Abstract
Leaf rolling is considered an important agronomic trait in rice (Oryza sativa) breeding. To understand the molecular mechanism controlling leaf rolling, we screened a rice T-DNA insertion population and isolated the outcurved leaf1 (oul1) mutant showing abaxial leaf rolling. The phenotypes were caused by knockout of Rice outermost cell-specific gene5 (Roc5), an ortholog of the Arabidopsis (Arabidopsis thaliana) homeodomain leucine zipper class IV gene GLABRA2. Interestingly, overexpression of Roc5 led to adaxially rolled leaves, whereas cosuppression of Roc5 resulted in abaxial leaf rolling. Bulliform cell number and size increased in oul1 and Roc5 cosuppression plants but were reduced in Roc5-overexpressing lines. The data indicate that Roc5 negatively regulates bulliform cell fate and development. Gene expression profiling, quantitative polymerase chain reaction, and RNA interference (RNAi) analyses revealed that Protodermal Factor Like (PFL) was probably down-regulated in oul1. The mRNA level of PFL was increased in Roc5-overexpressing lines, and PFL-RNAi transgenic plants exhibit reversely rolling leaves by reason of increases of bulliform cell number and size, indicating that Roc5 may have a conserved function. These are, to our knowledge, the first functional data for a gene encoding a homeodomain leucine zipper class IV transcriptional factor in rice that modulates leaf rolling.
Leaf functions, such as photosynthesis, respiration, and transpiration, are dependent on leaf shape or three-dimensional architecture (Govaerts et al., 1996; Zhang et al., 2009). Leaf shape has long been considered an important agronomic trait in rice (Oryza sativa; Yuan, 1997). Moderate leaf rolling in rice leads to erect leaf canopies and higher photosynthetic efficiency, improving stress responses by reducing transpirational water loss and radiant heat absorption (Lang et al., 2004; Zhang et al., 2009), thereby increasing grain yield. Therefore, moderate leaf rolling is an ideal trait for rice breeding (Price et al., 1997). To date, 12 rice mutants with rolled leaves (rl) have been isolated and reported in several different studies, for which six genes (rl1–rl6) were mapped on corresponding rice chromosomes through conventional genetic screening, and rl7 to rl12 were mapped to chromosomes 5 (rl7 and rl8; Li et al., 2000), 7 (rl11; Shi et al., 2009), 9 (rl9 [Yan et al., 2006] and rl10 [Luo et al., 2007]), and 10 (rl12; Luo et al., 2009). However, only a few mutant genes have been cloned and characterized, and most of these leaf-rolling genes are associated with leaf adaxial-abaxial polarity establishment. RL9 and SHALLOT-LIKE1 (SLL1) are the same gene (Yan et al., 2008; Zhang et al., 2009), and SLL1 encodes a SHAQKYF class MYB family transcription factor belonging to the KANADI family. Defective development of sclerenchymatous cells on the abaxial side of sll1 mutant leaves leads to extreme leaf rolling. SLL1 deficiency also leads to defective programmed cell death of abaxial mesophyll cells and suppresses the development of abaxial features (Zhang et al., 2009). The rice adaxialized leaf1 (adl1) mutant has abaxially rolled leaves. ADL1 is an ortholog of maize DEFECTIVE KERNEL1 (Becraft et al., 2002), which encodes a plant-specific calpain-like Cys protease and is required for establishment of the adaxial-abaxial axis in leaf primordia by promoting proper epidermal development, especially in bulliform cells (Lid et al., 2002; Hibara et al., 2009). The leaves of adl1 mutants are covered with bulliform cells on both adaxial and abaxial sides (Hibara et al., 2009). Bulliform cells are specialized epidermal cells on the adaxial leaf blade surface in all monocotyledonous orders except Helobiae (Metcalfe, 1960; Jane and Chiang, 1991). Shrinkage of bulliform cells in the adaxial epidermis near the midrib has been linked to leaf rolling in rice and other grasses (O’Toole et al., 1979; Kadioglu and Terzi, 2007). Overexpression of Abaxially Curled Leaf1 (ACL1) and its homolog ACL2 in rice leads to increased bulliform cell number and size and thus to leaf epidermal cell expansion, resulting in abaxial leaf curling (Li et al., 2010). However, direct genetic and molecular evidence is lacking for the involvement of bulliform cells in leaf rolling.
Homeodomain Leu zipper (HD-Zip) proteins are plant-specific transcription factors grouped into HD-Zip families I to IV based on sequence (Elhiti and Stasolla, 2009). Sixteen genes belong to the Arabidopsis (Arabidopsis thaliana) HD-Zip IV gene family (Abe et al., 2003). HD-Zip IV is also referred to as HD-GL2, because GLABRA2 (GL2) was the first gene identified in this family (Rerie et al., 1994). HD-Zip IV genes occur in species other than Arabidopsis, including maize (Zea mays; Ingram et al., 2000), rice (Ito et al., 2002), cotton (Gossypium hirsutum; Guan et al., 2008), and pine (Pinus spp.; Ingouff et al., 2001, 2003). The vast majority of HD-Zip IV genes are specifically expressed in the outer cell layer of plant organs (Vernoud et al., 2009). Identification of Arabidopsis GL2, ANL2, ATML1, and PDF2 from their corresponding mutants has highlighted the involvement of the HD-Zip IV transcription factors in the differentiation and maintenance of epidermal cell fate (Rerie et al., 1994; Lu et al., 1996; Kubo et al., 1999; Soppe et al., 2000; Ohashi et al., 2002; Abe et al., 2003). The functions of HD-Zip IV genes in monocots have only been shown for maize Outer Cell Layer1 (OCL1) and OCL4. ZmOCL1 may be involved in the specification of embryo protoderm identity, the organization of the primary root primordium, the maintenance of the L1 cell layer in the shoot apical meristem, and kernel development (Ingram et al., 1999; Khaled et al., 2005). ZmOCL4 is expressed in the leaf blade epidermis and inhibits trichome development in maize (Vernoud et al., 2009). Therefore, it is intriguing to determine whether the function of HD-Zip IV genes is evolutionarily conserved in other monocots. BLAST searches indicated that there are nine GL2-type Rice outermost cell-specific (Roc) genes in the rice genome (Ito et al., 2003). Full-length cDNAs for Roc1 to Roc5 have been cloned, and all five genes are specifically expressed in the rice epidermis with somewhat different temporal patterns (Ito et al., 2002, 2003). However, the biological functions of these Roc genes in rice morphogenesis and development remain to be determined.
In a large-scale screen of a rice T-DNA insertion population, we isolated the leaf-rolling mutant outcurved leaf1 (oul1). Here, we report that the phenotypes of oul1 are caused by T-DNA insertion in Roc5, a member of class IV HD-Zip genes (Ito et al., 2003). Knockout of Roc5 led to increased bulliform cell number and size on the adaxial leaf blade surface. Interestingly, Roc5 overexpression gave rise to opposite phenotypes of oul1, and cosuppression lines displayed abaxially rolled leaves like oul1. The data support a conserved function for HD-Zip IV family genes in dicots and monocots. Gene expression profiling, quantitative PCR, and RNA interference (RNAi) analyses revealed that Protodermal Factor Like (PFL) is probably down-regulated in oul1, indicating that Roc5 may have a conserved function. Our findings provide, to our knowledge, the first functional data for a gene encoding an HD-Zip IV transcriptional factor in rice and its role in modulating rice leaf rolling.
RESULTS
Isolation and Phenotypic Characterization of the Rice oul1 Mutant
To identify new genes modulating leaf rolling in rice, we screened more than 100,000 T-DNA insertion lines in rice (var Nipponbare) from several populations (Yang et al., 2004; Peng et al., 2005; Wan et al., 2009). A total of 312 individual mutant lines showing leaf-rolling phenotypes were isolated, identifying the oul1 mutant with leaves rolling toward the abaxial surface. In seedlings, oul1 leaves gradually curved toward the abaxial surface, displaying a slight spiral pattern in the middle of the leaf blade (Fig. 1, A and C). Leaf rolling became more evident during growth and finally became shallot like (Fig. 1, B, D, and F). Notably, shallot-like1 leaves roll adaxially (Zhang et al., 2009). Accordingly, leaf rolling index values (LRIs) in the oul1 mutant were 0.77 to 0.93 at later vegetative stages, whereas the LRIs in the flat wild-type leaves remained 0 (Fig. 1, E and H).
Figure 1.
