Abstract
We identified a gene responsible for tolerance to boron (B) toxicity in rice (Oryza sativa), named BORON EXCESS TOLERANT1. Using recombinant inbred lines derived from the B-toxicity-sensitive indica-ecotype cultivar IR36 and the tolerant japonica-ecotype cultivar Nekken 1, the region responsible for tolerance to B toxicity was narrowed to 49 kb on chromosome 4. Eight genes are annotated in this region. The DNA sequence in this region was compared between the B-toxicity-sensitive japonica cultivar Wataribune and the B-toxicity-tolerant japonica cultivar Nipponbare by eco-TILLING analysis and revealed a one-base insertion mutation in the open reading frame sequence of the gene Os04g0477300. The gene encodes a NAC (NAM, ATAF, and CUC)-like transcription factor and the function of the transcript is abolished in B-toxicity-tolerant cultivars. Transgenic plants in which the expression of Os04g0477300 is abolished by RNA interference gain tolerance to B toxicity.
Boron (B) is an essential micronutrient for higher plants, but excess amounts of B inhibit growth (B toxicity). As the optimal range of B concentrations in tissues is narrow (Blamey et al., 1997), B toxicity occurs in many plants at levels only slightly above that required for normal growth (Mengel and Kirkby, 2001). The mechanism of B toxicity is not yet fully clarified, but it is likely that boric acid binds spontaneously with diol groups in the cis-position (Power and Woods, 1997), and thus these cis-diol compounds, such as RNA, NADH, and ATP, might be the sites of injury (Reid et al., 2004).
Rice (Oryza sativa), one of the most important cereals in the humid tropics and subtropics, is sensitive to B toxicity. As is the case of other crop species, irrigation water with high B levels, which occurs both naturally and artificially through contamination by industrial wastewater, often causes B toxicity in rice (Ichikura and Maeda, 1966; Imaizumi and Okimura, 1981; Cayton, 1985). In pot culture experiments of rice plants, 10 mg B L−1 in the irrigation water decreased grain yield from 30% to 83% that of the control (Ochiai et al., 2008). Excess B has a greater effect on tillering than plant height in rice, and under moderate B toxicity, a decrease in the number of panicles is the main cause of the decreased grain yield (Imaizumi and Okimura, 1981; Ochiai et al., 2008). There may be a critical developmental stage that is particularly sensitive to excess B. Because of the nonionic nature and small molecular size of boric acid, a cost-effective technique for B removal from the irrigation water is not available, and therefore, a better understanding of the B-toxicity damage mechanism would be useful for breeding B-toxicity-tolerant rice plants.
There is large genetic variation in B-toxicity tolerance among species and cultivars. In wheat (Triticum aestivum), barley (Hordeum vulgare; Nable, 1988; Paull et al., 1988), and oilseed rape (Brassica rape; Kaur et al., 2006), the shoot B concentration is higher in the B-toxicity-sensitive cultivars than in the B-toxicity-tolerant cultivars. Therefore, the ability to exclude B from tissues seems to be important for B-toxicity tolerance in these species, and indeed, in wheat and barley, quantitative trait loci (QTL) responsible for the shoot B concentration and relative root length (B toxicity inhibits root elongation) under B toxicity are detected at the same chromosomal location (Jefferies et al., 1999, 2000). In barley, Bot1, a gene responsible for the B-tolerance QTL on chromosome 4H, is a B-efflux transporter (Sutton et al., 2007). For the B-tolerance QTL on barley chromosome 6H, a multifunctional aquaporin HvNIP2;1 is responsible, and it is proposed that the lower expression of HvNip2;1 prevents passive influx of B under high B conditions (Schnurbusch et al., 2010). In Arabidopsis (Arabidopsis thaliana), constitutive overexpression of the B-efflux transporter BOR4 increases B-toxicity tolerance (Miwa et al., 2007).
In a transcriptome analysis using Arabidopsis plants under B toxicity, nine genes encoding multidrug and toxic compound extrusion transporters, a zinc-finger family transcription factor, a heat-shock protein-like protein, a NAC (NAM, ATAF, and CUC)-like transcription factor, and unknown proteins, were induced (Kasajima and Fujiwara, 2007), although the functions of these proteins are not yet known.
