LOW SOIL PHOSPHORUS AVAILABILITY IS A PRIMARY CONSTRAINT TO PLANT PRODUCTIVITY
Plant growth in the vast majority of terrestrial ecosystems is limited by low phosphorus availability. Over 70% of all terrestrial biomass occurs in low-phosphorus soils, including over half of agricultural land (Fig. 1). Phosphorus availability is declining in many systems because of soil degradation, which has affected over half of global agricultural land and 75% of agricultural land in Africa. Intensive phosphorus fertilization is uncommon in the low-input agriculture common in poor nations and has limitations as a long-term strategy because of limited reserves of high-grade phosphate ore deposits, the energy costs of producing fertilizer, and the environmental cost associated with intensive fertilization. The development of crops with greater phosphorus efficiency, defined as the ability to grow and yield in soils with reduced phosphorus availability, would substantially improve food security in developing nations, while enhancing the sustainability of agriculture in rich nations (Lynch, 2007).
Figure 1.
Map of global soil phosphorus availability. The dominance of red and light-gray colors, indicating suboptimal phosphorus availability for the growth of many plant species, indicates the importance of phosphorus availability as a primary limitation to plant productivity in terrestrial environments (from Jaramillo-Velastagui, 2011).
PHOSPHORUS IS AN IMMOBILE SOIL NUTRIENT
Phosphate is highly immobile in soil, because it reacts with many chemical and biological soil constituents. Plant strategies to acquire phosphorus are therefore oriented around two basic themes: (1) soil exploration and (2) mobilization of phosphate from poorly available phosphorus pools in the rhizosphere. This Update focuses on phenes controlling soil exploration by roots, since they are subject to selection in crop breeding programs, and since root deployment is senior to many other root phenes affecting phosphorus acquisition, by determining the placement of root exudates and symbionts in specific soil domains, and thereby their functional benefit.
PHENES AFFECTING SOIL EXPLORATION FOR PHOSPHORUS ACQUISITION
Topsoil Foraging
Because of the immobility of phosphorus in soil, surface soil strata, enriched by the deposition of plant residues over time, generally have greater phosphorus availability than subsoil strata. Root traits that enhance topsoil foraging are therefore important for phosphorus acquisition. Substantial differences exist in topsoil foraging within and among species. Architectural traits associated with enhanced topsoil foraging include shallower growth angles of axial roots, enhanced adventitious rooting, a greater number of axial roots, and greater dispersion of lateral roots (Fig. 2).
Figure 2.
Root phenes associated with genotypic differences in adaptation to low phosphorus (from Lynch, 2007).
Shallower Root Growth Angle
In maize (Zea mays), bean (Phaseolus vulgaris), and soybean (Glycine max), shallower root growth angles (RGAs) of axial roots (basal roots in legumes, seminal and crown roots in maize; Fig. 3) increase topsoil foraging and thereby phosphorus acquisition. To determine the value of genotypic variation in RGA for phosphorus acquisition, it is useful to compare plants that have varying RGA but have otherwise similar phenotypes. Since RGA is controlled by multiple genes, useful tools for this analysis are recombinant inbred lines (RILs), created by repeated selfing of F2 progeny from parents contrasting for the phene of interest, resulting in a set of typically 100 or 200 homozygous inbred lines segregating for RGA yet sharing the same genetic background. RILs are powerful tools for the analysis of quantitative phenes like RGA since they permit the evaluation of a phene in an array of related yet distinct genomes. RILs are also useful for identifying genetic loci associated with phene expression (quantitative trait loci [QTL]). Cosegregation of QTL controlling a phene with phosphorus acquisition is strong evidence that the phene is important for phosphorus acquisition in a range of phenotypes. Analysis of maize and bean RILs varying in RGA shows that this phene has a dominant influence on phosphorus acquisition, accounting for up to 6-fold variation in phosphorus acquisition and 3-fold variation in yield of bean in low-phosphorus soil (Lynch and Brown, 2001), and 2-fold variation in phosphorus acquisition in maize (Zhu et al., 2005b). QTL associated with RGA in bean cosegregate with yield under phosphorus stress (Liao et al., 2004). Functional-structural modeling supports a positive role for RGA in phosphorus acquisition, showing that in stratified soils shallow RGA increases phosphorus acquisition by increasing topsoil foraging, and that in soils with uniform phosphorus distribution shallow RGA improves phosphorus acquisition by reducing competition for phosphorus among roots of the same plant (Lynch and Brown, 2001). RGA of axial roots is an important phene for topsoil foraging and phosphorus acquisition in annual crops.