Characterization of oul1 and wild-type (wt) morphology. A and C, At the five-leaf stage, oul1 leaves spiraled slightly in the middle of the leaf blade, whereas wild-type leaves were flat. B and D to F, In mature plants (B), the leaves rolled abaxially to form a cylinder-like shape in oul1 plants compared with the flat wild-type leaves (D–F). ab, Abaxial; ad, adaxial. Bars = 1 cm (A), 10 cm (B), 1 mm (C), and 5 mm (D–F). G and J, The spikelet in oul1 was longer than in the wild type. H and I, LRIs (H) and LEIs (I) of the wild type and oul1 are shown. K and L, The spikelet number per panicle (plump + empty) in oul1 (n = 136) was higher than in the wild type (n = 106; K), whereas oul1 had a lower (66%) seed-setting rate than the wild type (88%; L).
The leaf erection index values (LEIs) in oul1 were significantly higher than in the wild type and remained constant from the sixth leaf stage to the flowering stage (Fig. 1I), suggesting that the abaxially rolled leaves could enhance leaf erection. Leaf erection can optimize canopy light transmission in the middle and later growing stages, thereby increasing photosynthetic efficiency and consequently increasing grain yields (Price et al., 1997; Lang et al., 2004; Zhang et al., 2009). The stomatal conductance and photosynthetic rate of oul1 were significantly higher than in the wild type (Table I). Interestingly, unlike most leaf-rolling mutants (Lang et al., 2004), oul1 exhibited a higher transpiration rate compared with the wild type (Table I). oul1 had longer spikelets (Fig. 1G) and a higher spikelet number per panicle (136) than in the wild type (106; Fig. 1K), although oul1 had a lower seed-setting rate (64%) than in the wild type (88%; Fig. 1L). These results indicated that OUL1 affected rice yields through photosynthesis and other factors.
Table I. Measurements of photosynthetic rate, transpiration rate, and stomatal conductance.
Samples were collected from the mid region of wild-type and oul1 flag leaves. Data show means ± sd (n > 40) using the heteroscedastic t test to show significant differences (* P < 0.05, ** P < 0.01) compared with the wild type.
| Leaf | Transpiration Rate | CO2 Stomatal Conductance | Photosynthetic Rate |
| mmol m−2 s−1 | mol m−2 s−1 | μmol m−2 s−1 | |
| Wild type | 2.90 ± 0.52 | 0.189 ± 0.022 | 11.47 ± 0.73 |
| oul1 | 3.26 ± 0.33* | 0.224 ± 0.020** | 13.03 ± 0.77** |
Bulliform Cell Number and Size Are Increased in oul1
To investigate the formation of abaxial leaf rolling in oul1, cross sections from mature 10th leaves were analyzed. In the wild type, bulliform cells occurred between two vascular bundle ridges in parallel with the more adaxially localized veins. In cross sections, wild-type bulliform cells were typically arranged in groups of 4 ± 1 cells, with the middle cells larger than those on either side (Fig. 2, C and E). The oul1 mutant had groups of 7 ± 1 bulliform cells located between two vascular bundle ridges (Fig. 2, D and E). In cross sections taken from similar positions, the average bulliform cell area was significantly larger in oul1 (5,128.21 ± 1,166.33 μm2) than in the wild type(2,298.55 ± 447.26 μm2; Fig. 2F). Bulliform cells mainly consist of water-filled vacuoles, and as expected, the water content of the oul1 leaves was higher than in the wild type (Fig. 2G).
Figure 2.
oul1 has increased bulliform cell number and size. A and B, Adaxial epidermal peels abutting the small veins of the wild type (wt; A) and oul1 (B). C to F, Cross sections of wild-type (C) and oul1 (D) mature leaf blades show significantly increased oul1 bulliform cell number (E) and area (F) between vascular bundle ridges. ab, Abaxial; ad, adaxial. Red lines (C and D) show the bulliform cells. Data show means and sd values of biological replicates (n > 23) and statistical analysis by heteroscedastic t test indicating significant differences (** P < 0.01). Bars = 20 μm (A–D). G, Relative water content of the 10th leaf of 120-d-old greenhouse plants grown in the soil. oul1 had higher water content than the wild type. The data are means and sd (n > 5), with statistical analysis using the heteroscedastic t test showing significant differences (** P < 0.01).
The bulliform cell phenotype was obvious throughout the whole oul1 mature blade, with no differences along the proximodistal and centrolateral axes (Supplemental Fig. S1). Other leaf cell types in oul1 mutants appeared normal. oul1 mutants do not have altered adaxial-abaxial identity of the internal leaf structure (Zhang et al., 2009) or epidermal cells (Hibara et al., 2009). Chloroplast grana lamellae were disordered and irregular in rl9-1 mutant leaves (Yan et al., 2008), but cross sections of oul1 showed no significant difference in organelles, including chloroplast grana lamellae (Supplemental Fig. S2).
Toluidine blue O stains bulliform cells (Hernandez et al., 1999). The adaxial mature 10th leaf surfaces of oul1 and wild-type plants were peeled, and epidermal and bulliform cells were stained blue and purple, respectively, with toluidine blue O (Fig. 2, A and B), again demonstrating increased bulliform cell size and number in oul1 compared with the wild type (Fig. 2, D–F). The positions and numbers of linear bulliform cell files on the leaf blade were similar in the mutant, suggesting that the outcurved oul1 leaf phenotype may be caused by the increase in bulliform cell number and size.
Increased Bulliform Cell Area Correlates with oul1 Leaf Rolling
Bulliform cells are large, thin walled, and highly vacuolated (Jane and Chiang, 1991). Bulliform cells likely modulate leaf rolling (Li et al., 2010), with loss of turgor pressure causing leaves to roll or curl (Moulia, 1994; Moore et al., 1998) and cell shrinkage linked to rice leaf rolling (O’Toole et al., 1979; Kadioglu and Terzi, 2007). To investigate whether oul1 leaf rolling was due to changes in bulliform cells, we determined when the discrepancy of bulliform cell formation in different leaves at various developing stages occurred between the wild type and oul1. Cytological analyses of seedlings were performed on emerging (3 d), folded (5 d), and unfolded (8 d) first complete leaves and also on the second (14 d), third (22 d), and fourth (32 d) leaves. Compared with the wild type, transverse sections of oul1 first complete leaves revealed that bulliform cell sizes were similar before the leaves folded (Fig. 3, A–D) and had increased number and size at the unfolded stage (Fig. 3F). The average number of bulliform cells was seven (nine maximum) and five (six maximum) in oul1 and the wild type, respectively (Fig. 3, E–L), suggesting that differences in oul1 and wild-type bulliform cell development occurred after folding and before unfolding of the first leaf. Furthermore, bulliform cell area in oul1 gradually increased and became higher than in the wild type during leaf development (Fig. 3M), which was consistent with the highest OUL1 expression level correlating with the appearance of bulliform cell differences between oul1 and the wild type (Fig. 3N). oul1 plants had no visible phenotypes up to the four-leaf stage. However, oul1 leaves spiraled slightly in the middle of the leaf blade at the beginning of the five-leaf stage and gradually curved toward the abaxial surface to become shallot like, whereas wild-type leaves remained flat throughout development.
Figure 3.
Leaf rolling in oul1 is due to increased number and size of bulliform cells. A to L, Cross sections of the first leaves of 3-d (A and B), 5-d (C and D; folded leaves), and 8-d (E and F; unfolded leaves) seedlings and second (14 d; G and H), third (22 d; I and J), and fourth (32 d; K and L) leaves of wild-type (wt) and oul1 plants. Red arrowheads (C and D) and lines (E–L) indicate bulliform cells. In the first leaf, bulliform cells were undifferentiated at 3 d (A and B) and were evident in the folded leaf of 5-d-old seedlings (C and D), but bulliform cell number increased in oul1 compared with the wild type in the 8-d-old unfolded leaf, although the leaves of oul1 were flat (E and F). Bulliform cell number also increased in first unfolded (E and F), second (G and H), third (I and J), and fourth (K and L) leaves of oul1 compared with the wild type. Bars = 20 μm. M, Bulliform cell area between vascular ridges from the first folded leaf to the fourth leaf stage shows a higher increasing trend in oul1 than in the wild type. Data show means and sd of biological replicates (n > 23). N, qRT-PCR analysis of Roc5 expression in different developing leaves. Data show means and sd (n = 3).
oul1 Phenotypes Are Due to T-DNA Insertion in Roc5
The oul1 mutant was crossed to Nipponbare wild-type plants. Genetic analyses of heterozygous F1 progeny showed that the oul1 phenotype segregated in a 3:1 ratio (χ2 = 1.256 < χ20.05,1) of wild-type (208) and mutant-like (81) plants, indicating that the oul1 leaf-rolling phenotype was caused by a single recessive mutation.
Through PCR walking (Cottage et al., 2001; Peng et al., 2005), a genomic DNA fragment flanking the T-DNA insertion site in oul1 was isolated. BLAST search with the flanking sequence (http://blast.ncbi.nlm.nih.gov/Blast.cgi) identified the full sequence of the GL2-type homeobox gene Roc5 (P0657H12.28). Roc5, which spans approximately 7,049 bp on chromosome 2, contains nine exons and eight introns. The T-DNA was inserted into the sixth intron (Fig. 4A).
