Australia harbors some of the most nutrient-impoverished soils on Earth. Southwestern Australian soils are especially phosphorus (P) impoverished, due to the age of this ancient landscape and it being unaffected by major geological disturbance for millions of years (Hopper, 2009; Lambers et al., 2010). We are only now beginning to understand how plants acquire and use P in such highly infertile landscapes. At the same time, we are running out of nonrenewable, global phosphate resources in an era when we need more P fertilizers to produce more food and fiber to sustain a growing global population (Vance et al., 2003; Cordell et al., 2009; Gilbert, 2009). Can we learn valuable lessons for crop selection, breeding, and engineering from a flora that has evolved to function in soils with an extremely low availability of P (Lambers et al., 2006, 2008b, 2010)? Or are the traits in this flora highly suitable for the most P-impoverished soils but disadvantageous on the more fertile soils in which our crops currently grow? These questions can only be answered by learning more about the mechanisms that underpin the high plant P-efficiency traits that enable survival in P-impoverished landscapes as found in southwestern Australia. This Update explores traits in Proteaceae from soils with extremely low P availability and assesses whether these traits would be desirable for crops.
The ability to form root clusters and mycorrhizal associations are two adaptive traits that improve the ability of plants to acquire soil P (Lambers et al., 2008b; Smith et al., 2011). The root systems of species that develop cluster roots have a unique capacity for altered branch-root development (Shane and Lambers, 2005). Vast numbers of branch roots (“rootlets”) are initiated, which are compacted into specific regions along the axes of growing roots (Lambers et al., 2006). Species with root clusters (Shane and Lambers, 2005; Lambers et al., 2006) are relatively more abundant than mycorrhizal species on the most P-impoverished soils within old landscapes (Lambers et al., 2008b, 2010; Brundrett, 2009). Even though the majority of crop and forest species are mycorrhizal, some do form cluster roots, such as Macadamia integrifolia (macadamia nut), Aspalathus linearis (rooibos tea), Casuarina cunninghamiana (sheoak), Gevuina avellana, and Lupinus albus (white lupin); most of these species are nonmycorrhizal, but Casuarina species have both cluster roots and mycorrhizas (Halloy et al., 1996; Lambers and Shane, 2007).
In addition to P acquisition, we compare P use in plants that are common in old landscapes, particularly members of the Proteaceae, with that in plants from other regions of the world. We focus on Proteaceae because this is a plant family that is well represented in landscapes with a low P availability (Pate et al., 2001); consequently, much research attention has been devoted to their P nutrition (Lambers et al., 2010). We discuss the P-use efficiency of photosynthesis and growth, P-remobilization efficiency and proficiency, and P allocation to seeds. More sustainable, P-efficient cropping systems are urgently needed, and knowledge about native plant physiology in ancient landscapes may guide us toward their development, either through directing the breeding or engineering of existing major crop species or through aiding the development as crops of species that originate from these P-impoverished landscapes (Ryan et al., 2009; Pang et al., 2010a, 2010b; Suriyagoda et al., 2010; Bell et al., 2011).
ROOT CLUSTERS
Root clusters combine specialized structure and physiology (Johnson et al., 1994; Shane et al., 2004b) to maximize P acquisition from soils of low P availability, especially when P is present in insoluble complexes (e.g. rock phosphate and iron phosphate; Shane and Lambers, 2005; Lambers et al., 2006). They occur in monocots (e.g. “dauciform” roots in Cyperaceae; Lamont, 1982; Shane et al., 2005; Playsted et al., 2006) as well as dicots (e.g. “proteoid” roots in numerous families, including Proteaceae; Purnell, 1960; Gardner et al., 1983; Lamont, 2003; Lambers et al., 2006). Root clusters effectively “mine” soil P (Fig. 1A), as opposed to the “scavenging” strategy of mycorrhizas, and hence species that produce cluster roots dominate on the most P-depauperate soils (Lambers et al., 2008b, 2010).
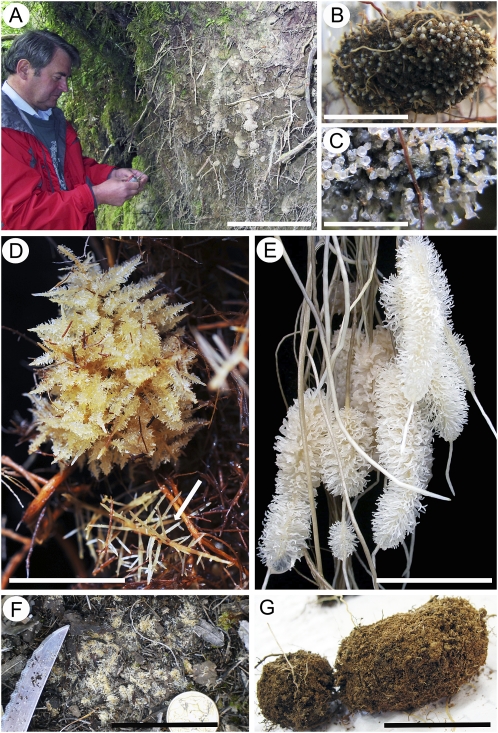
Figure 1.
Simple and compound types of proteoid root clusters of Proteaceae. Both types have large numbers of ephemeral rootlets arising from a persistent mother root. A to C, Roots of G. avellana (in Parque Katalapi, Chile). A, Soil profile beneath G. avellana showing numerous white clumps of simple proteoid roots at the trench face. B, Young proteoid root with a high density of growing rootlet tips and normal-shaped apices. C, Mature proteoid rootlets, after the soil has been washed off in water, showing “claviform” apices. D, Roots of Banksia repens, native to southwestern Australia, hydroponically grown at a very low [P] (1 μm or less), with compound proteoid roots. Mature Christmas tree-like morphology is shown at the top, with a developing cluster at bottom (arrow). E, Simple proteoid root clusters of H. prostrata, native to southwestern Australia, each with thousands of rootlets. Plants were grown hydroponically at a very low [P] (1 μm or less). This group of root clusters, about 8 to 12 d old, is likely at its peak in exudation of P-mobilizing carboxylates. Rootlets develop abundant root hairs. Unbranched noncluster roots release very little carboxylate. F, Banksia attenuata, native to southwestern Australia, develops in the field in dense mats of compound proteoid root clusters with rootlets and root hairs just below and in the litter layer. G, Simple proteoid roots of Hakea trifurcata, from the Jurien Bay area of southwestern Australia. Mature rootlets develop abundant root hairs that entrap soil and organic matter, forming sand sausages. Bars = 240 mm in A, 20 mm in B, 8 mm in C, 17 mm in D, 40 mm in E, 50 mm in F, and 30 mm in G.