In a previous report (Ochiai et al., 2008), we showed that modern japonica rice cultivars such as Nipponbare, Koshihikari, and Sasanishiki are more tolerant to B toxicity than indica cultivars such as IR36 and Kasalath. The shoot and root B concentrations of japonica Nekken-1 and indica IR36 are not different under 0 to 27 g B m−3 culture solutions, although Nekken-1 is more tolerant to excess B than IR36. Therefore, in Nekken-1 and IR36, mechanisms other than B exclusion might be important for the difference in the B-toxicity tolerance. A QTL for B-toxicity tolerance was detected on rice chromosome 4 in a population of recombinant inbred lines derived from a cross of Nekken-1 and IR36 (Ochiai et al., 2008), while four ortholog genes of the Arabidopsis B-efflux transporter gene BOR1 are located on rice chromosomes 1, 5, and 12 (Nakagawa et al., 2007).
We named the QTL gene on chromosome 4 BORON EXCESS TOLERANT1 (BET1). Cloning of BET1 would provide new insight into the B-toxicity tolerance or B-toxicity mechanism. In this report, we attempted to clone BET1 using the map-based cloning method, and identified it as a NAC-like transcription factor gene that has not been previously reported to be an excess B-tolerance-related gene.
RESULTS
Fine Mapping of BET1
In a previous report, a gene (BET1) responsible for the QTL of excess B tolerance was mapped to the approximately 1,400-kb region between marker P-4 and Cht4 (Ochiai et al., 2008). The candidate region for BET1 was narrowed stepwise by the map-based cloning method. Self-pollinated seeds of a recombinant inbred line that was used for the QTL analysis and heterozygous at the BET1 region were used for the screening. In the first screening of approximately 500 plants, nine plants with a recombinant event between the markers in this region were selected. The relative shoot length (RSL) of the homozygous progenies of the selected plants was determined, and BET1 was mapped between P-1 and S-13322 (Fig. 1A). In the next step, more than 25,000 plants were screened. BET1 was eventually mapped to 49 kb between marker AA-1 and t44 (Fig. 1B). In this region, eight genes, Os04g0476400, Os04g0476500, Os04g0476600, Os04g0476700, Os04g0476800, Os04g0477000, Os04g0477200, \and Os04g0477300 are annotated (Rice Annotation Project Database: http://rapdb.dna.affrc.go.jp/; Tanaka et al., 2008). None of these genes, however, are known to be B-toxicity tolerance-related genes, such as for the efflux transporter, and all eight annotated genes contain polymorphisms between the DNA sequence of japonica Nekken-1 and indica IR36.
Figure 1.
Mapping of BET1. Plants with a recombinant event in the candidate region on chromosome 4 were screened from self-pollinated seeds of one of the line in the recombinant inbred line population derived from Nekken-1 and IR36 and used for QTL analysis. A, Left, graphical genotype of the homozygous progenies of plants selected in the first screening. Black bars indicate the Nekken-1 type, and white bars indicate IR36 type. Physical positions of the markers are indicated on the vertical axis. Right, RSL. Values are the mean of 10 plants ± sd. B, Graphical genotype and RSL in the second screening. The lines followed by A and B indicate the progeny of the parent lines. The white square indicates the mapped region of BET1.
B-Toxicity Tolerance and DNA Polymorphisms among japonica Cultivars
B tolerance was examined in 44 japonica landraces. The RSL of the landraces ranged from 0.1 to 0.45. The landraces were roughly separated into two groups, sensitive landraces with an RSL value lower than approximately 0.3, and tolerant landraces (Fig. 2A). The candidate region for BET1 in Nipponbare and the 44 landraces was intensively searched for DNA polymorphisms using the eco-TILLING method. An unanalyzed gap of 1.46 kb remained in the noncoding region flanking the 5′ side of Os04g0476400 due to difficulties in PCR amplification. Association of RSL with genotype was eyeball fitted and analyzed statistically using ANOVA. Among the detected DNA polymorphisms in the candidate region, polymorphisms at four positions were the most consistent with the difference in B-toxicity tolerance (P = 8.84 × 10−14). Thirty cultivars were Nipponbare type and 14 cultivars were Wataribune type at these polymorphic positions. The mean RSL value of the Nipponbare-type plants was 0.37 ± 0.07 and the mean RSL value of the Wataribune-type plants was 0.12 ± 0.06 (Fig. 2B). The differences between the genomic DNA sequences of the sensitive cultivar Wataribune and the tolerant cultivar Nipponbare were: a four-base insertion in the noncoding region between Os04g0476700 and Os04g0476800, a single nucleotide polymorphism in the 3′-untranslated region of Os04g0477000, a single nucleotide polymorphism in the intron of Os04g0477300, and a one-base insertion in the open reading frame (ORF) of Os04g0477300. Because a major change in the amino acid sequence is anticipated by the one-base insertion mutation in the ORF sequence of Os04g0477300, it is likely that Os04g0477300 is BET1. The B-toxicity-sensitive indica cultivar IR36 also had the same insertion.