Figure 4.
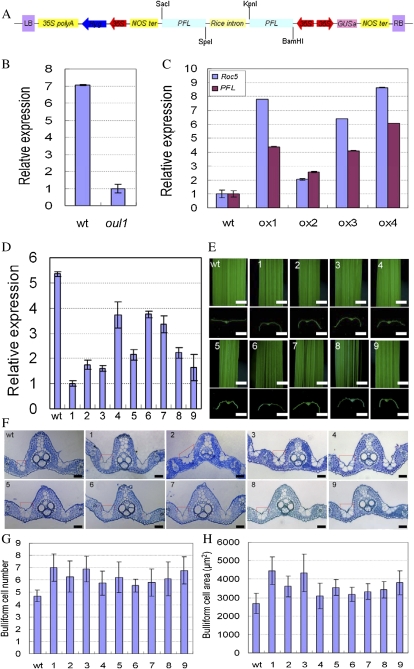
Roc5 characterization and nuclear localization of Roc5 in onion epidermal cells. A, Schematic representation of the T-DNA insertion into the sixth intron of Roc5 (exons = black boxes; introns = white boxes) and Roc5 protein domain organization (http://www.uniprot.org/uniprot/Q6EPF0). L and R represent the left and right T-DNA borders, respectively. Arrows indicate the primers used for genotyping with AS39/LB2/S39 and with Q1f and Q1r. UTR, Untranslated region. B, RT-PCR analyses of Roc5 expression in the wild type (wt) and oul1, using ACTIN as a control. One RT-PCR primer for Roc5 spans the sixth intron, and genomic DNA (gDNA) served as a negative control. C to H, 35S::Roc5-GFP (C–E) and 35S::GFP (F–H) constructs were transiently expressed in onion epidermal cells, showing bright-field (C and F), GFP (D and G), and merged (E and H) signals.
To confirm that the T-DNA insertion in Roc5 cosegregated with the oul1 mutant phenotype, more than 100 segregating individual plants were tested by PCR. Gene-specific PCR primers (AS39 and S39) flanking the insertion in Roc5 were used in combination with a primer to the T-DNA left border (primer LB2; Fig. 4A). Under these conditions, PCR amplification using wild-type DNA or homozygous oul1-like DNA yielded only a fragment with the gene-specific primers or AS39/LB2 primers, whereas heterozygous DNA amplified two fragments with the gene-specific primers (AS39 and S39) and the combination primers (AS39 and LB2). PCR genotyping analysis confirmed that the oul1 mutant phenotype was due to the presence of a homozygous T-DNA insertion within Roc5. Furthermore, reverse transcription (RT)-PCR of RNA from the homozygous oul1 mutant did not detect full-length transcripts for Roc5 (Fig. 4B). The RT-PCR primers spanned an intron (Fig. 4A), and hence the genomic DNA should not have been amplified and thus was used as a control for RNA-specific amplification (Fig. 4B).
Previous sequence analysis revealed that Roc5 encodes an ortholog of Arabidopsis GL2, a member of the HD-Zip IV family of proteins (Ito et al., 2003). GL2 is a master regulator of epidermal trichome and non-root-hair cell development in Arabidopsis (Rerie et al., 1994; Di Cristina et al., 1996; Masucci et al., 1996; Szymanski et al., 1998). Roc5 contains the characterized Gly-rich, homeobox, and START domains (Fig. 4A; http://www.uniprot.org/uniprot/Q6EPF0; Ito et al., 2003). However, Roc5 does not contain a classical nuclear localization signal, although application of bioinformatics in the analysis found that Roc5 has two predicted nuclear localization signals (GGRMLGGG and RKRKK; https://www.predictprotein.org). To test whether Roc5 localized to the nucleus, the full-length Roc5 coding region was fused to the gene encoding GFP under the control of the cauliflower mosaic virus 35S promoter. Vectors to express the 35S::Roc5-GFP fusion protein and 35S::GFP (as a control) were introduced into onion (Allium cepa) epidermal cells by particle bombardment transformation. Roc5-GFP localized only in the nucleus, whereas GFP was detected throughout cells, suggesting that Roc5 is a nuclear protein (Fig. 4, C–H).
Complemented Expression of Roc5 Rescues the Mutant Phenotypes of oul1
To confirm that Roc5 disruption resulted in the oul1 mutant phenotype, calli derived from the oul1 background were transformed via Agrobacterium tumefaciens with pRoc5::Roc5, a construct with the full-length open reading frame of Roc5 driven by its own promoter. Of the numerous transgenic plants obtained, most showed the wild-type level of Roc5 expression as determined by quantitative real-time RT-PCR (qRT-PCR; Fig. 5A). oul1 plants expressing Roc5 restored the wild-type leaf shape and bulliform cell morphology, number, and area (Fig. 5). These results confirmed that the outcurling leaf and abnormal bulliform cell development in oul1 were due to T-DNA insertion in Roc5.
Figure 5.
Complemented expression of Roc5 rescues the mutant phenotypes of oul1. A, qRT-PCR analyses of Roc5 expression in the wild type (wt), oul1, and independent complementation plant 1 (cp1) and cp2, showing the expression of cp1 and cp2 near the wild-type level. Experiments are biological replicates with sd. B, Comparison of the wild type, oul1, and the complementation lines shows that Roc5 could rescue the leaf-rolling phenotype in oul1. ab, Abaxial; ad, adaxial. Bars = 10 cm (top) and 5 mm (middle and bottom). C to J, Toluidine blue O-stained adaxial epidermal peels abutting large (C–F) or small (G–J) veins of the wild type (C and G), oul1 (D and H), and cp1 and cp2 (E, F, I, and J). K to N, Cross sections of the wild type, oul1, and complementation lines show similar bulliform cell morphology in complementation plants (M and N) and the wild type (K). Line segments (red) highlight the bulliform cells. Bars = 20 μm (C–N). O to Q, LRIs of the second leaf from the top at flowering time (O), and the number (P) and area (Q) of bulliform cells in mature leaf blades for the wild type, cp1, and cp2.
Enhanced and Suppressed Expression of Roc5 Leads to Adaxially and Abaxially Rolled Leaves, Respectively
To further elucidate the role of Roc5 in bulliform cell development and leaf shape, the full-length Roc5 coding region driven by the 35S promoter was introduced into wild-type Nipponbare rice via A. tumefaciens-mediated transformation. Independent transgenic lines were generated and confirmed by PCR detection of the transgene. Roc5 expression was examined by qRT-PCR of total RNA extracted from leaves of each transgenic line. Roc5 mRNA levels were dramatically higher in overexpressing lines than in the wild type (Fig. 6A). Furthermore, mature leaves of all these Roc5-overexpressing lines were adaxially rolled (Fig. 6B). Adaxial epidermis stained by toluidine blue O showed a decreased number of bulliform cells in ox1 and ox2 plants (Fig. 6, E, F, K, and L). Cross sections of mature leaves showed decreased bulliform cell number and size between two vascular bundle ridges in Roc5-overexpressing lines compared with wild-type rice (Fig. 6, Q, R, U, and V). In some extreme cases, bulliform cell sizes were similar to epidermal cells (data not shown). Other leaf cell types in the Roc5-overexpressing lines appeared normal, and there was no alteration in the adaxial-abaxial arrangement of the internal leaf structure (Fig. 6, O, Q, and R). The transgenic lines with wild-type levels of Roc5 expression showed wild-type morphological phenotypes (data not shown), suggesting that the morphological changes in the overexpressing lines were not due to the transgenic procedure.
Figure 6.
Enhanced or suppressed expression of Roc5 leads to adaxially or abaxially rolled leaves, respectively. A, qRT-PCR analysis of Roc5 expression shows that the two independent overexpression lines (ox1 and ox2) had higher expression levels than the wild type (wt), whereas cosuppression (cs1 and cs2) transcripts were lower than the wild type, with the expression of cs2 near the oul1 level. B, Morphological phenotypes of wild-type, oul1, ox1, ox2, cs1, and cs2 plants show that the leaves of overexpression lines were adaxially rolled and cosuppression lines were abaxially rolled, whereas those of the wild type were flat. ab, Abaxial; ad, adaxial. Bars = 10 cm (top) and 5 mm (middle and bottom). C to N, Toluidine blue O-stained adaxial epidermal peels abutting large (C–H) or small (I–N) veins of the wild type (C and I) and oul1, ox1, ox2, cs1, and cs2 (D–H and J–N) showing purple-stained bulliform cells. O to T, Cross sections of leaves show that, compared with the wild type (O), bulliform cells in ox1 (Q) and ox2 (R) were smaller, whereas those in cs1 (S) and cs2 (T) plants were larger, with cs2 (T) similar to oul1 (P). Red lines highlight the bulliform cells. Bars = 20 μm (C–T). U and V, Measurement of numbers (U) and area (V) of bulliform cells in the wild type, ox1, ox2, cs1, and cs2. Data show means of biological replicates with sd (n > 30).