Proteoid roots in Proteaceae can be “simple” bottlebrush-like structures (Lamont, 1972a, 1972b; Shane et al., 2004b) or “compound” Christmas tree-like structures (Fig. 1, B, D, and E). The structures comprise rootlets emerging from primary or secondary roots that usually end in normal apices; the rootlets typically show abundant root hairs (Fig. 1B), except for the “claviform” apices of G. avellana (Ramirez et al., 2004; Fig. 1C). Cluster roots produce large amounts of carboxylates, which are released in an “exudative burst” (Watt and Evans, 1999; Shane et al., 2004b). Carboxylates release P from strongly sorbed forms by replacing P bound to aluminum or iron in acid soils or P bound to calcium in alkaline soils (Geelhoed et al., 1998; Veneklaas et al., 2003) or by locally reducing pH in highly alkaline soils (Dinkelaker et al., 1989). In addition, exudation of phosphatases from root clusters of Dryandra sessilis (Proteaceae) releases P from organic sources (Grierson and Comerford, 2000). Banksia species produce compound clusters (Fig. 1D; Purnell, 1960); the vast majority of these species occur in southwestern Australia (Mast et al., 2005), the most ancient and P-impoverished part of the continent (Mast et al., 2005; Lambers et al., 2010). Their root clusters tend to form root mats at the interface between mineral soil and the litter layer (Fig. 1F; Grierson and Attiwill, 1989; Denton et al., 2007b), which may reflect their dependency on litter as a P source in extremely weathered sandy soils. In comparison, simple cluster roots entrap soil and organic matter in “sand sausages” that tend to be close to the soil surface (Fig. 1, A and G). However, depending on P availability in the profile, cluster roots may also be found at depth (Fig. 1A; Pate et al., 2001). The cluster-root strategy, particularly that of compound cluster roots, may come at a very high carbon cost (Lambers et al., 2008b). This high carbon cost precludes a large carbon investment in leaf growth and hence is associated with slow growth, thus making this P-acquisition strategy disadvantageous in environments where P is more available (Lambers et al., 2006, 2008b, 2010).
In the context of managed systems, monocultures would minimize penalties associated with the diminished competitiveness associated with slow growth due to greater carbon allocation to cluster roots. On the other hand, using crop species with cluster roots in intercropping systems or crop rotations may confer benefits to the noncluster root species with the less efficient root system, as demonstrated by facilitated P uptake by wheat (Triticum aestivum) when intercropped with L. albus (Gardner and Boundy, 1983). In both cases, however, whether introducing root clusters into crop plants is desirable or not depends on the exact yield penalty associated with root-cluster formation and on the fate of the P that is removed. Cluster roots in crop and pasture plants may well be advantageous, compared with mycorrhizal associations, in soils that have a high level of total P but where most of this is only sparingly available, such as young volcanic soils with low pH (Borie and Rubio, 2003). Examples of Proteaceae on such volcanic soils include Embothrium coccineum in Chile (Zúñiga-Feest et al., 2010). Old lateritic soils also contain high levels of occluded P (Tiessen et al., 1996), but it has yet to be investigated if any of this P is available to species with cluster roots. Crop and pasture plants with root clusters may provide a more reliable source of plant P than the variable benefits from mycorrhizal fungi (as well as utilizing a different soil P pool). For instance, in eastern Australia, mycorrhizas provide large benefits for P nutrition in the subtropical north (Thompson, 1987), whereas benefits are small and variable from year to year in temperate southern areas (Ryan et al., 2002, 2003, 2005; Ryan and Angus, 2003). Yet, despite these potential benefits, genetically transforming crops with the ability to form cluster roots by introducing genes from unrelated species is bound to be problematic, since we have yet to discover which genetic and molecular factors are responsible for cluster-root formation. That said, introducing cluster roots by crossing species with cluster roots with species without cluster roots appears to be possible. Interspecific crosses between Lupinus species with cluster roots (e.g. L. albus) with ones that lack them is certainly possible (Roy and Gladstones, 1985; Clements et al., 2008). Moreover, some Lupinus species that do not form true cluster roots produce “cluster-like” structures, which function just the same as true cluster roots (Hocking and Jeffery, 2004). Therefore, in the genus Lupinus, there is a wide range of genetic material with desirable P-efficient traits to choose from for crop improvement, without the immediate need of genetic transformation.
Variation in investment in cluster roots and sensitivity of cluster-root development to high shoot P status among Lupinus species suggests that the investment of carbon in cluster-root functioning associated with mining for P could be optimized to minimize the cost of P acquisition under cropping conditions (Pearse et al., 2006a, 2006b, 2007). Root clusters of L. albus, the best-studied cluster-root-forming crop species, do more than mine P; they show an exudative burst of isoflavonoids prior to the peak of organic acid exudation (Weisskopf et al., 2006b). Consequently, bacterial abundance in the surrounding soil is decreased at the stage when cluster roots exude large amounts of citrate and protons (Weisskopf et al., 2005). While flavonoids from L. albus roots mobilize inorganic phosphorus (Pi), they also decrease soil microbial respiration, citrate mineralization, and soil phosphohydrolase activities (Tomasi et al., 2008, 2009). Exudation of phenolic compounds, mainly isoflavonoids, induces fungal sporulation, thus reducing fungal vegetative growth and potential citrate consumption (Weisskopf et al., 2006b). In addition, the activity of two exuded antifungal cell wall-degrading enzymes, chitinase and glucanase, is highest at the stage preceding citrate excretion (Weisskopf et al., 2006a; Cesco et al., 2010). Do Proteaceae exhibit a similarly complex strategy to reduce microbial degradation of phosphate-solubilizing agents and to inhibit microbial P uptake (Grierson and Attiwill, 1989)? The roots of many Australian species exude malonate (Playsted et al., 2006; Pang et al., 2010a), a potent respiratory inhibitor, previously found in chickpea (Cicer arietinum; Veneklaas et al., 2003). Their ability to exude other compounds with the potential to inhibit microbial growth, such as flavonoids and other phenolics, needs to be investigated. Potentially, cluster-root-forming crop plants may not only be better at solubilizing P from sparingly soluble forms in soil but may also be able to restrict the amount of P that becomes incorporated into soil microbial pools as well as to access P from these pools. Breeding or development of crop plants that better compete against microbes for soil P could be very important if this translates into increases in yield. An alternative would be not to inhibit but to promote the growth of microorganisms that hydrolyze phytate, a compound that is not available to most higher plants. The challenge would be to reduce microbes that compete for P without reducing beneficial microbes. The research on lupin clusters demonstrates that this requires a complex and tightly coordinated series of biochemical processes.