Figure 2.
B tolerance in japonica landraces. A, Frequency distribution of the RSL in 44 japonica landraces in the core collection of Japanese landraces. Values are the mean of five plants. B, Genotypic difference in the RSL of japonica landraces at four polymorphic loci in the candidate region. Values are the mean ± sd of 30 landraces for Nipponbare type and 14 landraces for Wataribune type.
Characterization of the Candidate Gene
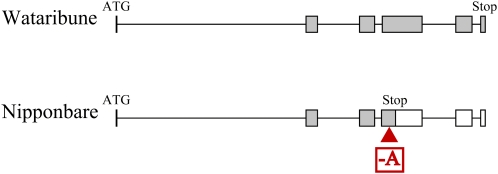
The mRNA sequence was confirmed by direct sequencing of the reverse transcription (RT)-PCR product prepared from the roots of Wataribune and Nipponbare. The candidate gene comprised six exons and five introns (Fig. 3). The gene of the B-sensitive landrace Wataribune encoded a 362-amino acid protein, and a NAC domain that contained a characteristic plant-specific NAC transcription factor. On the other hand, an adenine in the ORF was deleted in the B-tolerant Nipponbare allele, which introduced a premature stop codon at the 170th amino acid (Fig. 3).
Figure 3.
Schematic diagrams of the BET1 candidate gene for the B-sensitive Wataribune and B-tolerant Nipponbare alleles. Boxes represent exons, and gray color indicates the ORF. The triangle indicates the position of the one-base deletion of adenine in the Nipponbare allele.
In the transcriptional activating assay in yeast (Saccharomyces cerevisiae), yeast strain MaV203, which was transformed to express a fusion protein of the GAL4 DNA-binding domain and the ORF of the Wataribune allele, could grow on the synthetic complete medium lacking His or uracil, but yeast transformed with the Nipponbare allele did not (Fig. 4). Quantitative RT-PCR analysis revealed that the gene was expressed mainly in the roots and the expression level was less in the shoots in 7-d-old seedlings (P < 0.05; Fig. 5). The mean expression level in the sensitive cultivar group, Wataribune, IR36, and Kasalath was higher than that in the tolerant cultivar group, Koshihikari and Nipponbare both in the shoots and roots (P < 0.05), suggesting that mRNA digestion was promoted in the tolerant cultivars because of the nonsense mutation. These results suggest that the gene product acts as a transcriptional activator in the sensitive cultivars, whereas this function is lost in the tolerant cultivars.
Figure 4.
Transactivation analysis of the BET1 candidate gene fused with the GAL4 DNA-binding domain in yeast. Transgenic yeasts were grown on a control medium (Control) or a selection medium lacking His (−His) or uracil (−Ura).
Figure 5.
Quantitative RT-PCR analysis of the BET1 candidate gene in the shoots and roots of 7-d-old rice seedlings. Five rice cultivars, Nipponbare, Nekken-1, Wataribune, IR36, and Kasalath, were hydroponically grown under the control condition. Ubiquitin and actin were used as the internal control. Values are the mean ± sd (n = 3).
Suppression of BET1 Expression Induced by RNA Interference
The candidate gene was functional in the B-sensitive cultivars, but nonfunctional in B-tolerant cultivars. If Os04g0477300 is BET1, inhibition of the expression of this gene would make sensitive cultivars tolerant to B toxicity. Therefore, transgenic plants generated by RNA interference (RNAi) were made to test this hypothesis.