Unexpectedly, some plants with abaxially rolled leaves were observed in the transgenic lines (Fig. 6B). qRT-PCR analysis of two independent lines exhibiting abaxially rolled leaves (cs1 and cs2) showed that Roc5 mRNA levels were decreased compared with the wild type (Fig. 6A). The outcurved leaves of cs1 and cs2 had an increased number and size of bulliform cells (Fig. 6, G, H, M, N, and S–V), as for ocu1 (Fig. 6P), suggesting that endogenous Roc5 expression was probably suppressed by exogenous Roc5 cDNA, which has been referred to as a cosuppression (Napoli et al., 1990; van der Krol et al., 1990; Chen et al., 2007). These results and the phenotypes of oul1 suggested that Roc5 negatively controls the development of bulliform cells, which in turn modulate leaf rolling.
PFL-RNAi Transgenic Plants Exhibit Reversely Rolling Leaves, and PFL Is a Putative Target of Roc5
To understand how Roc5 regulates downstream gene expression, expression profiling was performed using GeneChips (Affymetrix) to screen for putative target genes of Roc5. Triplicate mRNA samples of 8-d-old seedlings of oul1 mutants and the wild type were harvested when the first complete leaf unfolded, because Roc5 expression was highest at this stage (Fig. 3N). Microarray analysis indicated that 54 genes were up-regulated and 64 genes were down-regulated in oul1 mutants, with differentially expressed genes having more than 2-fold change and P < 0.01 detection values (Supplemental Table S1).
The L1 box is well conserved within promoter regions of down-regulated target genes of all L1-specific HD-Zip IV genes analyzed to date (Abe et al., 2001, 2003; Ohashi et al., 2003; Tominaga-Wada et al., 2009). Among the differentially expressed genes, 12 genes contained an L1 box in their promoter regions (Table II).
Table II. Twelve genes that are significantly expressed in oul1, and their candidate L1 box-binding motif in the promoter region.
Italics indicated down-regulated genes in oul1 mutants; the others were up-regulated. RAP, Rice Annotation Project; TIGR, The Institute for Genomic Research; wt, wild type.
| RAP or TIGR Locus Identifier | Intensity Value | Fold Change (oul1/Wild Type) | P | Putative Roc5 Binding Site in the Promoter Region | Binding Motif | Description | |||||
| oul1-1 | oul1-2 | oul1-3 | wt1 | wt2 | wt3 | ||||||
| LOC_Os10g30670 | 234.9 | 217.7 | 183.1 | 10.22 | 7.37 | 9.65 | 23.3 | 5E-08 | −910 to −903 bp | AACATTTA | Putative transposon protein |
| LOC_Os01g35330 | 901 | 804.1 | 736.9 | 91.88 | 116.8 | 94.96 | 8.04 | 7E-08 | −976 to −969 bp | TAAATGTT | Unknown protein |
| LOC_Os03g19600 | 56.4 | 70.55 | 69.86 | 9.01 | 11.36 | 7.69 | 7.01 | 6E-06 | −687 to −680 bp and −413 to −406 bp | TGCATTTA TAAATGCA | Unknown protein |
| Os01g0591000 | 481 | 466.2 | 454.5 | 68.86 | 64.15 | 68.59 | 6.95 | 7E-07 | −754 to −747 bp and −469 to −462 bp | TAAATGTT | Cytosolic aldehyde dehydrogenase |
| Osllg0592100 | 547 | 670 | 536.4 | 85.12 | 87.7 | 80.52 | 6.92 | 5E-07 | −879 to −872 bp | TAAATGTT | Cell wall macromolecule catabolic process |
| Os10g0504900 | 951 | 730.9 | 328.2 | 55.44 | 195.6 | 52.94 | 6.61 | 0.0004 | −561 to −554 bp | AACATTTA | Nonspecific lipid-transfer protein 2 |
| Os07g0241600 | 123 | 159.4 | 130.5 | 25.08 | 33.03 | 25.83 | 4.92 | 2E-05 | −973 to −966 bp | AACATTTA | Transferring hexosyl groups |
| Os05g0161500 | 3,474 | 3,168 | 2,435 | 784.41 | 550.2 | 917.1 | 4.03 | 1E-05 | −894 to −887 bp | TAAATGCA | Putative uncharacterized protein |
| Os05g0225800 | 136 | 121 | 88.12 | 23.03 | 42.68 | 32.41 | 3.52 | 9E-06 | −833 to −826 bp | AACATTTA | Putative uncharacterized protein |
| Os11g0226800 | 10.1 | 17.42 | 14.57 | 90.85 | 85.22 | 80.22 | 0.16 | 1E-06 | −653 to−646 bp | TAAATGCA | NBS-LRR-like protein (YR5) |
| Os06g0553200 | 9.01 | 11.36 | 7.69 | 56.4 | 70.55 | 69.86 | 0.14 | 7E-07 | −494 to−487 bp and −1,906 to−1,898 bp | AACATTTA | Protodermal factor-like protein |
| Os08g0473900 | 64.4 | 44.9 | 26.21 | 477.5 | 444.4 | 421.7 | 0.1 | 8E-06 | −803 to−796 bp | TAAATGCT | α-Amylase isozyme 3D |
To determine putative Roc5 targets in these 12 genes, RNAi constructs were made (Fig. 7A) and introduced into the wild-type rice calli through A. tumefaciens-mediated transformation. The transgenic T0 plants were grown in a paddy field of the Chinese Academy of Agricultural Sciences in Beijing. In the paddy field, only PFL-RNAi transgenic plants displayed abaxially rolled leaves (Fig. 7E). If Roc5 regulates PFL (GenBank accession no. AP003682.3), the expression of PFL should be higher in overexpression lines and lower in oul1 than in the wild type. This was confirmed by qRT-PCR (Fig. 7, B and C). Furthermore, all abaxially rolled leaves of PFL-RNAi transgenic plants exhibited decreased PFL expression compared with those without curling (Fig. 7D), which was consistent with microarray data. The bulliform cell number and area beside large veins of abaxially rolled leaves increased to varying degrees in transgenic plants (Fig. 7, F–H). All these results suggested that the L1 box sequence [AACATTT(T)A; from approximately –1,906 to –1,898 bp and approximately –487 to –494 bp of the transcription start site] located in the PFL promoter region was probably recognized by Roc5.
Figure 7.
Construction of 35S::PFL-RNAi and characterization of transgenic plants. A, Schematic structure of 35S::PFL-RNAi vector includes the inverted repeat sequence of the 3′ end regions of PFL DNA. LB, Left border; Hyg, hygromycin; NOS ter, A. tumefaciens nopaline synthase terminator; RB, right border. B to D, qRT-PCR analyses of PFL (B and D) and Roc5 (C) expression show that, compared with the wild type (wt), PFL expression was decreased in oul1 (B), increased in overexpression (ox) lines, similar to Roc5 (C), and decreased in nine independent T0 PFL-RNAi transgenic plants from which total leaf RNA was analyzed (D). E and F, Morphological phenotypes (E) and cross sections (F) of the flat wild-type and abaxially rolled PFL-RNAi leaves show varying increases in bulliform cell size (F; highlighted by red lines). Bars = 5 mm (E) and 20 μm (F). G and H, Measurement of number (G) and area (H) of bulliform cells in wild-type and PFL-RNAi lines. Graphs show means of biological replicates and sd (n > 30).
DISCUSSION
The epidermis is the outermost cell layer covering the plant body, which prevents water loss and pathogen invasion (Martin and Glover, 2007). Epidermal cells are specialized cells, which differentiate from the early undifferentiated epidermis in significant patterns and frequencies (Glover, 2000). Intensive genetic analyses have established the molecular mechanism regulating epidermal cell fate in Arabidopsis (Ishida et al., 2008). However, it is unknown whether the same mechanism works in other plant species, especially monocots, which have different epidermal cell types and organization. In this study, we isolated a rice leaf-rolling mutant, oul1, and the corresponding cloned gene was Roc5 (Fig. 4A), a member of the HD-GL2 (HD-Zip IV) gene family. Our work shows that Roc5 has essential roles in the formation and development of epidermal bulliform cells in rice. Therefore, HD-Zip IV family genes seem to have a conserved function in regulating epidermal cell fate both in monocots and dicots.