LEAF P CONCENTRATIONS
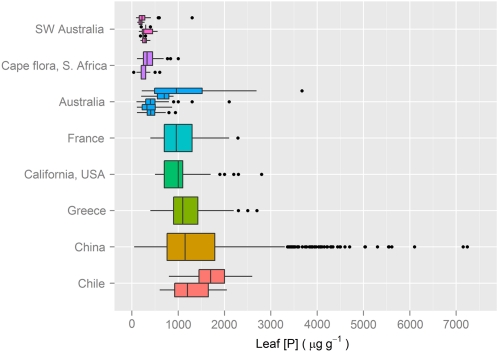
Leaf P concentrations ([P]) in southwestern Australian Banksia species (Denton et al., 2007a) and plants in general (Lambers et al., 2010) are considerably lower than global average values. Slightly greater leaf [P] values are found in fynbos vegetation in the Cape Region of South Africa and for plants in other regions of Australia (Fig. 2). While these Banksia leaves have a very high leaf mass per unit of leaf area (LMA; Hassiotou et al., 2009b), their high nitrogen-P ratios indicate that the low [P] is not simply the result of “dilution” by a high LMA but point to severe P limitation (Lambers et al., 2010). Despite their low leaf [P], area-based rates of photosynthesis are similar to global averages; in contrast, when expressed per unit of leaf P, rates of photosynthesis are extremely high (Denton et al., 2007a; Lambers et al., 2010). Understanding adaptations that allow for P-efficient photosynthesis of Proteaceae from southwestern Australia may provide valuable knowledge that can be used to improve the photosynthetic P-use efficiency (PPUE) of crops.
Figure 2.
Concentrations of P in leaves of plants in different regions of the world. Data are based on sources used by Lambers et al. (2010) with some additional values (Pate and Dell, 1984; Diehl et al., 2003; Niinemets et al., 2009). Values for Australia are for various regions on the continent, except southwestern Australia, which are presented separately. The central vertical bar in each box shows the median, the box represents the interquartile range, the whiskers show the location of the most extreme data points that are still within a factor of 1.5 of the upper or lower quartiles, and the black points are outliers that fall outside the values of the “extreme limits” described above.
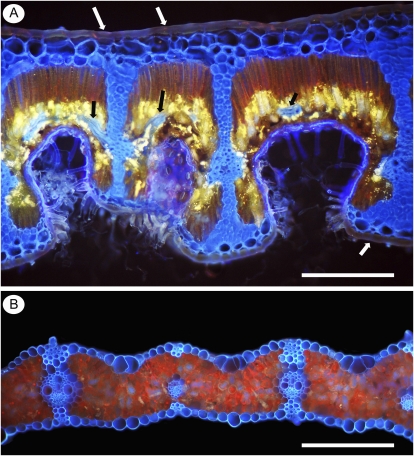
How can leaves exhibit ordinary rates of area-based photosynthesis at extraordinarily low leaf [P]? Because the LMA of barley (Hordeum vulgare; Gunn et al., 1999) is 11 times lower than that of Banksia (Denton et al., 2007a), the difference in P per unit of leaf area is actually smaller. Using rates of photosynthesis for barley leaves at different P supplies (Fay et al., 1996), we expect rates of photosynthesis to decrease from 22 to 15 μmol CO2 m−2 s−1 with decreasing P supply and the rate of photosynthesis per unit of P to be about 210 μmol CO2 g−1 P s−1, remarkably similar to the average for Banksia leaves (Denton et al., 2007a) and about twice as high as global average values (Lambers et al., 2010). This shows that both thin leaves with a low LMA, such as in barley (Fig. 3B), and thick leaves with a very high LMA, such as in Banksia (Fig. 3A), can use P equally efficiently in terms of photosynthesis. The high-LMA leaves live considerably longer, so the photosynthetic return per unit of P over a leaf’s lifetime is considerably higher in Banksia. Moreover, the cells and their functional components that account for the extra mass in the high-LMA Banksia leaves (Fig. 3A) must contain some P in essential compounds, such as nucleic acids. This implies that the photosynthetic cells of Banksia leaves must invest P very efficiently, even more so than barley.
Figure 3.
Hand-cut transverse sections of mature leaves. The lower leaf surface is at the bottom in each micrograph. UV-induced autofluorescence is shown. A, Scleromorphic leaf of B. repens (Proteaceae). Heavily thickened cell walls of epidermis, fibers, and vascular tissues fluorescence blue. Transverse veins (black arrows) connect longitudinal veins that separate each stomatal crypt. Chlorophyll in palisade parenchyma fluoresces red. Bright yellow fluorescence of some vacuolar contents is typical, but its identity is unknown. Upper and lower cuticles are thick (white arrows). Entrances to stomatal crypts are filled with long, thick-walled hairs. B, Mesophytic leaf of barley. The relatively thin-walled longitudinal veins (from left to right: large, small, and large intermediate lateral veins) and fibers fluorescence blue, and chlorophyll in mesophyll parenchyma fluorescences red. The outer epidermal cell wall and cuticle are thin. Bars = 440 μm in A and 240 μm in B.
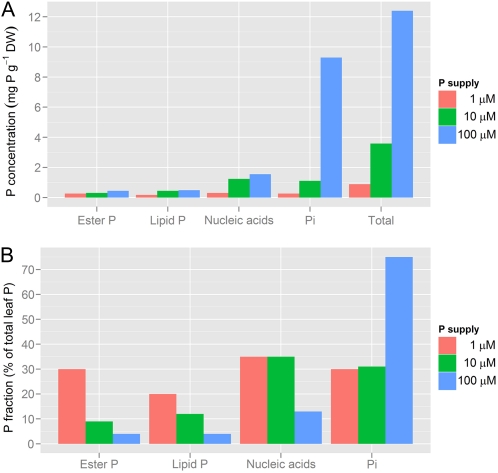
What is the biochemical mechanism underpinning the high PPUE of thick Banksia leaves with a very high LMA? To address this question, we first consider what P is used for in leaves of a mesophytic crop plant such as barley (Fig. 4). When grown with an optimum amount of P (100 μm), 75% of the total P in leaves is Pi (Fig. 4B; Chapin and Bieleski, 1982), most of which is located in the vacuole (Foyer and Spencer, 1986). When the P supply is decreased to 10 or 1 μm, the leaf Pi concentration ([Pi]) in barley leaves declines to 30% of total [P]. At the lowest P supply, the total leaf [P] is 8.86 mg g−1 dry weight in barley (Fig. 4A), still much higher than the approximately 200 μg g−1 dry weight found in leaves of a range of Banksia species (Denton et al., 2007a). Looking at the various P fractions in barley leaves (Fig. 4), Banksia leaves could economize by accumulating very little Pi and allocating it preferentially to photosynthetically active cells.
Figure 4.
P fractions in barley leaves as dependent on P supply, both in absolute (A) and in relative (B) terms. DW, Dry weight. (Based on data from Chapin and Bieleski [1982].)
Vacuolar [Pi] in Hakea prostrata (Proteaceae) leaves, which have a low leaf [P], about twice that of the leaf [P] in Banksia species referred to above, is below the detection limit of cryoscanning electron microscopy (Shane et al., 2004c). However, that technique is relatively insensitive, with a detection limit of about 10 mm (Ryan et al., 2003; Shane et al., 2004c). The vacuolar [P] in maize (Zea mays) leaves grown at very low P supply is about 5 mm, measured using 31P NMR (Loughman et al., 1989). Therefore, little accumulation of Pi in vacuoles is something that requires further exploration in Proteaceae, using 31P NMR.