Figure 3.
Shallow versus deep basal RGAs in two common bean genotypes grown in the field in South Africa.
Basal Root Whorl Number
The main structural roots of annual legumes are the primary root emerging from the seed, and basal roots appearing at the base of the subterranean hypocotyl. In bean, basal roots occur at distinct nodes or whorls along the base of the hypocotyl. Basal root whorl number (BRWN) varies among genotypes from one to four, with each whorl typically generating four basal roots (Fig. 4). Uppermost whorls produce roots with shallower RGA, and lower whorls produce roots of progressively steeper angle. Therefore, greater BRWN may increase soil exploration by increasing the vertical range of root deployment. In a field study on low-phosphorus soil in Mozambique, RILs with three whorls accumulated 60% greater biomass than related RILs with two whorls (Miguel, 2011). BRWN appears to be under relatively simple genetic control—three QTL explain 58% of phenotypic variance for BRWN in bean (Miguel, 2011).
Figure 4.
Variation in BRWN in common bean is related to the number of basal roots produced and therefore soil exploration.
Adventitious Rooting
Adventitious roots emerging from subterranean shoot tissue may increase phosphorus acquisition because they typically have shallow RGA and are metabolically cheaper than other root classes. In bean, adventitious roots have greater specific root length, lower tissue construction cost, more aerenchyma (see below), and less lateral branching than other axial roots, which permits them to explore the soil for less metabolical investment (Lynch and Ho, 2005). Bean genotypes with greater adventitious root formation had greater growth and phosphorus acquisition in low-phosphorus soil. However, excessive adventitious rooting may decrease phosphorus acquisition by diverting carbohydrates from lateral branches of basal roots, thereby decreasing total soil exploration (Walk et al., 2006). In bean adventitious root formation is under strong genetic control, with 19 QTL accounting for 19% to 61% of phenotypic variation in the field (Ochoa et al., 2006).
Lateral Branching
The formation of lateral roots diverts root foraging from axial elongation and thereby exploration of new soil domains to root proliferation and soil exploitation near the existing location of the axial root. Theoretically phosphorus acquisition would be optimized if roots had reduced lateral branching in low-phosphorus soil domains, permitting greater axial elongation, and greater lateral branching in high-phosphorus patches. This is evident in bean and maize, in which low phosphorus reduces lateral rooting more than it reduces axial elongation (Borch et al., 1999; Mollier and Pellerin, 1999). In young plants with few axial roots, lateral branching may increase exploration of soil domains not reached by axial roots. This may account for the observation that increased lateral rooting among young maize RILs under phosphorus stress was associated with substantially greater phosphorus accumulation and growth (Zhu and Lynch, 2004). Lateral branching is under complex genetic control in maize, where 15 relatively small effect QTL have been identified (Zhu et al., 2005a). The plasticity of lateral rooting in response to phosphorus availability is under genetic control, indicating that plasticity is a potential selection criterion in crop breeding (Zhu et al., 2005a).
Reducing the Metabolic Cost of Soil Exploration
The metabolic cost of soil exploration is an important component of plant growth under low-phosphorus availability (Lynch and Ho, 2005). Variation among RILs for root costs is associated with phosphorus acquisition in maize and bean. Genotypic variation for root costs may be caused by several types of phenes. Anatomical variation may reduce root respiration by changing the proportion of active and inactive tissue. Architecture phenes can reduce root costs by reducing competition for phosphorus within and among plants, and by regulating biomass allocation to root classes of varying metabolic cost. Morphological phenes such as root hairs can increase phosphorus acquisition at minimal metabolic expense.
Aerenchyma Reduces the Metabolic Costs of Soil Exploration
Root cortical aerenchyma (RCA) varies constitutively among genotypes (Fig. 5A), and is induced by suboptimal availability of oxygen, water, nitrogen, phosphorus, and sulfur. RCA is an important adaptive response to hypoxia by improving oxygenation of root tissue, and may be generally useful for soil resource acquisition by converting living cortical tissue to air space, thereby reducing the nutrient and carbon costs of soil exploration. Genotypes of maize and bean vary in RCA formation, which in maize is strongly related to root phosphorus content and respiration, and root growth maintenance in low-phosphorus soil (Fig. 5B). SimRoot modeling indicates that RCA could increase growth 70% in maize under phosphorus stress and 14% in bean, primarily by reducing the phosphorus content of root tissue, and secondarily by reducing root respiration (Fig. 6; Postma and Lynch, 2010). RCA formation in maize is under the control of several QTL accounting for about half of phenotypic variation (Mano et al., 2007).