The Roc5 T-DNA insertion knockout mutant oul1 had significantly increased bulliform cell number and size than the wild type (Fig. 2, C–F). However, the position and number of linear bulliform cell files on leaf blades did not change in oul1 (Fig. 2, A and B), indicating that Roc5 plays a role in bulliform cell fate but not its patterning. Consistent with this role, Roc5 is mainly expressed in the L1 layer of the meristem but not in mature leaves and other organs (Ito et al., 2003), suggesting that Roc5 may be involved in the establishment of bulliform cells rather than their maintenance in the adaxial epidermis. Interestingly, Roc5 overexpression led to the adaxially curved leaves and decreased number/size of bulliform cells (Fig. 6, B, Q, R, U, and V). In contrast, the Roc5 cosuppression lines had abaxially rolled leaves, just like oul1 (Fig. 6, B, P, S, and T). Overall, several lines of evidence indicate that Roc5 negatively regulates bulliform cell formation and development in rice.
Although Roc5 has no classical nuclear localization signal, transient expression in onion cells showed that Roc5 is a nuclear protein (Fig. 4, C–H). Roc5 has no transcription activity in yeast (Ito et al., 2003) and was a negative regulator in bulliform cell formation, suggesting that Roc5 may function as a transcriptional repressor. Phylogenetic analyses grouped Roc5 into a subfamily with Roc4, Roc6, ZmOCL1, ZmOCL3, AtANL2, and AtHDG1 (Supplemental Fig. S3; Ito et al., 2003; Khaled et al., 2005). Roc5’s closest homolog is maize OCL1, with 85.1% amino acid identity. Our study provides further evidence that class IV HD-Zip transcription factors have a conserved function in controlling epidermal cell development in dicots and monocots, indicating that some gene duplications took place before their divergence.
Clearly, Roc5 is not the only gene controlling bulliform cell development. Bulliform cells generally occur intercostally as long strips several cells wide. Clonal analysis of maize leaf development suggested that the linear patterning of bulliform cells may be directed by positional information, just like trichome pattern formation in Arabidopsis leaves (Hernandez et al., 1999). In Arabidopsis, a network of interacting factors (MYB-BHLH-TTG-GL2) promotes different epidermal cell fates (Serna, 2004). Ectopic expression of the maize R gene, a basic helix-loop-helix family member, in Arabidopsis induced trichome and root hair formation (Lloyd et al., 1992), suggesting that some of these genes have conserved functions in monocots and dicots. Initiation of bulliform cells correlates with procambium formation (Jane and Chiang, 1991). Therefore, it would be intriguing to identify rice orthologs corresponding to Arabidopsis genes and investigate their functions in epidermal cell specification. In Arabidopsis, GL2 positively regulates the formation of epidermal cells (trichomes) in aerial plant parts, but here we showed that Roc5 negatively regulates rice leaf bulliform cell formation. Moreover, unlike Arabidopsis trichomes and root hairs, which are single cells and never connected to each other, rice bulliform cells occur as clusters of several cells, and the clusters are not adjacent to each other. Expression of maize OCL4 under the control of the GL2 promoter enhanced the glabrous phenotype of the Arabidopsis gl2 mutant (Vernoud et al., 2009). Therefore, identification of genetic and molecular interacting partners of Roc5 would determine if Roc5 has a different function in epidermal cell formation than GL2.
Roc5 interacts with Roc2 in yeast (Ito et al., 2003), and hence it will be interesting to study the role of Roc2 in bulliform cell development. Several genes affect bulliform cell development in rice. Loss of function of BRD1, encoding a protein that catalyzes the C-6 oxidation step in brassinosteroid synthesis, increased the number of bulliform cells but did not cause the leaves to roll because the brd1 mutation also affected internal leaf cells, resulting in stiff leaf blades (Hong et al., 2002). The YABBY family gene YAB1 is involved in feedback regulation of GA3 biosynthesis in rice. In YAB1 cosuppression plants, blades were abaxially rolled to form a cylinder-like structure because of the increased number of bulliform cells between the vascular bundles on the adaxial surface, whereas the abaxial-adaxial identity in the internal leaf structure was not affected. Hence, the rolled leaf phenotype may be due to increases in bulliform cell number, and YAB1 may have a function in bulliform cell division and differentiation (Dai et al., 2007). The rice adl1 mutant, mutated in phytocalpain, showed defects in leaf polarity and abaxially rolled leaves (Hibara et al., 2009). adl1 mutants have increased bulliform cell number present also on the abaxial blade surface, demonstrating that both differentiation and patterning of bulliform cells are affected. qRT-PCR analyses of the above three genes showed no significant difference in their expression between wild-type and oul1 mutant plants (data not shown), indicating that Roc5 regulates bulliform cell differentiation either through a different pathway or downstream of these genes.
In the large, thin-walled, highly vacuolated bulliform cells (Jane and Chiang, 1991), loss of turgor pressure (Moulia, 1994; Moore et al., 1998) and shrinkage (O’Toole et al., 1979; Kadioglu and Terzi, 2007) have been linked to leaf rolling. However, several other studies claimed that nonbulliform leaf cell types control leaf rolling under water stress (Shields, 1951; Metcalfe, 1960), and Linsbauer (1930) suggested that bulliform cells are just for water storage. Both the oul1 mutant (Fig. 2, A–F) and cosuppression lines (Fig. 6, B and S–V) had increased bulliform cell numbers, leading to abaxial leaf rolling, whereas decreased bulliform cell numbers in Roc5-overexpressing lines resulted in adaxial leaf rolling (Fig. 6, B, Q, R, U, and V). In these Roc5 mutant and transgenic lines, no other epidermal cells or internal leaf structures were changed (Figs. 2, C and D, and 6, O–T). Therefore, our study provides molecular genetic evidence supporting a direct role for bulliform cells in controlling leaf shape. Leaf rolling decreases the leaf surface area, which is believed to reduce water loss during drought. The transpiration rate was higher in oul1 than in the wild type (Table I), indicating that, at least under some conditions, there is no benefit of leaf rolling under water stress. On the other hand, the photosynthetic rate of oul1 was significantly higher than in the wild type (Table I), suggesting that altering leaf structure benefits photosynthesis.
As a transcription regulator, Roc5, mainly expressed in the L1 layer of the meristem (Ito et al., 2003), probably regulates PFL by binding the L1 box motif in its promoter region (Fig. 7). PFL, which spans approximately 1,392 bp on chromosome 6 and contains three exons and two introns, encodes a protodermal factor-like protein (protein identifier BAD53672.1). A protein BLAST search (http://blast.ncbi.nlm.nih.gov/Blast.cgi) identified three hypothetical proteins (SORBIDRAFT_10g021810 [Sorghum bicolor] and LOC100502555 and LOC100382257 [maize]) with 79%, 78%, and 72%, respectively, amino acid sequence identity to PFL, although none of these potentially homologous proteins have putative conserved domains.
To date, several GL2-type homeobox genes have been cloned. ATML1 is expressed in protodermal cells from an early stage of embryogenesis (Lu et al., 1996). Similarly, ZmOCL1 is expressed in the protodermal cells from an early stage of embryogenesis and is also expressed in the outer layer of the developing root apical meristem (Ingram et al., 1999). Although amino acid sequence comparison and phylogenic analysis showed that Roc5 is most similar to Arabidopsis ANL2 (Supplemental Fig. S3; Ito et al., 2003), our study supports a Roc5 function that is similar to Arabidopsis ATM, indicating that ANL2 function may have arisen after the divergence of monocots and dicots. However, delineating the involvement of cis-regulatory elements (L1 box) in multiple DNA-protein interactions is a complex process. Whether PFL is a downstream target of Roc5 remains to be determined.
MATERIALS AND METHODS
Plant Materials and Measurements
Rice (Oryza sativa spp. japonica) cv Nipponbare plants were grown in a greenhouse at 30°C (16 h of light) and 22°C (8 h of dark) or in a paddy field of the Chinese Academy of Agricultural Sciences in Beijing from May to October of each year. LRI and LEI were measured as described by Shi et al. (2007). Wild-type and oul1 photosynthetic rate, transpiration rate, and stomatal conductance were measured at full heading stage using the portable photosynthetic LCPRO+ instrument (ADC Bioscientific), with 500 μmol s–1 flow velocity, 30°C leaf chamber, and 1,800 μmol s–1 light quantum flux density. Water content of wild-type and oul1 leaves (the 10th leaf of 120-d-old plants) was measured as described by Barrs and Weatherley (1962). Three leaf weights were taken: W1, immediately after leaf excision; W2, after wiping off water following a 4-h water saturation of excised leaves at room temperature; and W0, after subsequently drying leaves for 12 h at 70°C. Each measurement had at least five replicates. The formula for calculating the relative water content is as follows: relative water content (%) = (W1 – W0)/(W2 – W0) × 100%.