Are Proteaceae investing less in phospholipids, replacing these with galactolipids or sulfolipids (Andersson et al., 2003; Raven, 2008)? Based on data from barley leaves (Fig. 4), such a replacement may only have a minor effect, because the fraction of total P in lipids is relatively small. Is there less investment in P esters, which play a role in photosynthetic and respiratory carbon metabolism? Can these metabolic pathways operate at lower substrate concentrations than are present in plants found on higher P soils? If so, how has the enzymology of metabolism adapted to accommodate smaller substrate pools (e.g. by increasing amounts of enzymes in these metabolic pathways)? Indeed, nitrogen-P ratios of Australian plants from severely impoverished soils are high (Lambers et al., 2010), and the photosynthetic nitrogen-use efficiency of leaves of some Australian plants from drier locations is relatively low (Wright et al., 2001). Wright et al. (2001) interpreted this as a water-conserving strategy, because greater investment in Rubisco and leaf nitrogen would allow photosynthesis to operate at lower internal CO2 concentrations. However, it might also enhance PPUE. A lower photosynthetic nitrogen-use efficiency, reflecting higher investment in Rubisco and other photosynthetic enzymes, which represent a major component of all organic nitrogen in C3 leaves (Evans, 1989), might allow the Calvin cycle to operate at lower levels of phosphorylated intermediates. Is this something we want in crop plants, or would it cause greater reliance on nitrogen inputs and hence be undesirable?
We should bear in mind that most photosynthetically active leaves on evergreen species, such as Banksia species, are mature and fully expanded; they only require protein synthesis associated with protein repair, turnover, and acclimation. Can a lower investment in P-rich nucleic acids (e.g. rRNA) explain a high PPUE? The “growth-rate hypothesis,” which is based on data for nonphotosynthetic microbes and metazoa (Elser et al., 1996; Acharya et al., 2004), predicts a direct proportionality of growth rate with rRNA content and a declining protein-rRNA ratio with increasing growth rate. Thus, the low leaf [P] among some Proteaceae is predicted to reflect low rRNA amounts, and thus a limit on the rate of protein synthesis. Although the predictions of the growth-rate hypothesis do not agree as well with data from photosynthetic organisms as with those from nonphotosynthetic organisms (Sterner and Elser, 2002; Matzek and Vitousek, 2009; Flynn et al., 2010), a very low [P] in biomass necessarily implies a low rRNA content and, hence, even with the maximum known catalytic activity of ribosomes, a low rate of protein synthesis. Is this a trait worth pursuing in crop plants, or would it jeopardize a plant’s acclimation potential if such acclimation requires rapid protein synthesis?
Given the high LMA of Banksia species, their high rates of photosynthesis are remarkable, because high-LMA leaves tend to have low area-based rates of photosynthesis (Galmés et al., 2005). High-LMA leaves also tend to have a Rubisco with greater specificity to compensate for this (Galmés et al., 2005). Thick Banksia leaves, on the other hand, have stomatal crypts (Fig. 3A; Hassiotou et al., 2009a). Since Rubisco enzymes with greater specificity tend to have a lower catalytic capacity (Tcherkez et al., 2006), the strategy of stomatal crypts in thick leaves might allow a Rubisco with “normal” catalytic properties, thus increasing PPUE by allowing photosynthesis at lower levels of protein and rRNA. It is likely that low-LMA crop plants already have a Rubisco with high catalytic capacity and that little can be gained by changing its kinetic properties.
In summary, the adaptive physiology underpinning a high PPUE among some Australian Proteaceae remains unknown. It will require considerable investigation before we can assess the significance of this knowledge for improving the PPUE of crops.
An additional consideration is that leaf [P] can become very high in many Proteaceae when fertilized with a readily available source of P (Handreck, 1991, 1997; Parks et al., 2000; Lambers et al., 2002; Shane et al., 2004c). This is accounted for by their low capacity to down-regulate their P uptake (Shane et al., 2004a, 2008; Shane and Lambers, 2006). It highlights that it is the down-regulation of the expression of P transporters that is of ecological significance (to avoid P toxicity), rather than their up-regulation at low P (which would do little to acquire more P when soil processes, rather than kinetic properties of the roots’ P transporters, are major limiting factors; Shane et al., 2004a; Lambers et al., 2006). A low capacity to down-regulate P uptake at elevated soil P availability would appear to be a highly undesirable trait in crop and pasture plants, but this requires further investigation. For example, the Australian native herb Ptilotus polystachyus (Amaranthaceae), a fast-growing plant with an apparent low capacity to down-regulate its P uptake, accumulates P to very high concentrations, approximately 40 mg g−1 shoot dry weight, without signs of P toxicity (Ryan et al., 2009).
P REMOBILIZATION DURING TISSUE SENESCENCE: P-REMOBILIZATION EFFICIENCY AND PROFICIENCY
Some of the species that exhibit high rates of photosynthesis at low leaf [P] are also extremely efficient at remobilizing P from senescing leaves. In particular, some Banksia species can remobilize over 80% of their leaf P (Denton et al., 2007a), whereas global average values are about 50% (Lambers et al., 2008a). Considering that Banksia leaves have very low leaf [P] when mature, their P proficiency is extremely high (i.e. the level to which P is depleted is very low). That high proficiency is, to some extent, explained by high LMA values, as discussed above for leaf [P]. Even so, the P-remobilization proficiency values are much higher than values reported before in the literature (Killingbeck, 1996; Lambers et al., 2008a). Remobilization from cluster roots of H. prostrata (Proteaceae) is equally efficient and proficient (Shane et al., 2004b). A high P-resorption proficiency from senescing clusters is thought to be important, because these structures represent significant P sinks but live for only about 21 d in Proteaceae (Shane et al., 2004b, 2006; Playsted et al., 2006).
Proficient and efficient P remobilization from senescent leaves would be a desirable trait for the P economy of crops and pastures for several reasons. First, it would enable better use of P during plant development (e.g. through allocation of P to younger leaves with higher PPUE). Second, remobilization into stems or root systems conserves P for future use in the plant through further internal cycling, especially in perennial crops, or through mineralization of crop residues. Third, remobilization from senescing plant parts into seeds could enhance seed production or seed P content. High seed P content, however, is not always a positive outcome, as discussed below.