Figure 5.
A, Cross sections of seminal roots of maize showing genotypic difference in cortical aerenchyma formation, which replaces living cortical cells (left) with air-filled lacunae (right). Genotypes are closely related progeny (RILs) of the same two parents. B, Maintenance of root growth in a low-phosphorus field as related to cortical aerenchyma formation in unrelated maize genotypes. Root weights are expressed as the proportion of corresponding high-phosphorus roots. Each point is the mean of four replicates.
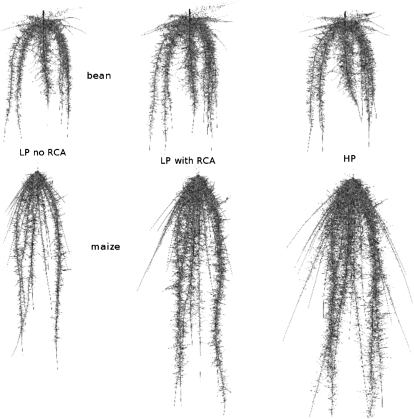
Figure 6.
Visualization of the simulated root architecture of common bean and maize at 40 d after germination. HP, High soil phosphorus (18 μm); LP, low soil phosphorus (3 μm). HP visualization shows a root system without RCA formation, as the root system with RCA formation was visually not different (from Postma and Lynch, 2010).
Root Etiolation
Bean roots under phosphorus stress show reduced secondary development and radial expansion in favor of continued root elongation, a process called root etiolation (Fig. 7A). Bean genotypes with greater root etiolation under phosphorus stress have reduced root metabolic costs and increased soil exploration (Morrow de la Riva, 2010). SimRoot modeling indicates that this phene may increase shoot growth by 38% over the first 40 d of growth under low phosphorus (Fig. 7B).
Figure 7.
A, Root etiolation: reduced secondary development of common bean roots in response to phosphorus stress. Root cross sections are from tissues of equivalent age in equivalent root classes of the same genotype. B, Effect of root etiolation on shoot biomass accumulation in low-phosphorus plants 40 d after germination as modeled in SimRoot (from J.A. Postma and J.P. Lynch, unpublished data).
Root Hairs
It is well established that root hairs are important for phosphorus acquisition by expanding the effective phosphorus depletion zones around the root. Root hairs are especially important for phosphorus acquisition in nonmycorrhizal plants, since mycorrhizal hyphae fulfill some of the same functions as root hairs. However, genotypic variation in root hair length and density is important for phosphorus acquisition regardless of the mycorrhizal status of the plant (Miguel, 2004). Physiological analysis of wild-type and hairless Arabidopsis (Arabidopsis thaliana) genotypes, and contrasting RILs of maize, indicates that the direct metabolic cost of root hairs is negligible (Lynch and Ho, 2005). Root hair length and density are attractive targets for crop breeding programs because they vary substantially among genotypes (Fig. 8), are directly associated with phosphorus acquisition regardless of mycorrhizal status (Fig. 9), they are under relatively simple genetic control, and are amenable to direct phenotypic selection (Lynch, 2007). Root hair length and density in maize and bean are controlled by several QTL, which in bean show cosegregation with yield in low-phosphorus soils (Yan et al., 2004; Zhu et al., 2005c).
Figure 8.
Genotypic variation for root hair length and density in common bean. The genotype on the top is the result of scientific breeding and is an important commercial cultivar in Central America. The genotype on the bottom is a Peruvian landrace.
Figure 9.
Longer root hairs improve phosphorus acquisition in the presence and absence of mycorrhizal inoculation in common bean. Plants were grown for 28 d in low-phosphorus soil in pots with (+VAM) or without (−VAM) mycorrhizal inoculum. Genotypes are RILs having long or short root hairs. Each bar is the mean of four replicates, bars = sem. (from Miguel, 2004).