Histology and Microscopy Observation
For paraffin section analysis, the basal half of each mature 10th leaf was collected and fixed in Carnoy’s solution (60:30:10, ethanol:chloroform:glacial acetic acid, v/v/v) for 12 h. Samples were washed with 60% (v/v) ethanol, immersed in 30% (v/v) hydrofluoric acid for approximately 7 to 10 d, and then dehydrated with a graded ethanol series and embedded in paraffin. Sections (approximately 5–10 μm thick) were cut with a microtome (Leica RM2155), stained with 1% (w/v) safranin O (Amresco) and 1% (w/v) fast green FCF (Merck), examined with a fluorescence microscope (Zeiss AXIO Imager A1), and photographed. Bulliform cell numbers between two vascular bundle ridges from the midrib to the margin were counted, and their area was measured with AxioVision release 4.6 software using six separate leaves for each sample.
To analyze bulliform cell arrangement, approximately 1-cm2 adaxial surfaces were peeled approximately midway along the mature rice blade (10th leaf), gently abraded with an aqueous silica mixture, and then soaked in 95% (v/v) ethanol for approximately 1 d, changing the solution every 4 h to remove chlorophyll. Leaves were then rinsed in water and soaked for 12 h in 1 n NaOH to extract cell contents, rinsed again in water, and then fixed in Carnoy’s solution for 12 h. Samples were then stained overnight in 1% (w/v) toluidine blue O (Hernandez et al., 1999). After washing with water, ribs were removed and leaves were sealed for microscopy (Zeiss AXIO Imager A1) and photographed.
Roc5 Isolation and Complementation
Genomic DNA flanking the T-DNA left border was cloned using PCR walking with nesting-specific primer pairs according to Cottage et al. (2001) and Peng et al. (2005). The primers used were LB1 (5′-CGATGGCTGTGTAGAAGTACTCGC-3′), LB2 (5′-GTTCCTATAGGGTTTCGCTCATGTGTTG-3′), AP1 (5′-GGATCCTAATACGAGTCACTATAGCGC-3′), and AP2 (5′-CTATAGCGCTCGAGCGGC-3′). PCR products were directly sequenced. The T-DNA insertion site in Roc5 was identified using National Center for Biotechnology Information BLAST searches of the rice genome database (http://www.ncbi.nlm.nih.gov/Blast/) of the rescued flanking sequences. The full-length cDNA of Roc5 was cloned into the vector carrying a 1.8-kb Roc5 promoter to generate pRoc5::Roc5. The vector was introduced into the Agrobacterium tumefaciens strain AGL1 using the heat shock method and then was transformed into oul1 mutant calli.
Genotyping of oul1 with PCR
Genotyping of the oul1 segregating population was performed by PCR using the following primers: AS39 (5′-CCACTACTTCTCCACTACCACTATCAC-3′), S39 (5′-TCAATCATTTCGATCAAGAGTGCAAC-3′), and LB2 (T-DNA left border). PCR was conducted with an initial step of 94°C incubation for 3 min and 30 cycles of 94°C for 30 s, 56°C for 30 s, and 72°C for 1 min.
Transient Expression in Onion Epidermal Cells
The 2.5-kb full-length coding sequence of Roc5 was amplified by PCR with primers Roc5-GFPF (5′-CATGCCATGGAAGAGGACTTTCTCGAG-3′) and Roc5-GFPR (5′-GGAAGATCTTTGATGGTGCAGGAGATGAG-3′) from Roc5 mRNA and cloned into pCAMBIA1302 to generate 35S::Roc5-GFP. The fusion plasmid and pCAMBIA1302 (35S::GFP, as a control) were transformed into onion (Allium cepa) epidermal cells with the Bio-Rad PDS-1000/He device (Bio-Rad). Bombarded epidermal cells were incubated for 20 h at 25°C in the dark. The cell layers were then examined with laser scanning confocal microscopy (Leica TCS SP2). GFP fluorescence was imaged using excitation with the 488-nm line of the argon laser and a 505- to 530-nm band-pass emission filter.
RT-PCR and qRT-PCR
Total RNA was extracted using TRIzol solution (Invitrogen) from leaves of wild-type and oul1 plants. Total RNA (2 μg) from each sample was reverse transcribed with oligo(dT) primer and PrimeScript RT Enzyme (TaKaRa) according to the manufacturer’s instructions. For RT-PCR, the PCR primers for amplifying Roc5 were Q1f (5′-ATGGGCAGTAGTTGATGTGTC-3′) and Q1r (5′-CGAAGGAGTGGACGGTAGAG-3′), and primers for actin were actinf (5′-GACCTTGCTGGGCGTGATCTC-3′) and actinr (5′-GATGGGCCAGACTCGTCGTAC-3′). PCR conditions were as follows: preincubation at 94°C for 2.5 min, then 30 cycles at 94°C for 30 s, 52°C for 30 s, and 72°C for 1 min. qRT-PCR was performed on the iQ5 Muticolor Real-Time PCR Detection System (Bio-Rad) with real-time PCR Master Mixture (SYBR Green Mix). The primers for Roc5 were Roc5F (5′-CGCAAGAGGAAGAAGCGATAC-3′) and Roc5R (5′-GCTCCAGTTGCGTCTTCATC-3′). The primers for PFL were PFLf (5′-ATCTCTCCGGCCATCTCTC-3′) and PFLr (5′-AAACGCACACAAGCCACA-3′). qRT-PCR was performed in triplicate for each individual line, and threshold cycle values were quantified by qRT-PCR by calculating means of normalized expression using the relative quantification method (Livak and Schmittgen, 2001). The rice ACTIN gene, amplified with primers actinF (5′-TGCTATGTACGTCGCCATCCAG-3′) and actinR (5′-AATGAGTAACCACGCTCCGTCA-3′), was selected as an internal standard to normalize the expression of Roc5.
Overexpression Construct of Roc5
The 2.5-kb Roc5 full-length coding region was amplified by PCR with the primers Roc5-5′SmaI (5′-CCCGGGGATCCAAGGAAGAGGACTTTCTCG-3′) and Roc5-3′XbaI (5′-TCTAGACGTTCTTGACTCAGGCTCGTC-3′). The PCR product was cloned into the pEASY-Blunt simple cloning vector (Transgen Biotech) and sequenced, then excised from the vector by SmaI and XbaI digestion and subcloned into pCAMBIA 23A. In pCAMBIA 23A, Roc5 was downstream of the 35S promoter. The 35S::Roc5 construct was introduced into wild-type rice calli by A. tumefaciens-mediated transformation.
Microarray and Data Analysis
Total RNA of both Nipponbare and oul1 was extracted using TRIzol reagent, and GeneChip expression analysis was performed by CapitalBio. Each sample was assayed in triplicate. This array contains 22,582 probe sets representing 10,127 positive genes. In brief, total RNA (1–15 μg) was first reverse transcribed using a T7 oligo(dT) promoter primer in the first-strand cDNA synthesis reaction. Following RNase H-mediated second-strand cDNA synthesis, the double-stranded cDNA was purified and served as a template for in vitro transcription, which was performed with T7 RNA polymerase and a biotinylated nucleotide analog/ribonucleotide mix for copy RNA amplification and biotin labeling. The biotinylated copy RNA targets were then cleaned up, fragmented, and hybridized to GeneChip expression arrays (GeneChip Expression Analysis Technical Manual; CapitalBio). After the arrays were washed and stained with streptavidin-PE in a GeneChip-Fluidics Station 450 (Affymetrix), the hybridized microarrays were scanned using the Affymetrix GeneChip Scanner 3000 and converted into DAT/CEL images for analysis (Zou et al., 2009).
Data normalization and comparison were performed for the GeneChip expression experiment (Bolstad et al., 2003; Irizarry et al., 2003a, 2003b; Smyth, 2004). Genes with a greater than 2-fold change and a detection value of P < 0.01 were defined as differentially expressed genes. Sequences of 2 kb upstream of the transcription start site (ATG) in all differentially expressed genes were identified from ftp://ftp.plantbiology.msu.edu/pub/data/Eukaryotic_Projects/o_sativa/annotation_dbs/pseudomolecules/version_6.1/all.dir/all.2kUpstream or annotation files of Affymetrix microarray and defined as promoter regions of differentially expressed genes. Promoters containing an L1 box were selected, and their corresponding genes were termed as candidate targets of Roc5 (Abe et al., 2001, 2003; Ohashi et al., 2003; Tominaga-Wada et al., 2009).