P STORAGE IN SEEDS
Contrary to the very low [P] in leaves, seed [P] is often very high in species naturally occurring on severely P-impoverished soils (Groom and Lamont, 2010). Seed P represents up to half of the total aboveground P in Banksia hookeriana (Proteaceae), which grows on severely P-impoverished sands in southwestern Australia (Witkowski and Lamont, 1996). While high seed P is advantageous to plants that need to establish offspring in a P-impoverished environment, this is not necessarily desirable within the context of a cropping system, where removal of P in grain must be matched with inputs of fertilizer P. A very important P-storage compound in seeds is a mixed cation salt of myoinositol hexakis-phosphoric acid (phytate; Lott et al., 2000). It is the major source of P during early seedling growth in maize (Nadeem et al., 2011). A high phytate concentration in grains may render micronutrients such as iron and zinc less available, leading to micronutrient disorders in humans (Welch and Graham, 2004). Most of the P in the human diet is excreted again, so is not required for human health (Cordell et al., 2009). Breeding for low-phytate content in grain is an option (Raboy et al., 2000; Raboy, 2001), but at least in rice (Oryza sativa), low phytic acid content is associated with reduced grain yield and seed viability (Zhao et al., 2008), suggesting a tradeoff between human health benefits and crop performance. In summary, the high seed [P] often found in species native to P-impoverished soils is not a desirable trait in crop plants, where low seed [P] may contribute to more efficient use of P fertilizer and better nutritional value. Instead, the challenge is to ensure that more seeds are produced at the expense of seed P content.
CONCLUDING REMARKS
Most species in the Proteaceae are nonmycorrhizal and extremely efficient at acquiring P from P-impoverished soils. Their P-mobilizing cluster roots are a P-mining strategy, as opposed to the scavenging strategy of mycorrhizal plants (Lambers et al., 2008b). Some of these species are also very efficient at photosynthesizing at very low leaf [P], but the current lack of knowledge of P pools in their leaves and their involvement in the photosynthetic process limit our understanding of their very high photosynthetic P-use efficiency. P remobilization from senescing tissues is among the highest recorded, and again, a biochemical explanation is currently not available. Seed [P] in Proteaceae is very high, allowing seedlings to grow independent of soil P for a long time, but high seed [P] may not be a desirable trait for crop plants, due to negative impacts on human health from high phytate and the need to replace P removed in grain with fertilizer P. Understanding the functioning of Proteaceae adapted to P-impoverished landscapes, particularly their desirable traits for P-utilization or -acquisition efficiency such as photosynthesizing at low leaf [P] and cluster-root development, will provide knowledge that can be used toward the development of more P-efficient crops.
Several strategies are available to us for improving P efficiency in cropping systems. First, we can make greater use of crop species with cluster roots in environments with large amounts of total P but low P availability (e.g. young volcanic soils in Chile, where L. albus is a profitable crop with minimal fertilizer input; Huyghe, 1997; Von Baer, 2006). Second, we should also further explore the potential of native species with P-efficient traits for use as crops or pastures. Third, at least in some of our present crop species, there is substantial variation in P-acquisition or P-use efficiency; that variation could be exploited through marker-assisted breeding. Fourth, there is the option of intercropping and crop rotation to provide benefits of P-efficient crops to less efficient ones. Finally, a better understanding of the molecular basis of the P-efficient traits may, in the long term, allow us to engineer plants that are more P efficient. A molecular approach will be challenging, but the possible benefits in the long term are enormous. Pursuing a combination of the above approaches is critical to improving the productivity of crop production in an era of rapid population growth where P fertilizers are becoming scarcer and more costly.
Acknowledgments
We thank Margaret McCully for her critical input into the figure legends, Alejandra Zúñiga-Feest for her kind invitation to explore cluster roots of Proteaceae in Chile, Marion Cambridge for providing the photographs of G. avellana, Luis Corcuera for being a great host while H.L. visited Parque Katalapi, and two anonymous reviewers for thoughtful and constructive comments.
References
- Acharya K, Kyle M, Elser JJ. (2004) Biological stoichiometry of Daphnia growth: an ecophysiological test of the growth rate hypothesis. Limnol Oceanogr 49: 656–665 [Google Scholar]
- Andersson MX, Stridh MH, Larsson KE, Liljenberg C, Sandelius AS. (2003) Phosphate-deficient oat replaces a major portion of the plasma membrane phospholipids with the galactolipid digalactosyldiacylglycerol. FEBS Lett 537: 128–132 [DOI] [PubMed] [Google Scholar]
- Bell LW, Bennett RG, Ryan MH, Clarke H. (2011) The potential of herbaceous native Australian legumes as grain crops: a review. Renew Agric Food Syst 26: 72–91 [Google Scholar]
- Borie F, Rubio R. (2003) Total and organic phosphorus in Chilean volcanic soils. Gayana Bot 60: 69–73 [Google Scholar]
- Brundrett M. (2009) Mycorrhizal associations and other means of nutrition of vascular plants: understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil 320: 37–77 [Google Scholar]
- Cesco S, Neumann G, Tomasi N, Pinton R, Weisskopf L. (2010) Release of plant-borne flavonoids into the rhizosphere and their role in plant nutrition. Plant Soil 329: 1–25 [Google Scholar]
- Chapin FS, Bieleski RL. (1982) Mild phosphorus stress in barley and a related low-phosphorus-adapted barleygrass: phosphorus fractions and phosphate absorption in relation to growth. Physiol Plant 54: 309–317 [Google Scholar]
- Clements J, Prilyuk L, Quealy J, Francis G. (2008) Interspecific crossing among the New World lupin species for Lupinus mutabilis crop improvement. Palta JA, Berger JB, , Lupins for Health and Wealth: Proceedings of the 12th International Lupin Conference, 14–18 Sept. 2008, Fremantle, Western Australia. International Lupin Association, Canterbury, New Zealand, pp 324–327 [Google Scholar]