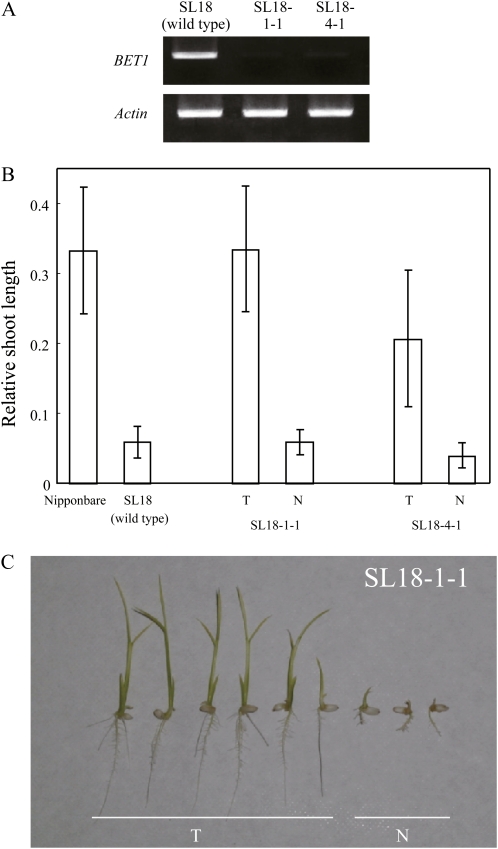
A chromosome segment substitution line SL18 was used of which the parents are a B-toxicity-tolerant cultivar Nipponbare and a moderately sensitive cultivar Kasalath. The genetic background of SL18 is Nipponbare, and it carries a Kasalath chromosomal segment on chromosome 4 that contains the candidate gene. The RSL of SL18 was significantly lower than that of Nipponbare under B toxicity (Ochiai et al., 2008; Fig. 6B). In two independent lines of RNAi transgenic plants made from SL18, the expression level of the candidate BET1 was suppressed (Fig. 6A). Under the control condition, shoot length of transgenic T1 plants was not different from that of null segregants (the mean values were 11.9 ± 4.1 and 12.8 ± 3.6 for the SL18-1-1 transgenic plants and null segregants, and 14.5 ± 2.5 and 15.8 ± 1.2 for the SL18-4-1 transgenic plants and null segregants, n = 3, 3, 5, and 2, respectively). The RSL of transgenic T1 plants was significantly (P < 0.05) higher than that of their null segregants (Fig. 6, B and C). Suppression of the gene expression induced tolerance to B toxicity in SL18, which indicates that Os04g0477300 encoding the NAC-like transcriptional factor is BET1.
Figure 6.
B-toxicity tolerance of the RNAi plants. A, Semiquantitative RT-PCR analysis of the gene expression of BET1 for SL18 and two independent RNAi lines. The cDNA was prepared from roots of 8-d-old seedlings grown under the control condition, and PCR amplification was performed using 25 cycles for actin and 30 cycles for BET1. B, RSL values of Nipponbare, SL18 (wild type), and two independent lines of RNAi T1 plants, SL18-1-1 and SL18-4-1. In RNAi plants, T, transgenic plants; and N, null segregants. Values are the mean of 10, 10, six, three, six, and four plants for Nipponbare, SL18, SL18-1-1-T, SL18-1-1N, SL18-4-1-T, and SL18-4-1-N, respectively, ± sd. The differences between transgenic plants and null segregants were significant (P < 0.05) in both lines. C, Seven-day-old SL18-1-1 plants grown under 60 mg B L−1. Individual T1 plants were examined for the presence of the transgene by PCR detection after determining the shoot length. The six plants on the left are transgenic and the three plants on the right are null segregants.
DISCUSSION
In a previous report, we demonstrated significant differences in the tolerance to B toxicity between two rice cultivars Nekken-1 (tolerant) and IR36 (sensitive), measured by shoot elongation, while their shoot and root B concentrations were not significantly different (Ochiai et al., 2008). This finding led us to hypothesize that mechanisms other than B exclusion are involved in B-toxicity tolerance in rice plants, and BET1, a QTL gene responsible for the cultivar difference in B-toxicity tolerance at the seedling stage, was isolated in this study.
BET1 encodes a NAC-like transcription factor protein. The NAC is a plant-specific transcriptional factor and comprises a gene family, 151 nonredundant NAC genes in rice and 117 genes in Arabidopsis (Nuruzzaman et al., 2010). The function of the NAC proteins is highly diverse; they act in the development of shoot meristems (Souer et al., 1996; Aida et al., 1997), secondary cell wall synthesis (Zhong et al., 2006), leaf senescence (Uauy et al., 2006), and stress responses (Hu et al., 2006; Nakashima et al., 2007; Kaneda et al., 2009). Some NAC transcription factors function to promote survival under abiotic stresses such as salinity and drought (Hu et al., 2006; Nakashima et al., 2007), and iron deficiency (Ogo et al., 2008), but the function of most NAC genes remains unknown.
Involvement of BET1 in the B-toxicity tolerance reported here is a newly found function of the family. In a comprehensive study of rice NAC family gene, NAC proteins were classified into 16 subgroups based on the similarities in the NAC domain structures (Nuruzzaman et al., 2010). BET1 belongs to the ONAC4 subgroup, which is comprised of 14 NAC genes, and the functions of the other 13 genes are not clarified yet. Microarray analyses showed that BET1 is up-regulated under several abiotic and biotic stresses, such as cold and infection by the rice stripe virus. Gibberellic acid also enhances expression, and the expression is neither affected by osmotic, salt, submergence stresses, nor infection by the rice tungro spherical virus (Nuruzzaman et al., 2010). Whether BET1 is induced under B toxicity is being studied.