Generation of 35S::PFL-RNAi Transgenic Rice Plants
PFL (GenBank accession no. P0427B07.25) DNA encompassing 165 bp of the 3′ end (999–1,164 bp) was amplified with primers 5′-GGGGTACCACTAGTATCTCTCCGGCCATCTCTC-3′ and 5′-CGGGATCCGAGCTCAAACGCACACAAGCCACA-3′ and inserted into the pTCK309 vector using SacI, SpeI, KpnI, and BamHI sites in inverted orientations. The PFL-RNAi construct was transferred to A. tumefaciens strain AGL1 by electroporation and then introduced into wild-type rice calli through A. tumefaciens-mediated transformation. The regenerated T0 plants were grown in a paddy field at the Chinese Academy of Agricultural Sciences in Beijing. The abaxially rolling leaves of PFL-RNAi transgenic plants were selected and analyzed.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession number EU267976.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. The bulliform cell phenotype in wild-type and oul1 plants.
Supplemental Figure S2. Transmission electron microscopy analysis of wild-type (A) and oul1 (B) chloroplast grana lamellae (arrows).
Supplemental Figure S3. Phylogenetic tree showing the predicted relationships between almost all plant members of class IV HD-Zip proteins analyzed to date.
Supplemental Table S1. Microarray data for the 118 differentially expressed genes.
Supplemental Materials and Methods S1. Transmission electron microscopy analysis.
Supplementary Material
Acknowledgments
We thank Dr. Chengcai Chu for providing the binary vector.
References
- Abe M, Katsumata H, Komeda Y, Takahashi T. (2003) Regulation of shoot epidermal cell differentiation by a pair of homeodomain proteins in Arabidopsis. Development 130: 635–643 [DOI] [PubMed] [Google Scholar]
- Abe M, Takahashi T, Komeda Y. (2001) Identification of a cis-regulatory element for L1 layer-specific gene expression, which is targeted by an L1-specific homeodomain protein. Plant J 26: 487–494 [DOI] [PubMed] [Google Scholar]
- Barrs HD, Weatherley PE. (1962) A re-examination of the relative turgidity technique for estimating water deficits in leaves. J Biol Sci 15: 415–428 [Google Scholar]
- Becraft PW, Li K, Dey N, Asuncion-Crabb Y. (2002) The maize dek1 gene functions in embryonic pattern formation and cell fate specification. Development 129: 5217–5225 [DOI] [PubMed] [Google Scholar]
- Bolstad BM, Irizarry RA, Astrand M, Speed TP. (2003) A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19: 185–193 [DOI] [PubMed] [Google Scholar]
- Chen R, Zhao X, Shao Z, Wei Z, Wang Y, Zhu L, Zhao J, Sun M, He R, He G. (2007) Rice UDP-glucose pyrophosphorylase1 is essential for pollen callose deposition and its cosuppression results in a new type of thermosensitive genic male sterility. Plant Cell 19: 847–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottage A, Yang A, Maunders H, de Lacy RC, Ramsay NA. (2001) Identification of DNA sequences flanking T-DNA insertions by PCR-walking. Plant Mol Biol Rep 19: 321–327 [Google Scholar]
- Dai M, Zhao Y, Ma Q, Hu Y, Hedden P, Zhang Q, Zhou DX. (2007) The rice YABBY1 gene is involved in the feedback regulation of gibberellin metabolism. Plant Physiol 144: 121–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cristina M, Sessa G, Dolan L, Linstead P, Baima S, Ruberti I, Morelli G. (1996) The Arabidopsis Athb-10 (GLABRA2) is an HD-Zip protein required for regulation of root hair development. Plant J 10: 393–402 [DOI] [PubMed] [Google Scholar]
- Elhiti M, Stasolla C. (2009) Structure and function of homodomain-leucine zipper (HD-Zip) proteins. Plant Signal Behav 4: 86–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover BJ. (2000) Differentiation in plant epidermal cells. J Exp Bot 51: 497–505 [DOI] [PubMed] [Google Scholar]
- Govaerts YM, Jacquemoud S, Verstraete MM, Ustin SL. (1996) Three-dimensional radiation transfer modeling in a dicotyledon leaf. Appl Opt 35: 6585–6598 [DOI] [PubMed] [Google Scholar]
- Guan XY, Li QJ, Shan CM, Wang S, Mao YB, Wang LJ, Chen XY. (2008) The HD-Zip IV gene GaHOX1 from cotton is a functional homologue of the Arabidopsis GLABRA2. Physiol Plant 134: 174–182 [DOI] [PubMed] [Google Scholar]
- Hernandez ML, Passas HJ, Smith LG. (1999) Clonal analysis of epidermal patterning during maize leaf development. Dev Biol 216: 646–658 [DOI] [PubMed] [Google Scholar]
- Hibara K, Obara M, Hayashida E, Abe M, Ishimaru T, Satoh H, Itoh J, Nagato Y. (2009) The ADAXIALIZED LEAF1 gene functions in leaf and embryonic pattern formation in rice. Dev Biol 334: 345–354 [DOI] [PubMed] [Google Scholar]
- Hong Z, Ueguchi-Tanaka M, Shimizu-Sato S, Inukai Y, Fujioka S, Shimada Y, Takatsuto S, Agetsuma M, Yoshida S, Watanabe Y, et al. (2002) Loss-of-function of a rice brassinosteroid biosynthetic enzyme, C-6 oxidase, prevents the organized arrangement and polar elongation of cells in the leaves and stem. Plant J 32: 495–508 [DOI] [PubMed] [Google Scholar]
- Ingouff M, Farbos I, Lagercrantz U, von Arnold S. (2001) PaHB1 is an evolutionary conserved HD-GL2 homeobox gene expressed in the protoderm during Norway spruce embryo development. Genesis 30: 220–230 [DOI] [PubMed] [Google Scholar]
- Ingouff M, Farbos I, Wiweger M, von Arnold S. (2003) The molecular characterization of PaHB2, a homeobox gene of the HD-GL2 family expressed during embryo development in Norway spruce. J Exp Bot 54: 1343–1350 [DOI] [PubMed] [Google Scholar]
- Ingram GC, Boisnard-Lorig C, Dumas C, Rogowsky PM. (2000) Expression patterns of genes encoding HD-ZipIV homeo domain proteins define specific domains in maize embryos and meristems. Plant J 22: 401–414 [DOI] [PubMed] [Google Scholar]
- Ingram GC, Magnard JL, Vergne P, Dumas C, Rogowsky PM. (1999) ZmOCL1, an HDGL2 family homeobox gene, is expressed in the outer cell layer throughout maize development. Plant Mol Biol 40: 343–354 [DOI] [PubMed] [Google Scholar]
- Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. (2003a) Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res 31: e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. (2003b) Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4: 249–264 [DOI] [PubMed] [Google Scholar]
- Ishida T, Kurata T, Okada K, Wada T. (2008) A genetic regulatory network in the development of trichomes and root hairs. Annu Rev Plant Biol 59: 365–386 [DOI] [PubMed] [Google Scholar]
- Ito M, Sentoku N, Nishimura A, Hong SK, Sato Y, Matsuoka M. (2002) Position dependent expression of GL2-type homeobox gene, Roc1: significance for protoderm differentiation and radial pattern formation in early rice embryogenesis. Plant J 29: 497–507 [DOI] [PubMed] [Google Scholar]
- Ito M, Sentoku N, Nishimura A, Hong SK, Sato Y, Matsuoka M. (2003) Roles of rice GL2-type homeobox genes in epidermis differentiation. Breed Sci 53: 245–253 [Google Scholar]
- Jane WN, Chiang SHT. (1991) Morphology and development of bulliform cells in Arundo formosana Hack. Taiwania Int J Life Sci 36: 85–97 [Google Scholar]
- Kadioglu A, Terzi R. (2007) A dehydration avoidance mechanism: leaf rolling. Bot Rev 73: 290–302 [Google Scholar]
- Khaled AS, Vernoud V, Ingram GC, Perez P, Sarda X, Rogowsky PM. (2005) Engrailed-ZmOCL1 fusions cause a transient reduction of kernel size in maize. Plant Mol Biol 58: 123–139 [DOI] [PubMed] [Google Scholar]
- Kubo H, Peeters AJ, Aarts MG, Pereira A, Koornneef M. (1999) ANTHOCYANINLESS2, a homeobox gene affecting anthocyanin distribution and root development in Arabidopsis. Plant Cell 11: 1217–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang YZ, Zhang ZJ, Gu XY, Yang JC, Zhu QS. (2004) A physiological and ecological effect of crimpy leaf character in rice (Oryza sativa L.). II. Photosynthetic character, dry mass production and yield forming. Acta Agron Sin 30: 883–887 [Google Scholar]