- Cordell D, Drangert J-O, White S. (2009) The story of phosphorus: global food security and food for thought. Glob Environ Change 19: 292–305 [Google Scholar]
- Denton MD, Veneklaas EJ, Freimoser FM, Lambers H. (2007a) Banksia species (Proteaceae) from severely phosphorus-impoverished soils exhibit extreme efficiency in the use and re-mobilization of phosphorus. Plant Cell Environ 30: 1557–1565 [DOI] [PubMed] [Google Scholar]
- Denton MD, Veneklaas EJ, Lambers H. (2007b) Does phenotypic plasticity in carboxylate exudation differ among rare and widespread Banksia species (Proteaceae)? New Phytol 173: 592–599 [DOI] [PubMed] [Google Scholar]
- Diehl P, Mazzarino MJ, Funes F, Fontenla S, Gobbi M, Ferrari J. (2003) Nutrient conservation strategies in native Andean-Patagonian forests. J Veg Sci 14: 63–70 [Google Scholar]
- Dinkelaker B, Römheld V, Marschner H. (1989) Citric acid excretion and precipitation of calcium citrate in the rhizosphere of white lupin (Lupinus albus L.). Plant Cell Environ 12: 285–292 [Google Scholar]
- Elser JJ, Dobberfuhl D, MacKay NA, Schampel JH. (1996) Organism size, life history, and N:P stoichiometry: towards a unified view of cellular and ecosystem processes. Bioscience 46: 674–684 [Google Scholar]
- Evans JR. (1989) Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 78: 9–19 [DOI] [PubMed] [Google Scholar]
- Fay P, Mitchell DT, Osborne BA. (1996) Photosynthesis and nutrient-use efficiency of barley in response to low arbuscular mycorrhizal colonization and addition of phosphorus. New Phytol 132: 425–433 [DOI] [PubMed] [Google Scholar]
- Flynn K, Raven J, Rees T, Finkel Z, Quigg A, Beardall J. (2010) Is the growth rate hypothesis applicable to microalgae? J Phycol 46: 1–12 [Google Scholar]
- Foyer C, Spencer C. (1986) The relationship between phosphate status and photosynthesis in leaves. Planta 167: 369–375 [DOI] [PubMed] [Google Scholar]
- Galmés J, Flexas J, Keys AJ, Cifre J, Mitchell RAC, Madgwick PJ, Haslam RP, Medrano H, Parry MAJ. (2005) Rubisco specificity factor tends to be larger in plant species from drier habitats and in species with persistent leaves. Plant Cell Environ 28: 571–579 [Google Scholar]
- Gardner WK, Barber DA, Parbery DG. (1983) The acquisition of phosphorus by Lupinus albus L. III. The probable mechanism by which phosphorus movement in the soil/root interface is enhanced. Plant Soil 70: 107–124 [Google Scholar]
- Gardner WK, Boundy KA. (1983) The acquisition of phosphorus by Lupinus albus L. IV. The effect of interplanting wheat and white lupin on the growth and mineral composition of the two species. Plant Soil 70: 391–402 [Google Scholar]
- Geelhoed JS, Hiemstra T, Van Riemsdijk WH. (1998) Competitive interaction between phosphate and citrate on goethite. Environ Sci Technol 32: 2119–2123 [Google Scholar]
- Gilbert N. (2009) The disappearing nutrient. Nature 461: 716–718 [DOI] [PubMed] [Google Scholar]
- Grierson PF, Attiwill PM. (1989) Chemical characteristics of the proteoid root mat of Banksia integrifolia L. Aust J Bot 37: 137–143 [Google Scholar]
- Grierson PF, Comerford NB. (2000) Non-destructive measurement of acid phosphatase activity in the rhizosphere using nitrocellulose membranes and image analysis. Plant Soil 218: 49–57 [Google Scholar]
- Groom PK, Lamont BB. (2010) Phosphorus accumulation in Proteaceae seeds: a synthesis. Plant Soil 334: 61–72 [Google Scholar]
- Gunn S, Farrar JF, Collis BE, Nason M. (1999) Specific leaf area in barley: individual leaves versus whole plants. New Phytol 143: 45–51 [Google Scholar]
- Halloy S, Grau A, McKenzie B. (1996) Gevuina nut (Gevuina avellana, Proteaceae), a cool climate alternative to macadamia. Econ Bot 50: 224–235 [Google Scholar]
- Handreck K. (1991) Interactions between iron and phosphorus in the nutrition of Banksia ericifolia L. f var ericifolia (Proteaceae) in soil-less potting media. Aust J Bot 39: 373–384 [Google Scholar]
- Handreck KA. (1997) Phosphorus requirements of Australian native plants. Aust J Soil Res 35: 241–290 [Google Scholar]
- Hassiotou F, Evans JR, Ludwig M, Veneklaas EJ. (2009a) Stomatal crypts may facilitate diffusion of CO2 to adaxial mesophyll cells in thick sclerophylls. Plant Cell Environ 32: 1596–1611 [DOI] [PubMed] [Google Scholar]
- Hassiotou F, Ludwig M, Renton M, Veneklaas EJ, Evans JR. (2009b) Influence of leaf dry mass per area, CO2, and irradiance on mesophyll conductance in sclerophylls. J Exp Bot 60: 2303–2314 [DOI] [PubMed] [Google Scholar]
- Hocking PJ, Jeffery S. (2004) Cluster-root production and organic anion exudation in a group of Old-World lupins and a New-World lupin. Plant Soil 258: 135–150 [Google Scholar]
- Hopper SD. (2009) OCBIL theory: towards an integrated understanding of the evolution, ecology and conservation of biodiversity on old, climatically buffered, infertile landscapes. Plant Soil 322: 49–86 [Google Scholar]
- Huyghe C. (1997) White lupin (Lupinus albus L.). Field Crops Res 53: 147–160 [Google Scholar]
- Johnson JF, Allan DL, Vance CP. (1994) Phosphorus stress-induced proteoid roots show altered metabolism in Lupinus albus. Plant Physiol 104: 657–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killingbeck KT. (1996) Nutrients in senesced leaves: keys to the search for potential resorption and resorption proficiency. Ecology 77: 1716–1727 [Google Scholar]
- Lambers H, Brundrett MC, Raven JA, Hopper SD. (2010) Plant mineral nutrition in ancient landscapes: high plant species diversity on infertile soils is linked to functional diversity for nutritional strategies. Plant Soil 334: 11–31 [Google Scholar]
- Lambers H, Chapin FS, Pons TL. (2008a) Plant Physiological Ecology, Ed 2. Springer, New York [Google Scholar]
- Lambers H, Juniper D, Cawthray GR, Veneklaas EJ, Martínez-Ferri E. (2002) The pattern of carboxylate exudation in Banksia grandis (Proteaceae) is affected by the form of phosphate added to the soil. Plant Soil 238: 111–122 [Google Scholar]
- Lambers H, Raven JA, Shaver GR, Smith SE. (2008b) Plant nutrient-acquisition strategies change with soil age. Trends Ecol Evol 23: 95–103 [DOI] [PubMed] [Google Scholar]
- Lambers H, Shane MW. (2007) Role of root clusters in phosphorus acquisition and increasing biological diversity in agriculture. Spiertz JHJ, Struik PC, Van Laar HH, , Scale and Complexity in Plant Systems Research: Gene-Plant-Crop Relations. Springer, Dordrecht, The Netherlands, pp 237–250 [Google Scholar]