In a transcriptome analysis using Arabidopsis plants under B toxicity, a NAC-like transcription factor gene At1g32870 was induced (Kasajima and Fujiwara, 2007). Phylogenetic analyses of NAC genes indicate that gene products of At1g32870 and BET1 are classified in different groups (Ooka et al., 2003; Fang et al., 2008). Therefore, it is unclear whether BET1 functions similarly as At1g32870 under B-toxicity tolerance.
The BET1 gene product of the sensitive cultivars had transcriptional activator activity in yeast, but the function was lost in the allele of B-toxicity-tolerant cultivars because of the nonsense mutation. One of the rice NAC, OsNAC4, regulates hypersensitive cell death due to infection of Acidvorax avena strain N1141. OsNAC4 up-regulates 139 genes including a heat-shock protein gene OsHSP90 and Ca2+-dependent nuclease gene IREN, then, the activated genes bring about hypersensitive reactions, that is, loss of plasma membrane integrity and DNA fragmentation (Kaneda et al., 2009). Our results suggest that functional BET1 acts to increase the sensitivity of rice plants to excess B. In B-toxicity-sensitive rice, excess B might trigger a fatal cascade; on the other hand, the loss-of-function mutation in BET1 interrupts this cascade in B-toxicity-tolerant cultivars, allowing the tolerant cultivars to continue growing. Continuous growth also confers the benefit of diluting the internal B concentrations. BET1 might make the rice hypersensitive to excess B. For the better understanding of the molecular mechanisms of B toxicity, we are planning to identify the target genes of BET1 using microarray analyses.
The results reported here indicate that tolerance can be induced in excess B-sensitive rice cultivars by destroying BET1. Many japonica elite cultivars, such as Koshihikari and Nipponbare, have nonfunctional BET1, and therefore the destruction of BET1 would not be expected to affect rice growth or yield. Marker-assisted breeding of excess B-tolerant near-isogenic lines of the popular indica cultivars IR36 and IR64 in which the BET1 region is substituted by the Koshihikari allele is now in progress.
MATERIALS AND METHODS
Plant Materials
Self-pollinated progenies of a line of recombinant inbred lines derived from Nekken-1 and IR36 (Ochiai et al., 2008), which was heterozygous at the QTL region, were used for the fine mapping of BET1.
SL18 seeds were obtained from the Rice Genome Resource Center, Tsukuba, Japan. SL18 has a genetic background of japonica Nipponbare and carries chromosome segments of indica Kasalath at the QTL region on chromosome 4 (http://www.rgrc.dna.affrc.go.jp/).
Seeds of landraces in the Japanese rice (Oryza sativa) core collection were obtained from National Institute of Agrobiological Sciences GenBank, Tsukuba, Japan (Ebana et al., 2008).
B Tolerance in Water Culture
Plants were grown as previously described (Ochiai et al., 2008). A batch of 20 seeds of a cultivar, landrace, or line was soaked for 3 d at 30°C in distilled water supplemented with a fungicide (3% w/v, TORIFUMIN; Nippon Soda Co., Ltd.). Ten seeds were sown on a nylon mesh (18 mesh, 24 × 36 mm) stretched on a plastic frame. Eight meshes were floated on 2 L of culture solution in a plastic container. The other batch of 10 seeds of the same line or cultivar was raised similarly, but the culture solution was supplemented with high B levels. The plants on the mesh were raised for 7 d in a growth chamber (NS-280 FHW; Takayama Seisakusyo) under the following conditions: temperature 30°C, relative humidity 80%, photoperiod 12 h, and light intensity 350 μmol m−2 s−1. The culture solution contained 0.5 mol m−3 (NH4)2SO4, 0.13 mol m−3 KH2PO4, 0.25 mol m−3 KCl, 0.25 mol m−3 CaCl2, and 0.25 mol m−3 MgCl2, pH 6.0. Iron was supplied at 2.5 g iron m−3 as FeNa-EDTA. Micronutrients were supplied according to Arnon’s formula (Hewitt, 1966) at half strength. B was supplied as boric acid at 0.5 g B m−3 for the controls and at 60 g B m−3 as high B treatment. Seven days after sowing, the shoot length was determined, and the RSL (the shoot length at 60 g B m−3 divided by the shoot length of the control) was used as an indicator of B tolerance.