Phenology
An important limitation to phosphorus acquisition by roots is the slowness of phosphorus diffusion to the root and the time required for recharge of solution phosphorus from soil phosphorus pools. Plants that are able to extend the length of time that roots are deployed in the soil will benefit by increased phosphorus acquisition as well as increased duration and therefore utility of acquired phosphorus in plant tissue. Annual plants typically delay flowering and maturation in response to phosphorus stress. In Arabidopsis, genotypic variation in phenology, and phenological delays caused by phosphorus stress, were strongly correlated with phosphorus acquisition (Nord and Lynch, 2008). Extending crop phenology, and therefore the duration of root foraging, may therefore increase phosphorus acquisition in those environments where temperature or water availability do not curtail reproduction.
PHENE INTERACTIONS
The value of a phene for phosphorus acquisition may depend on the expression of other phenes in the plant phenotype. Such interactions may be synergistic, neutral, or negative. For example, four distinct phenes associated with root hair phenotypes in Arabidopsis: root hair length, root hair density, the distance from the root tip to the first appearance of root hairs, and the pattern of root-hair-bearing epidermal cells (trichoblasts) among non-hair-bearing cells (atrichoblasts), have a combined effect on phosphorus acquisition 371% greater than their additive effects (Lynch and Ho, 2005). Morphological, anatomical, symbiotic, and biochemical phenes expressed by root axes should have significant synergies with architectural phenes, since architectural phenes determine the position of root axes in time and space, and therefore the soil domain in which spatially localized phenes are expressed. For example, longer root hairs are twice as beneficial for phosphorus acquisition and plant growth under phosphorus stress when located on shallow roots as they are when located on deep roots, because of the greater phosphorus availability in surface soil strata (Miguel, 2011). Similarly, phosphatases that release phosphorus from organic compounds would be more useful if produced by shallow roots than by deep roots, since soil organic matter typically decreases with depth. In contrast, carboxylates capable of releasing phosphorus from iron and aluminum oxides may be more useful when released into deeper soil horizons where these forms of phosphorus predominate. Root architectural phenes interact by altering the extent of competition within and among plants, which is an important determinant of phosphorus acquisition (Lynch and Brown, 2008). Architectural phenes may negatively interact by generating competition for internal resources, as is evident in the case of abundant adventitious rooting reducing plant phosphorus acquisition by reducing assimilate supply to basal root laterals in bean (Walk et al., 2006). Likewise anatomical phenes that reduce root costs may show positive synergism with architectural traits by reducing competition for internal resources among roots of the same plant. Our understanding of such interactions is very limited. The large number of possible phene interactions in integrated phenotypes (e.g. 10 traits each existing in two states would result in 210 integrated phenotypes) makes simulation modeling an attractive complement to empirical research.
TRADEOFFS
Evaluation of the utility of a phene for phosphorus acquisition must consider potential tradeoffs for other plant processes. Direct tradeoffs for many architectural phenes result from diversion of internal plant resources from other functions, as is evident in the competition for assimilates between adventitious and basal root laterals, in which excessive biomass allocation to one root class diverts resources from another root class, resulting in reduced overall phosphorus acquisition. Architectural tradeoffs can also result if concentration of foraging effort in one soil domain reduces exploitation of other soil domains. This is especially important for phenes related to phosphorus acquisition, since phosphorus availability is greater in surface soil strata, but water and nitrate are typically located in deep soil strata. Architectural tradeoffs for phosphorus and water have been demonstrated for RGA in bean, in which shallower genotypes had superior growth under phosphorus stress, but deep-rooted genotypes had superior growth under water stress (Ho et al., 2005). The direct and indirect tradeoffs for root phenes related to phosphorus acquisition are poorly understood. It is likely that the large genotypic variation observed for most root phenes is related to the diversity of soil environments in which plants have evolved, as well as tradeoffs associated with specific phenes. A better understanding of fitness tradeoffs for root phenes is needed for their intelligent deployment in crop breeding programs.
BELOWGROUND COMPETITION
Root phenes affecting phosphorus acquisition are also likely to affect plant performance in competitive environments, such as those confronted by the majority of wild and agricultural plants. The effects of root phenes on belowground competition may be a direct result of resource competition for phosphorus, and may also be an indirect result of altered plant growth. An example of growth-mediated effects of a root phene on competition is the positive effect of root hairs on plant performance in mixed stands of Arabidopsis at low phosphorus but not at high phosphorus (Bates and Lynch, 2001). An example of the direct effect of a root phene on resource competition for phosphorus is the observation that bean genotypes with shallow roots outcompete genotypes with deep roots in low-phosphorus fields, because of enhanced topsoil exploitation and reduced competition among roots of the same plant (Lynch and Brown, 2008). The effects of root phenes on belowground competition are poorly understood, and represent a knowledge gap that must be addressed for the informed deployment of root phenes in crop breeding programs.