- Lambers H, Shane MW, Cramer MD, Pearse SJ, Veneklaas EJ. (2006) Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Ann Bot (Lond) 98: 693–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont B. (1972a) The effect of soil nutrients on the production of proteoid roots by Hakea species. Aust J Bot 20: 27–40 [Google Scholar]
- Lamont B. (1972b) The morphology and anatomy of proteoid roots in the genus Hakea. Aust J Bot 20: 155–174 [Google Scholar]
- Lamont B. (1982) Mechanisms for enhancing nutrient uptake in plants, with particular reference to Mediterranean South Africa and Western Australia. Bot Rev 48: 597–689 [Google Scholar]
- Lamont BB. (2003) Structure, ecology and physiology of root clusters: a review. Plant Soil 248: 1–19 [Google Scholar]
- Lott JNA, Ockenden I, Raboy V, Batten GD. (2000) Phytic acid and phosphorus in crop seeds and fruits: a global estimate. Seed Sci Res 10: 11–33 [Google Scholar]
- Loughman BC, Ratcliffe RG, Southon TE. (1989) Observations on the cytoplasmic and vacuolar orthophosphate pools in leaf tissues using in vivo 31P-NMR spectroscopy. FEBS Lett 242: 279–284 [Google Scholar]
- Mast AR, Jones EH, Havery SP. (2005) An assessment of old and new DNA sequence evidence for the paraphyly of Banksia with respect to Dryandra (Proteaceae). Aust Syst Bot 18: 75–88 [Google Scholar]
- Matzek V, Vitousek PM. (2009) N:P stoichiometry and protein:RNA ratios in vascular plants: an evaluation of the growth-rate hypothesis. Ecol Lett 12: 765–771 [DOI] [PubMed] [Google Scholar]
- Nadeem M, Mollier A, Morel C, Vives A, Prud’Homme L, Pellerin S. (2011) Relative contribution of seed phosphorus reserves and exogenous phosphorus uptake to maize (Zea mays L.) nutrition during early growth stages. Plant Soil (in press) [Google Scholar]
- Niinemets U, Wright IJ, Evans JR. (2009) Leaf mesophyll diffusion conductance in 35 Australian sclerophylls covering a broad range of foliage structural and physiological variation. J Exp Bot 60: 2433–2449 [DOI] [PubMed] [Google Scholar]
- Pang J, Ryan MH, Tibbett M, Cawthray GR, Siddique KHM, Bolland MDA, Denton MD, Lambers H. (2010a) Variation in morphological and physiological parameters in herbaceous perennial legumes in response to phosphorus supply. Plant Soil 331: 241–255 [Google Scholar]
- Pang J, Tibbett M, Denton MD, Lambers H, Siddique KHM, Bolland MDA, Revell C, Ryan MH. (2010b) Variation in seedling growth of 11 perennial legumes in response to phosphorus supply. Plant Soil 328: 133–143 [Google Scholar]
- Parks SE, Haigh AM, Cresswell GC. (2000) Stem tissue phosphorus as an index of the phosphorus status of Banksia ericifolia L. f. Plant Soil 227: 59–65 [Google Scholar]
- Pate JS, Dell B. (1984) Economy of mineral nutrients in sandplain species. Pate JS, Beard JS, , Kwongan: Plant Life of the Sandplain. University of Western Australia Press, Nedlands, Australia, pp 227–252 [Google Scholar]
- Pate JS, Verboom WH, Galloway PD. (2001) Cooccurrence of Proteaceae, laterite and related oligotrophic soils: coincidental associations or causative inter-relationships? Aust J Bot 49: 529–560 [Google Scholar]
- Pearse SJ, Veneklaas EJ, Cawthray GR, Bolland MDA, Lambers H. (2006a) Carboxylate release of wheat, canola and 11 grain legume species as affected by phosphorus status. Plant Soil 288: 127–139 [Google Scholar]
- Pearse SJ, Veneklaas EJ, Cawthray GR, Bolland MDA, Lambers H. (2006b) Triticum aestivum shows a greater biomass response to a supply of aluminium phosphate than Lupinus albus, despite releasing fewer carboxylates into the rhizosphere. New Phytol 169: 515–524 [DOI] [PubMed] [Google Scholar]
- Pearse SJ, Veneklaas EJ, Cawthray GR, Bolland MDA, Lambers H. (2007) Carboxylate composition of root exudates does not relate consistently to a crop species’ ability to use phosphorus from aluminium, iron or calcium phosphate sources. New Phytol 173: 181–190 [DOI] [PubMed] [Google Scholar]
- Playsted CWS, Johnston ME, Ramage CM, Edwards DG, Cawthray GR, Lambers H. (2006) Functional significance of dauciform roots: exudation of carboxylates and acid phosphatase under phosphorus deficiency in Caustis blakei (Cyperaceae). New Phytol 170: 491–500 [DOI] [PubMed] [Google Scholar]
- Purnell H. (1960) Studies of the family Proteaceae. I. Anatomy and morphology of the roots of some Victorian species. Aust J Bot 8: 38–50 [Google Scholar]
- Raboy V. (2001) Seeds for a better future: ‘low phytate’ grains help to overcome malnutrition and reduce pollution. Trends Plant Sci 6: 458–462 [DOI] [PubMed] [Google Scholar]
- Raboy V, Gerbasi PF, Young KA, Stoneberg SD, Pickett SG, Bauman AT, Murthy PPN, Sheridan WF, Ertl DS. (2000) Origin and seed phenotype of maize low phytic acid 1-1 and low phytic acid 2-1. Plant Physiol 124: 355–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez GC, Valenzuela FE, San Martin PC. (2004) Nuevos antecedentes sobre desarrollo temprano, morfología y anatomía de las raíces proteiformes de Gevuina avellana. Agro Sur 32: 33–44 [Google Scholar]
- Raven JA. (2008) Phosphorus and the future. White PJ, Hammond JP, , The Ecophysiology of Plant-Phosphorus Interactions. Springer, Dordrecht, The Netherlands, pp 271–283 [Google Scholar]
- Roy NN, Gladstones JS. (1985) Prospects for interspecific hybridization of Lupinus atlanticus Gladst. with L. cosentini Guss. Theor Appl Genet 71: 238–241 [DOI] [PubMed] [Google Scholar]
- Ryan MH, Angus JF. (2003) Arbuscular mycorrhizae in wheat and field pea crops on a low P soil: increased Zn-uptake but no increase in P-uptake or yield. Plant Soil 250: 225–239 [Google Scholar]
- Ryan MH, Ehrenberg S, Bennett RG, Tibbett M. (2009) Putting the P in Ptilotus: a phosphorus-accumulating herb native to Australia. Ann Bot (Lond) 103: 901–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan MH, McCully ME, Huang CX. (2003) Location and quantification of phosphorus and other elements in fully hydrated, soil-grown arbuscular mycorrhizas: a cryo-analytical scanning electron microscopy study. New Phytol 160: 429–441 [DOI] [PubMed] [Google Scholar]
- Ryan MH, Norton RM, Kirkegaard JA, McCormick KM, Knights SE, Angus JF. (2002) Increasing mycorrhizal colonisation does not improve growth and nutrition of wheat on Vertosols in south-eastern Australia. Aust J Agric Res 53: 1173–1181 [Google Scholar]
- Ryan MH, Van Herwaarden AF, Angus JF, Kirkegaard JA. (2005) Reduced growth of autumn-sown wheat in a low-P soil is associated with high colonisation by arbuscular mycorrhizal fungi. Plant Soil 270: 275–286 [Google Scholar]