Fine Mapping of BET1
For individual progeny plants of a recombinant inbred line derived from Nekken-1 and IR36 that was still heterozygous for the QTL region, genotypes in the candidate region were determined using PCR-based DNA markers to screen for plants with a recombination between the markers. Selected plants were grown in a green house to obtain self-pollinated seeds, and B tolerance of their homozygous progeny was examined.
Eco-TILLING Assay
Nipponbare was used as a reference cultivar. Fragments of 1 to 2 kb were amplified by PCR from genomic DNA. Eighty-seven primer pairs were used to cover the 49-kb candidate region, and a 1.46-kb gap that could not be amplified remained in the noncoding region. Aliquots of 5 μL of the PCR product of each cultivar or landrace were mixed with that of Nipponbare, then the mixture was denatured for 10 min at 98°C, and annealed. Preparation of celery (Apium graveolens) juice extract and digestion of single-strand DNA using the celery juice extract was performed as described by Till et al. (2004). Aliquots of 20 μL of the reaction were electrophoresed on a 2% agarose gel and stained by ethidium bromide. In this assay, DNA polymorphisms were detected as bands smaller than those of the PCR products.
cDNA Synthesis
Plants were grown in water culture under control conditions. Total RNA was extracted from the shoots or roots of 7-d-old seedlings using an RNeasy plant mini kit (Qiagen). First-strand cDNA was synthesized from total RNA using an oligo dT primer.
Gene Expression Analysis
Quantitative real-time RT-PCR was performed with a thermal cycler dice real time system (Takara Bio), using SYBR Premix EX Taq II (Takara Bio). Three independent cDNA was prepared for each sample, and duplicate quantitative assays were performed on each cDNA sample. Relative expression level was calculated by the comparative CT method using ubiquitin and actin as an internal control. The difference between the sensitive cultivar group, Wataribune, IR36, and Kasalath, and the tolerant cultivar group, Nipponbare and Koshihikari, and between the shoot and root as a whole were statistically tested by the t test.
Transcriptional Activation Analysis in Yeast
Insert DNA was amplified by PCR from cDNA prepared from B-sensitive Wataribune and B-tolerant Nipponbare. Subcloned PCR products in vector pENTR/D-TOPO were transferred to vector pDEST32 (Invitrogen) by the LR reaction to express the fusion proteins of the GAL4 DNA-binding domain and BET1. Yeast (Saccharomyces cerevisiae) strain MaV203 was transformed and activation of the expression of reporter genes HIS3 and URA3 was examined.
Plasmid Construction and Rice Transformation
Insert DNA was amplified by PCR using cDNA prepared from roots of B-sensitive cultivar Wataribune as a template. The PCR products were subcloned to pENTR/D-TOPO (Invitrogen), and then transferred to a binary vector pANDA (Miki et al., 2005) for the RNAi plant by LR reaction. The vector plasmid pANDA was a kind gift from Dr. K. Shimamoto, Nara Institute of Science and Technology. Transformation of rice was mediated by Agrobacterium as described by Toki et al. (2006), using Agrobacterium tumefaciens strain EHA105 (Hood et al., 1993).
PCR Primers
The sequences (5′ to 3′) of primers used in the fine mapping of BET1 are as follows: for P-1, GGAAGGGAAGCCTAACCTGA and TGCTCAGCAATCCATCACTC; for AA-1, CTCCAGCTTTAGGGCCTCTT and CATGATGCATCCAGCTATGC; and for t44, TTCTTCCTCTCCAAGCCAAA and CAGCCCAATCAAACGGTAAT. The primers used for the transformation of yeast are CACCATGGCAAGGCCATGGATTATAGC and TTACGTGTGATCCCCATCCAC. The primers used for the transformation of rice are CACCCGCCTGAAATGGACTC and GCTCACTAGAGTGGCGAACC. The primers used in semiquantitative RT-PCR analysis are as follows: for BET1, CCTCTCGCTCTCCTCCTCT and CTGTGGAAGAAATGGCTGCT; and for Actin, TCCATCTTGGCATCTCTCAG and GTACCCTCATCAGGCATCTG. The primers used in the quantitative real-time PCR analysis are as follows: for BET1, CCTCTCGCTTCTCCTCCTCT and GCAATTACCTTTCCACCAGCTA; for Ubiquitin, AGAAGGAGTCCACCCTCCACC and GCATCCAGCACAGTAAAACACG; and for Actin, ATCCTTGTATGCTAGCGGTCGA and ATCCAACCGGAGGATAGCATG.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers AB630330 (Wataribune BET1) and AB630331 (Nipponbare BET1).