DEPLOYMENT OF ROOT PHENES IN CROP BREEDING PROGRAMS
The development of crop genotypes with enhanced phosphorus acquisition efficiency (PAE) represents an important opportunity to improve food security in developing nations, where crop yields are severely limited by low phosphorus availability (Lynch, 2007). Simple screening for yield in low-phosphorus soils has not been an effective breeding strategy to improve PAE, because of the confounding effects of spatial variability in phosphorus availability, cooccurring biotic and abiotic stresses, and the improbability of identifying genotypes possessing useful phenes in a setting in which the possession of many distinct, yet interacting, phenes is necessary for organismal success. Furthermore, elite crop germplasm has been subject to decades of selection with irrigation and fertilizer, meaning that sources of useful root phenes are more likely to be found in landraces (e.g. Fig. 8), which may not have other agronomic attributes conferring success in field screening, such as disease resistance or high yield potential. For these reasons, a physiological breeding strategy, targeting the improvement of specific phenes known to increase phosphorus acquisition, is more likely to be fruitful than simple yield screening of elite germplasm under phosphorus stress. The prerequisites for this strategy are (1) identification of the target phene(s), which implies a comprehensive understanding of how the phene affects crop performance in specific agroecosystems, (2) sources of genetic variation for the target phenes, (3) phenotyping platforms permitting the efficient evaluation of phene expression in many genotypes, and (4) an understanding of the likely effects of improved genotypes on the productivity and sustainability of the agroecosystems of interest. In cases where these prerequisites are in place, root phenes for phosphorus acquisition are being employed in crop breeding programs. For example, RGA, root hair length and density, and BRWN are all being employed as direct phenotypic selection criteria in bean breeding programs in Central America and Mozambique. In these breeding programs the use of root phenes as selection criteria has permitted the identification of excellent germplasm sources for these phenes among otherwise poorly adapted landraces, has enabled rapid phenotypic screening of young seedlings grown in the lab, and has permitted focused breeding strategies such as phene introgression into elite lines. New bean lines emerging from these programs typically have 20% to 40% greater yield under stress than current cultivars (e.g. IIAM, 2010). New soybean lines possessing root architectural traits for superior phosphorus acquisition are being deployed in Southern China (Wang et al., 2010). Homology among root phenes enhancing phosphorus acquisition in common bean, soybean, and maize suggests that these phenes would have general application in the breeding of annual crops with greater PAE.
Architectural Multilines
The development of architectural multilines consisting of closely related genotypes having contrasting root architecture may offer several advantages over traditional monocultures for stressful environments. For example, multilines consisting of shallow and deep-rooted genotypes may benefit from reduced interplant competition, and greater yield stability in environments in which both drought and low soil fertility are important. This hypothesis is supported by an analysis of architectural multilines of bean grown in stressful environments in Central America, in which multilines tended to have greater yields than the average yield of their monoculture components (Henry et al., 2010b).
High-Throughput Phenotyping for Root Architectural Phenes
The precise definition of root phenes controlling phosphorus acquisition greatly facilitates the development of efficient, high-throughput phenotyping platforms. Traits expressed in young seedlings such as RGA, BRWN, and root hair length and density may be directly and easily phenotyped in growth pouches or roll ups in the lab (http://roots.psu.edu/). More complex traits expressed later in plant development such as adventitious root formation are amenable to marker-assisted selection using QTL or other molecular markers, although these tools have limited availability in many developing countries, where the need for phosphorus-efficient genotypes is greatest. A number of important root architectural phenes can be rapidly phenotyped in the field using a simple manual method called shovelomics, in which root crowns are excavated and visually scored for several root phenes at a rate of 2 min/plot (Trachsel et al., 2010).
Functional Structural Modeling
In silico analysis of the relationship between specific root phenes and soil resource acquisition through functional structural modeling is a useful tool to guide crop breeding. As noted above, simulation models are capable of evaluating a large number of integrated phenotypes in a large number of soil environments, identifying a subset of potential phenotypes deserving empirical investigation. Modeling is the only practical way to assess the large number of phene interactions with other phenes and with environmental variables in integrated phenotypes, a critical step in defining breeding priorities. Modeling also permits the evaluation of the utility of specific phenes and integrated phenotypes in future climate scenarios, which is especially important in the stressful environments of developing nations, which could be seriously affected by global climate change (St. Clair and Lynch, 2010).