- Li L, Shi ZY, Li L, Shen GZ, Wang XQ, An LS, Zhang JL. (2010) Overexpression of ACL1 (abaxially curled leaf 1) increased bulliform cells and induced abaxial curling of leaf blades in rice. Mol Plant 3: 807–817 [DOI] [PubMed] [Google Scholar]
- Li SG, He P, Wang YP, Li HY, Cheng Y, Zhou KD, Zhu LH. (2000) Genetic analysis and gene mapping of the leaf traits in rice (Oryza sativa L.). Acta Agron Sin 26: 261–265 [Google Scholar]
- Lid SE, Gruis D, Jung R, Lorentzen JA, Ananiev E, Chamberlin M, Niu X, Meeley R, Nichols S, Olsen OA. (2002) The defective kernel 1 (dek1) gene required for aleurone cell development in the endosperm of maize grains encodes a membrane protein of the calpain gene superfamily. Proc Natl Acad Sci USA 99: 5460–5465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsbauer K. (1930) Die epidermis. Linsbauer K , Encyclopedia of Plant Anatomy, Vol. 4(2) Gebrüder Borntraeger, Berlin [Google Scholar]
- Livak KJ, Schmittgen TD. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- Lloyd AM, Walbot V, Davis RW. (1992) Arabidopsis and Nicotiana anthocyanin production activated by maize regulators R and C1. Science 258: 1773–1775 [DOI] [PubMed] [Google Scholar]
- Lu P, Porat R, Nadeau JA, O’Neill SD. (1996) Identification of a meristem L1 layer-specific gene in Arabidopsis that is expressed during embryonic pattern formation and defines a new class of homeobox genes. Plant Cell 8: 2155–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo YZ, Zhao FM, Sang XC, Ling YH, Yang ZL, He GH. (2009) Genetic analysis and gene mapping of a novel rolled leaf mutant rl12(t) in rice. Acta Agron Sin 35: 1967–1972 [Google Scholar]
- Luo Z, Yang Z, Zhong B, Li Y, Xie R, Zhao F, Ling Y, He G. (2007) Genetic analysis and fine mapping of a dynamic rolled leaf gene, RL10(t), in rice (Oryza sativa L.). Genome 50: 811–817 [DOI] [PubMed] [Google Scholar]
- Martin C, Glover BJ. (2007) Functional aspects of cell patterning in aerial epidermis. Curr Opin Plant Biol 10: 70–82 [DOI] [PubMed] [Google Scholar]
- Masucci JD, Rerie WG, Foreman DR, Zhang M, Galway ME, Marks MD, Schiefelbein JW. (1996) The homeobox gene GLABRA2 is required for position-dependent cell differentiation in the root epidermis of Arabidopsis thaliana. Development 122: 1253–1260 [DOI] [PubMed] [Google Scholar]
- Metcalfe CR. (1960) Anatomy of Monocotyledons. I. Gramineae. Claredon Press, Oxford [Google Scholar]
- Moore R, Clark WD. (1998) Botany, Ed 2. WCB/McGraw Hill, Dubuque, Iowa [Google Scholar]
- Moulia B. (1994) The biomechanics of leaf rolling. Biomimetics 2: 267–281 [Google Scholar]
- Napoli C, Lemieux C, Jorgensen R. (1990) Introduction of a chimeric chalcone synthase gene into petunia results in reversible co-suppression of homologous genes in trans. Plant Cell 2: 279–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi Y, Oka A, Rodrigues-Pousada R, Possenti M, Ruberti I, Morelli G, Aoyama T. (2003) Modulation of phospholipid signaling by GLABRA2 in root-hair pattern formation. Science 300: 1427–1430 [DOI] [PubMed] [Google Scholar]
- Ohashi Y, Oka A, Ruberti I, Morelli G, Aoyama T. (2002) Entopically additive expression of GLABRA2 alters the frequency and spacing of trichome initiation. Plant J 29: 359–369 [DOI] [PubMed] [Google Scholar]
- O’Toole JC, Cruz RT, Singh TN. (1979) Leaf rolling and transpiration. Plant Sci Lett 16: 111–114 [Google Scholar]
- Peng H, Huang H, Yang Y, Zhai Y, Wu J, Huang D, Lu T. (2005) Functional analysis of GUS expression patterns and T-DNA integration characteristics in rice enhancer trap lines. Plant Sci 168: 1571–1579 [Google Scholar]
- Price AH, Young EM, Tomos AD. (1997) Trait loci associated with stomatal conductance, leaf rolling and heading date mapped in upland rice (Oryza sativa). New Phytol 137: 83–91 [Google Scholar]
- Rerie WG, Feldmann KA, Marks MD. (1994) The GLABRA2 gene encodes a homeo domain protein required for normal trichome development in Arabidopsis. Genes Dev 8: 1388–1399 [DOI] [PubMed] [Google Scholar]
- Serna L. (2004) A network of interacting factors triggering different cell fates. Plant Cell 16: 2258–2263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Chen J, Liu W, Huang Q, Shen B, Leung H, Wu J. (2009) Genetic analysis and gene mapping of a new rolled-leaf mutant in rice (Oryza sativa L.). Sci China C Life Sci 52: 885–890 [DOI] [PubMed] [Google Scholar]
- Shi Z, Wang J, Wan X, Shen G, Wang X, Zhang J. (2007) Over-expression of rice OsAGO7 gene induces upward curling of the leaf blade that enhanced erect-leaf habit. Planta 226: 99–108 [DOI] [PubMed] [Google Scholar]
- Shields LM. (1951) The involution mechanism in leaf of certain xeric grasses. Pytomorphology 1: 225–241 [Google Scholar]
- Smyth GK. (2004) Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 3: Article 3 [DOI] [PubMed] [Google Scholar]
- Soppe WJ, Jacobsen SE, Alonso-Blanco C, Jackson JP, Kakutani T, Koornneef M, Peeters AJ. (2000) The late flowering phenotype of fwa mutants is caused by gain-of-function epigenetic alleles of a homeodomain gene. Mol Cell 6: 791–802 [DOI] [PubMed] [Google Scholar]
- Szymanski DB, Jilk RA, Pollock SM, Marks MD. (1998) Control of GL2 expression in Arabidopsis leaves and trichomes. Development 125: 1161–1171 [DOI] [PubMed] [Google Scholar]
- Tominaga-Wada R, Iwata M, Sugiyama J, Kotake T, Ishida T, Yokoyama R, Nishitani K, Okada K, Wada T. (2009) The GLABRA2 homeodomain protein directly regulates CESA5 and XTH17 gene expression in Arabidopsis roots. Plant J 60: 564–574 [DOI] [PubMed] [Google Scholar]
- van der Krol AR, Mur LA, Beld M, Mol JN, Stuitje AR. (1990) Flavonoid genes in petunia: addition of a limited number of gene copies may lead to a suppression of gene expression. Plant Cell 2: 291–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernoud V, Laigle G, Rozier F, Meeley RB, Perez P, Rogowsky PM. (2009) The HD-ZIP IV transcription factor OCL4 is necessary for trichome patterning and anther development in maize. Plant J 59: 883–894 [DOI] [PubMed] [Google Scholar]
- Wan S, Wu J, Zhang Z, Sun X, Lv Y, Gao C, Ning Y, Ma J, Guo Y, Zhang Q, et al. (2009) Activation tagging, an efficient tool for functional analysis of the rice genome. Plant Mol Biol 69: 69–80 [DOI] [PubMed] [Google Scholar]
- Yan CJ, Yan S, Zhang ZQ, Liang GH, Lu JF, Gu MH. (2006) Genetic analysis and gene fine mapping for a rice novel mutant rl9(t) with rolling leaf character. Chin Sci Bull 51: 63–69 (in Chinese) [Google Scholar]
- Yan S, Yan CJ, Zeng XH, Yang YC, Fang YW, Tian CY, Sun YW, Cheng ZK, Gu MH. (2008) ROLLED LEAF 9, encoding a GARP protein, regulates the leaf abaxial cell fate in rice. Plant Mol Biol 68: 239–250 [DOI] [PubMed] [Google Scholar]
- Yang Y, Peng H, Huang H, Wu J, Jia S, Huang D, Lu T. (2004) Large-scale production of enhancer trapping lines for rice functional genomics. Plant Sci 167: 281–288 [Google Scholar]
- Yuan LP. (1997) Super-high yield hybrid rice breeding. Hybrid Rice 12: 1–6 [Google Scholar]
- Zhang GH, Xu Q, Zhu XD, Qian Q, Xue HW. (2009) SHALLOT-LIKE1 is a KANADI transcription factor that modulates rice leaf rolling by regulating leaf abaxial cell development. Plant Cell 21: 719–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X, Zou L, Foldager C, Bendtsen M, Feng W, Bünger CE. (2009) Different mechanisms of spinal fusion using equine bone protein extract, rhBMP-2 and autograft during the process of anterior lumbar interbody fusion. Biomaterials 30: 991–1004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.