References
- Aida M, Ishida T, Fukaki H, Fujisawa H, Tasaka M. (1997) Genes involved in organ separation in Arabidopsis: an analysis of the cup-shaped cotyledon mutant. Plant Cell 9: 841–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blamey FPC, Asher CJ, Edwards DG. (1997) Boron deficiency in sunflower. Bell RW, Rerkasem B, , Boron in Soils and Plants. Kluwer, Dordrecht, The Netherlands, pp 145–149 [Google Scholar]
- Cayton MTC. (1985) Boron toxicity in rice. IRRI Res Pap Ser 113: 2–10 [Google Scholar]
- Ebana K, Kojima Y, Fukuoka S, Nagamine T, Kawase M. (2008) Development of mini core collection of Japanese rice landrace. Breed Sci 58: 281–291 [Google Scholar]
- Fang Y, You J, Xie K, Xie W, Xiong L. (2008) Systematic sequence analysis and identification of tissue-specific or stress-responsive genes of NAC transcription factor family in rice. Mol Genet Genomics 280: 547–563 [DOI] [PubMed] [Google Scholar]
- Hewitt EJ. (1966) The composition of the nutrient solution. Hewitt E, , Sand and Water Culture Methods Used in the Study of Plant Nutrition. Farnham Royal Bucks, Commonwealth Agricultural Bureaux, Slough, UK, pp 190 [Google Scholar]
- Hood EE, Gelvin SB, Melchers LS, Hoekema A. (1993) New Agrobacterium helper plasmids for gene transfer to plants. Transgenic Res 2: 208–218 [Google Scholar]
- Hu H, Dai M, Yao J, Xiao B, Li X, Zhang Q, Xiong L. (2006) Overexpressing a NAM, ATAF, and CUC (NAC) transcription factor enhances drought resistance and salt tolerance in rice. Proc Natl Acad Sci USA 103: 12987–12992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikura T, Maeda M. (1966) On the damage for crops of zinc and boron in the industrial waste water. Bull Osaka Prefect Agric Forest Res Cent 3: 1–10 [Google Scholar]
- Imaizumi M, Okimura I. (1981) Studies on the excess-boron harm to rice plant. Res Bull Aichi Agric Res Cent 13: 89–97 [Google Scholar]
- Jefferies SP, Barr AR, Karakousis A, Kretschmer JM, Manning S, Chalmers KJ, Nelson JC, Islam AKMR, Langridge P. (1999) Mapping of chromosome regions conferring boron toxicity tolerance in barley (Hordeum vulgare L.). Theor Appl Genet 98: 1293–1303 [Google Scholar]
- Jefferies SP, Pallotta MA, Paull JG, Karakousis A, Kretchmer JM, Manning S, Islam AKMR, Langridge P, Chalmers KJ. (2000) Mapping and validation of chromosome regions conferring boron toxicity tolerance in wheat (Triticum aestivum). Theor Appl Genet 101: 767–777 [Google Scholar]
- Kaneda T, Taga Y, Takai R, Iwano M, Matsui H, Takayama S, Isogai A, Che FS. (2009) The transcription factor OsNAC4 is a key positive regulator of plant hypersensitive cell death. EMBO J 28: 926–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasajima I, Fujiwara T. (2007) Identification of novel Arabidopsis thaliana genes which are induced by high levels of boron. Plant Biotechnol 24: 355–360 [Google Scholar]
- Kaur S, Nicolas ME, Ford R, Norton RM, Taylor PWJ. (2006) Selection of Brassica rapa genotypes for tolerance to boron toxicity. Plant Soil 285: 115–123 [Google Scholar]
- Mengel K, Kirkby EA. (2001) Boron. Mengel K, Kirkby E, , Principles of Plant Nutrition, Ed 5. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 621–638 [Google Scholar]
- Miki D, Itoh R, Shimamoto K. (2005) RNA silencing of single and multiple members in a gene family of rice. Plant Physiol 138: 1903–1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa K, Takano J, Omori H, Seki M, Shinozaki K, Fujiwara T. (2007) Plants tolerant of high boron levels. Science 318: 1417. [DOI] [PubMed] [Google Scholar]
- Nable R. (1988) Resistance to boron toxicity amongst several barley and wheat cultivars—a preliminary examination of the resistance mechanism. Plant Soil 112: 45–52 [Google Scholar]