System Impacts
Relatively little is known regarding the effects of phosphorus-efficient crop genotypes on the productivity and sustainability of agroecosystems. However, the available evidence indicates that these effects should be benign. In rich nations, such genotypes would require less fertilization, thereby reducing production costs and environmental pollution. In poor nations, characterized by low-input agriculture, crop genotypes with enhanced phosphorus extraction from the soil could potentially deplete soil fertility over the long term by soil mining. In bean, phosphorus-efficient genotypes may actually conserve soil fertility by reducing soil erosion through the formation of greater canopy biomass (Henry et al., 2010a). Phosphorus-efficient legumes may also contribute to soil fertility by enhanced biological nitrogen fixation, which is quite sensitive to phosphorus supply, and may have greater ability to utilize locally available phosphorus sources such as rock phosphorus. Economically, the greater productivity of phosphorus-efficient genotypes would permit third world farmers greater flexibility in soil management options, purchasing fertility inputs, etc., in addition to greater food security and household income. Therefore it does not appear that deployment of root phenes for enhanced phosphorus acquisition in crop genotypes will have negative consequences for agroecosystems.
PROSPECTS
The identification of root phenes for enhanced phosphorus acquisition is enabling crop breeders to develop new genotypes with better yield in low-fertility soils of Africa, Asia, and Latin America. With the advent of high-throughput phenotyping platforms for specific root phenes, it is now possible to discover the genetic basis of genotypic variation for these phenes, which will enable the development of more powerful molecular breeding strategies. Significant knowledge gaps remain in our understanding of the root phenome; how phenes interact to affect the fitness of integrated phenotypes, how phenes for enhanced phosphorus acquisition may incur tradeoffs for other plant functions, and how genotypes with improved phosphorus acquisition may affect the long-term productivity of agroecosystems, considering factors such as nutrient cycling, intercropping, and the socioeconomic impacts of improved genotypes on rural communities. These challenges call for a renewed focus on plant phenes and plant physiology at multiple levels of organization.
References
- Bates T, Lynch JP. (2001) Root hairs confer a competitive advantage under low phosphorus availability. Plant Soil 236: 243–250 [Google Scholar]
- Borch K, Bouma TJ, Lynch JP, Brown KM. (1999) Ethylene: a regulator of root architectural responses to soil phosphorus availability. Plant Cell Environ 22: 425–431 [Google Scholar]
- Henry A, Chaves NF, Kleinman PJA, Lynch JP. (2010a) Will nutrient-efficient genotypes mine the soil? Effects of genetic differences in root architecture in common bean (Phaseolus vulgaris L.) on soil phosphorus depletion in a low-input agro-ecosystem in Central America. Field Crops Res 115: 67–78 [Google Scholar]
- Henry A, Rosas JC, Beaver JS, Lynch JP. (2010b) Multiple stress response and belowground competition in multilines of common bean (Phaseolus vulgaris L.). Field Crops Res 117: 209–218 [Google Scholar]
- Ho M, Rosas J, Brown K, Lynch JP. (2005) Root architectural tradeoffs for water and phosphorus acquisition. Funct Plant Biol 32: 737–748 [DOI] [PubMed] [Google Scholar]
- IIAM (2010) Relatório de Actividades do Centro Zonal Sul. Centro Zonal Sul, Instituto de Investigação Agrária de Moçambique, Ministério da Agricultura, Chokwe, Gaza [Google Scholar]
- Jaramillo-Velastagui RE. (2011) The edaphic control of plant response to climate change: extent, interactions and mechanisms of adaptation. PhD thesis. The Pennsylvania State University, University Park, PA [Google Scholar]
- Liao H, Yan X, Rubio G, Beebe SE, Blair MW, Lynch JP. (2004) Genetic mapping of basal root gravitropism and phosphorus acquisition efficiency in common bean. Funct Plant Biol 31: 959–970 [DOI] [PubMed] [Google Scholar]