- Shane M, Szota C, Lambers H. (2004a) A root trait accounting for the extreme phosphorus sensitivity of Hakea prostrata (Proteaceae). Plant Cell Environ 27: 991–1004 [Google Scholar]
- Shane MW, Cawthray GR, Cramer MD, Kuo J, Lambers H. (2006) Specialized ‘dauciform’ roots of Cyperaceae are structurally distinct, but functionally analogous with ‘cluster’ roots. Plant Cell Environ 29: 1989–1999 [DOI] [PubMed] [Google Scholar]
- Shane MW, Cramer MD, Funayama-Noguchi S, Cawthray GR, Millar AH, Day DA, Lambers H. (2004b) Developmental physiology of cluster-root carboxylate synthesis and exudation in harsh hakea: expression of phosphoenolpyruvate carboxylase and the alternative oxidase. Plant Physiol 135: 549–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shane MW, Cramer MD, Lambers H. (2008) Root of edaphically controlled Proteaceae turnover on the Agulhas Plain, South Africa: phosphate uptake regulation and growth. Plant Cell Environ 31: 1825–1833 [DOI] [PubMed] [Google Scholar]
- Shane MW, Dixon KW, Lambers H. (2005) The occurrence of dauciform roots amongst Western Australian reeds, rushes and sedges, and the impact of phosphorus supply on dauciform-root development in Schoenus unispiculatus (Cyperaceae). New Phytol 165: 887–898 [DOI] [PubMed] [Google Scholar]
- Shane MW, Lambers H. (2005) Cluster roots: a curiosity in context. Plant Soil 274: 101–125 [Google Scholar]
- Shane MW, Lambers H. (2006) Systemic suppression of cluster-root formation and net P-uptake rates in Grevillea crithmifolia at elevated P supply: a proteacean with resistance for developing symptoms of ‘P toxicity’. J Exp Bot 57: 413–423 [DOI] [PubMed] [Google Scholar]
- Shane MW, McCully ME, Lambers H. (2004c) Tissue and cellular phosphorus storage during development of phosphorus toxicity in Hakea prostrata (Proteaceae). J Exp Bot 55: 1033–1044 [DOI] [PubMed] [Google Scholar]
- Smith SE, Jakobsen I, Groenlund M, Smith FA. (2011) Roles of arbuscular mycorrhizas in plant phosphorus nutrition: interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiol 156: 1050–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterner R, Elser JJ. (2002) Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere. Princeton University Press, Princeton, NJ [Google Scholar]
- Suriyagoda LD, Ryan MH, Renton M, Lambers H. (2010) Multiple adaptive responses of Australian native perennial legumes with pasture potential to grow in phosphorus- and moisture-limited environments. Ann Bot (Lond) 105: 755–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tcherkez GGB, Farquhar GD, Andrews TJ. (2006) Despite slow catalysis and confused substrate specificity, all ribulose bisphosphate carboxylases may be nearly perfectly optimized. Proc Natl Acad Sci USA 103: 7246–7251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. (1987) Decline of vesicular-arbuscular mycorrhizae in long fallow disorder of field crops and its expression in phosphorus deficiency of sunflower. Aust J Agric Res 38: 847–867 [Google Scholar]
- Tiessen H, Monaco S, Ramirez A, Santos M, Shang C. (1996) Phosphate minerals in a lateritic crust from Venezuela. Biogeochemistry 34: 1–17 [Google Scholar]
- Tomasi N, Kretzschmar T, Espen L, Weisskopf L, Fuglsang AT, Palmgren MG, Neumann G, Varanini Z, Pinton R, Martinoia E, et al. (2009) Plasma membrane H-ATPase-dependent citrate exudation from cluster roots of phosphate-deficient white lupin. Plant Cell Environ 32: 465–475 [DOI] [PubMed] [Google Scholar]
- Tomasi N, Weisskopf L, Renella G, Landi L, Pinton R, Varanini Z, Nannipieri P, Torrent J, Martinoia E, Cesco S. (2008) Flavonoids of white lupin roots participate in phosphorus mobilization from soil. Soil Biol Biochem 40: 1971–1974 [Google Scholar]
- Vance CP, Uhde-Stone C, Allan DL. (2003) Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytol 157: 423–447 [DOI] [PubMed] [Google Scholar]
- Veneklaas EJ, Stevens J, Cawthray GR, Turner S, Grigg AM, Lambers H. (2003) Chickpea and white lupin rhizosphere carboxylates vary with soil properties and enhance phosphorus uptake. Plant Soil 248: 187–197 [Google Scholar]
- Von Baer E. (2006) Relevant points for the production and use of sweet lupin in Chile. Palta JA, Berger JB, , Proceedings of the 12th International Lupin Conference, 14–18 Sept. 2008, Fremantle, Western Australia. International Lupin Association, Canterbury, New Zealand, pp 116–119 [Google Scholar]
- Watt M, Evans JR. (1999) Proteoid roots: physiology and development. Plant Physiol 121: 317–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisskopf L, Abou-Mansour E, Fromin N, Tomasi N, Santelia D, Edelkott I, Neumann G, Aragno M, Tabacchi R, Martinoia E. (2006a) White lupin has developed a complex strategy to limit microbial degradation of secreted citrate required for phosphate acquisition. Plant Cell Environ 29: 919–927 [DOI] [PubMed] [Google Scholar]
- Weisskopf L, Fromin N, Tomasi N, Aragno M, Martinoia E. (2005) Secretion activity of white lupin’s cluster roots influences bacterial abundance, function and community structure. Plant Soil 268: 181–194 [Google Scholar]
- Weisskopf L, Tomasi N, Santelia D, Martinoia E, Langlade NB, Tabacchi R, Abou-Mansour E. (2006b) Isoflavonoid exudation from white lupin roots is influenced by phosphate supply, root type and cluster-root stage. New Phytol 171: 657–668 [DOI] [PubMed] [Google Scholar]
- Welch RM, Graham RD. (2004) Breeding for micronutrients in staple food crops from a human nutrition perspective. J Exp Bot 55: 353–364 [DOI] [PubMed] [Google Scholar]
- Witkowski ETF, Lamont BB. (1996) Disproportionate allocation of mineral nutrients and carbon between vegetative and reproductive structures in Banksia hookeriana. Oecologia 105: 38–42 [DOI] [PubMed] [Google Scholar]
- Wright IJ, Reich PB, Westoby M. (2001) Strategy shifts in leaf physiology, structure and nutrient content between species of high- and low-rainfall and high- and low-nutrient habitats. Funct Ecol 15: 423–434 [Google Scholar]
- Zhao H-J, Liu Q-L, Fu H-W, Xu X-H, Wu D-X, Shu Q-Y. (2008) Effect of non-lethal low phytic acid mutations on grain yield and seed viability in rice. Field Crops Res 108: 206–211 [Google Scholar]
- Zúñiga-Feest A, Delgado M, Alberdi M. (2010) The effect of phosphorus on growth and cluster-root formation in the Chilean Proteaceae: Embothrium coccineum (R. et J. Forst.). Plant Soil 334: 113–121 [Google Scholar]