- Nakagawa Y, Hanaoka H, Kobayashi M, Miyoshi K, Miwa K, Fujiwara T. (2007) Cell-type specificity of the expression of Os BOR1, a rice efflux boron transporter gene, is regulated in response to boron availability for efficient boron uptake and xylem loading. Plant Cell 19: 2624–2635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima K, Tran LSP, Van Nguyen D, Fujita M, Maruyama K, Todaka D, Ito Y, Hayashi N, Shinozaki K, Yamaguchi-Shinozaki K. (2007) Functional analysis of a NAC-type transcription factor OsNAC6 involved in abiotic and biotic stress-responsive gene expression in rice. Plant J 51: 617–630 [DOI] [PubMed] [Google Scholar]
- Nuruzzaman M, Manimekalai R, Sharoni AM, Satoh K, Kondoh H, Ooka H, Kikuchi S. (2010) Genome-wide analysis of NAC transcription factor family in rice. Gene 465: 30–44 [DOI] [PubMed] [Google Scholar]
- Ochiai K, Uemura S, Shimizu A, Okumoto Y, Matoh T. (2008) Boron toxicity in rice (Oryza sativa L.). I. Quantitative trait locus (QTL) analysis of tolerance to boron toxicity. Theor Appl Genet 117: 125–133 [DOI] [PubMed] [Google Scholar]
- Ogo Y, Kobayashi T, Nakanishi Itai R, Nakanishi H, Kakei Y, Takahashi M, Toki S, Mori S, Nishizawa NK. (2008) A novel NAC transcription factor, IDEF2, that recognizes the iron deficiency-responsive element 2 regulates the genes involved in iron homeostasis in plants. J Biol Chem 283: 13407–13417 [DOI] [PubMed] [Google Scholar]
- Ooka H, Satoh K, Doi K, Nagata T, Otomo Y, Murakami K, Matsubara K, Osato N, Kawai J, Carninci P, et al. (2003) Comprehensive analysis of NAC family genes in Oryza sativa and Arabidopsis thaliana. DNA Res 10: 239–247 [DOI] [PubMed] [Google Scholar]
- Paull JG, Cartwright B, Rathjen AJ. (1988) Responses of wheat and barley genotypes to toxic concentrations of soil boron. Euphytica 39: 137–144 [Google Scholar]
- Power PP, Woods WG. (1997) The chemistry of boron and its speciation in plants. Plant Soil 193: 1–13 [Google Scholar]
- Reid RJ, Hayes JE, Post A, Stangoulis JCR, Graham RD. (2004) A critical analysis of the causes of boron toxicity in plants. Plant Cell Environ 25: 1405–1414 [Google Scholar]
- Schnurbusch T, Hayes J, Hrmova M, Baumann U, Ramesh SA, Tyerman SD, Langridge P, Sutton T. (2010) Boron toxicity tolerance in barley through reduced expression of the multifunctional aquaporin HvNIP2;1. Plant Physiol 153: 1706–1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souer E, van Houwelingen A, Kloos D, Mol J, Koes R. (1996) The no apical meristem gene of Petunia is required for pattern formation in embryos and flowers and is expressed at meristem and primordia boundaries. Cell 85: 159–170 [DOI] [PubMed] [Google Scholar]
- Sutton T, Baumann U, Hayes J, Collins NC, Shi BJ, Schnurbusch T, Hay A, Mayo G, Pallotta M, Tester M, et al. (2007) Boron-toxicity tolerance in barley arising from efflux transporter amplification. Science 318: 1446–1449 [DOI] [PubMed] [Google Scholar]
- Tanaka T, Antonio BA, Kikuchi S, Matsumoto T, Nagamura Y, Numa H, Sakai H, Wu J, Itoh T, Sasaki T, et al. (2008) The rice annotation project database (RAP-DB): 2008 update. Nucleic Acids Res 36: D1028–D1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till BJ, Burtner C, Comai L, Henikoff S. (2004) Mismatch cleavage by single-strand specific nucleases. Nucleic Acids Res 32: 2632–2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toki S, Hara N, Ono K, Onodera H, Tagiri A, Oka S, Tanaka H. (2006) Early infection of scutellum tissue with Agrobacterium allows high-speed transformation of rice. Plant J 47: 969–976 [DOI] [PubMed] [Google Scholar]
- Uauy C, Distelfeld A, Fahima T, Blechl A, Dubcovsky J. (2006) A NAC gene regulating senescence improves grain protein, zinc, and iron content in wheat. Science 314: 1298–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong R, Demura T, Ye ZH. (2006) SND1, a NAC domain transcription factor, is a key regulator of secondary wall synthesis in fibers of Arabidopsis. Plant Cell 18: 3158–3170 [DOI] [PMC free article] [PubMed] [Google Scholar]