- Lynch JP. (2007) Roots of the second green revolution. Aust J Bot; 55: 493–512 [Google Scholar]
- Lynch JP, Brown K. (2008) Root strategies for phosphorus acquisition. White P, Hammond J, , The Ecophysiology of Plant-Phosphorus Interactions. Springer Science, Dordrecht, The Netherlands, pp 83–116 [Google Scholar]
- Lynch JP, Brown KM. (2001) Topsoil foraging—an architectural adaptation of plants to low phosphorus availability. Plant Soil 237: 225–237 [Google Scholar]
- Lynch JP, Ho MD. (2005) Rhizoeconomics: carbon costs of phosphorus acquisition. Plant Soil 269: 45–56 [Google Scholar]
- Mano Y, Omori F, Takamizo T, Kindiger B, Bird RM, Loaisiga CH, Takahashi H. (2007) QTL mapping of root aerenchyma formation in seedlings of a maize x rare teosinte “Zea nicaraguensis” cross. Plant Soil 295: 103–113 [Google Scholar]
- Miguel M. (2004) Genotypic variation in root hairs and phosphorus efficiency in common bean (Phaseolus vulgaris L.). MSc thesis. The Pennsylvania State University, University Park, PA [Google Scholar]
- Miguel M. (2011) Functional role and synergistic effect of root traits for phosphorus acquisition efficiency and their genetic basis in common bean (Phaseolus vulgaris L.). PhD thesis. The Pennsylvania State University, University Park, PA [Google Scholar]
- Mollier A, Pellerin S. (1999) Maize root system growth and development as influenced by phosphorus deficiency. J Exp Bot 50: 487–497 [Google Scholar]
- Morrow de la Riva L. (2010) Root etiolation as a strategy for phosphorus acquisition in common bean. MSc thesis. The Pennsylvania State University, University Park, PA [Google Scholar]
- Nord E, Lynch JP. (2008) Delayed reproduction in Arabidopsis thaliana improves fitness in soil with suboptimal phosphorus availability. Plant Cell Environ 31: 1432–1441 [DOI] [PubMed] [Google Scholar]
- Ochoa I, Blair M, Lynch JP. (2006) QTL analysis of adventitious root formation in common bean (Phaseolus vulgaris L.) under contrasting phosphorus availability. Crop Sci 46: 1609–1621 [Google Scholar]
- Postma J, Lynch JP. (2010) Theoretical evidence for the functional benefit of root cortical aerenchyma in soils with low phosphorus availability. Ann Bot (Lond) 107: 829–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Clair S, Lynch JP. (2010) The opening of Pandora’s box: climate change impacts on soil fertility and crop nutrition in developing countries. Plant Soil 335: 101–115 [Google Scholar]
- Trachsel S, Kaeppler S, Brown K, Lynch JP. (2010) Shovelomics: high throughput phenotyping of maize (Zea mays L.) root architecture in the field. Plant Soil 341: 75–87 [Google Scholar]
- Walk T, Jaramillo R, Lynch JP. (2006) Architectural tradeoffs between adventitious and basal roots for phosphorus acquisition. Plant Soil 279: 347–366 [Google Scholar]
- Wang X, Yan X, Liao H. (2010) Genetic improvement for phosphorus efficiency in soybean: a radical approach. Ann Bot (Lond) 106: 215–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan XL, Liao H, Beebe SE, Blair MW, Lynch JP. (2004) QTL mapping of root hair and acid exudation traits and their relationship to phosphorus uptake in common bean. Plant Soil 265: 17–29 [Google Scholar]
- Zhu J, Kaeppler S, Lynch JP. (2005a) Mapping of QTLs for lateral root branching and length in maize (Zea mays L.) under differential phosphorus supply. Theor Appl Genet 111: 688–695 [DOI] [PubMed] [Google Scholar]
- Zhu J, Kaeppler S, Lynch JP. (2005b) Topsoil foraging and phosphorus acquisition efficiency in maize (Zea mays L.). Funct Plant Biol 32: 749–762 [DOI] [PubMed] [Google Scholar]
- Zhu J, Lynch JP. (2004) The contribution of lateral rooting to phosphorus acquisition efficiency in maize (Zea mays L.) seedlings. Funct Plant Biol 31: 949–958 [DOI] [PubMed] [Google Scholar]
- Zhu JM, Kaeppler SM, Lynch JP. (2005c) Mapping of QTL controlling root hair length in maize (Zea mays L.) under phosphorus deficiency. Plant Soil 270: 299–310 [Google Scholar]