related bHLH transcription factors using the pGIIK-LIC-VP40-3GFP-NOSt vector.
Supplemental Figure S2. Comparison between promoter expression patterns (pGIIK-LIC-VP40-3GFP-NOSt vector) and protein localization patterns (pGIIB-LIC-sYFP-NOSt vector).
Supplemental Data Set S1. Sequences of pPLV vectors in FASTA format.
Supplementary Material
References
- Bardóczy V, Géczi V, Sawasaki T, Endo Y, Mészáros T. (2008) A set of ligation-independent in vitro translation vectors for eukaryotic protein production. BMC Biotechnol 8: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MD, Grossniklaus U. (2003) A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol 133: 462–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle SA. (2005) High-throughput cloning for proteomics research. Methods Mol Biol 310: 107–113 [DOI] [PubMed] [Google Scholar]
- Eschenfeldt WH, Lucy S, Millard CS, Joachimiak A, Mark ID. (2009) A family of LIC vectors for high-throughput cloning and purification of proteins. Methods Mol Biol 498: 105–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauge B, Oggero C, Nguyen N, Fu C, Dong F. (2009) Single tube, high throughput cloning of inverted repeat constructs for double-stranded RNA expression. PLoS ONE 4: e7205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellens RP, Edwards EA, Leyland NR, Bean S, Mullineaux PM. (2000) pGreen: a versatile and flexible binary Ti vector for Agrobacterium-mediated plant transformation. Plant Mol Biol 42: 819–832 [DOI] [PubMed] [Google Scholar]
- Karimi M, Bleys A, Vanderhaeghen R, Hilson P. (2007) Building blocks for plant gene assembly. Plant Physiol 145: 1183–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremers GJ, Goedhart J, van Munster EB, Gadella TW., Jr (2006) Cyan and yellow super fluorescent proteins with improved brightness, protein folding, and FRET Förster radius. Biochemistry 45: 6570–6580 [DOI] [PubMed] [Google Scholar]
- Lee S, Lee S, Yang KY, Kim YM, Park SY, Kim SY, Soh MS. (2006) Overexpression of PRE1 and its homologous genes activates Gibberellin-dependent responses in Arabidopsis thaliana. Plant Cell Physiol 47: 591–600 [DOI] [PubMed] [Google Scholar]
- Li MZ, Elledge SJ. (2007) Harnessing homologous recombination in vitro to generate recombinant DNA via SLIC. Nat Methods 4: 251–256 [DOI] [PubMed] [Google Scholar]
- Mara CD, Huang T, Irish VF. (2010) The Arabidopsis floral homeotic proteins APETALA3 and PISTILLATA negatively regulate the BANQUO genes implicated in light signaling. Plant Cell 22: 690–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odell JT, Nagy F, Chua NH. (1985) Identification of DNA sequences required for activity of the cauliflower mosaic virus 35S promoter. Nature 313: 810–812 [DOI] [PubMed] [Google Scholar]
- Oh SK, Kim SB, Yeom SI, Lee HA, Choi D. (2010) Positive-selection and ligation-independent cloning vectors for large scale in planta expression for plant functional genomics. Mol Cells 30: 557–562 [DOI] [PubMed] [Google Scholar]
- Sawano A, Miyawaki A. (2000) Directed evolution of green fluorescent protein by a new versatile PCR strategy for site-directed and semi-random mutagenesis. Nucleic Acids Res 28: E78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlereth A, Möller B, Liu W, Kientz M, Flipse J, Rademacher EH, Schmid M, Jürgens G, Weijers D. (2010) MONOPTEROS controls embryonic root initiation by regulating a mobile transcription factor. Nature 464: 913–916 [DOI] [PubMed] [Google Scholar]
- Shaner NC, Campbell RE, Steinbach PA, Giepmans BN, Palmer AE, Tsien RY. (2004) Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol 22: 1567–1572 [DOI] [PubMed] [Google Scholar]
- Takada S, Jürgens G. (2007) Transcriptional regulation of epidermal cell fate in the Arabidopsis embryo. Development 134: 1141–1150 [DOI] [PubMed] [Google Scholar]
- Tsukagoshi H, Busch W, Benfey PN. (2010) Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell 143: 606–616 [DOI] [PubMed] [Google Scholar]
- Wang H, Zhu Y, Fujioka S, Asami T, Li J, Li J. (2009) Regulation of Arabidopsis brassinosteroid signaling by atypical basic helix-loop-helix proteins. Plant Cell 21: 3781–3791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weijers D, Franke-van Dijk M, Vencken RJ, Quint A, Hooykaas P, Offringa R. (2001) An Arabidopsis Minute-like phenotype caused by a semi-dominant mutation in a RIBOSOMAL PROTEIN S5 gene. Development 128: 4289–4299 [DOI] [PubMed] [Google Scholar]
- Weijers D, Van Hamburg JP, Van Rijn E, Hooykaas PJ, Offringa R. (2003) Diphtheria toxin-mediated cell ablation reveals interregional communication during Arabidopsis seed development. Plant Physiol 133: 1882–1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West RW, Jr, Yocum RR, Ptashne M. (1984) Saccharomyces cerevisiae GAL1-GAL10 divergent promoter region: location and function of the upstream activating sequence UASG. Mol Cell Biol 4: 2467–2478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G, Sui N, Tang Y, Xie K, Lai Y, Liu Y. (2010) One-step, zero-background ligation-independent cloning intron-containing hairpin RNA constructs for RNAi in plants. New Phytol 187: 240–250 [DOI] [PubMed] [Google Scholar]
- Zhou R, Benavente LM, Stepanova AN, Alonso JM. (2011) A recombineering-based gene tagging system for Arabidopsis. Plant J 66: 712–723 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.