Abstract
Whole plant senescence of monocarpic plants consists of three major processes: arrest of shoot apical meristem, organ senescence, and permanent suppression of axillary buds. At early stages of development, axillary buds are inhibited by shoot apex-produced auxin, a mechanism known as apical dominance. How the buds are suppressed as an essential part of whole plant senescence, especially when the shoot apexes are senescent, is not clear. Here, we report an AtMYB2-regulated post apical dominance mechanism by which Arabidopsis (Arabidopsis thaliana) inhibits the outgrowth of axillary buds as part of the whole plant senescence program. AtMYB2 is expressed in the compressed basal internode region of Arabidopsis at late stages of development to suppress the production of cytokinins, the group of hormones that are required for axillary bud outgrowth. atmyb2 T-DNA insertion lines have enhanced expression of cytokinin-synthesizing isopentenyltransferases genes, contain higher levels of cytokinins, and display a bushy phenotype at late stages of development. As a result of the continuous generation of new shoots, atmyb2 plants have a prolonged life span. The AtMYB2 promoter-directed cytokinin oxidase 1 gene in the T-DNA insertion lines reduces the endogenous cytokinin levels and restores the bushy phenotype to the wild type.
Plants, like many other organisms, exhibit various life history patterns and possess a broad spectrum of longevity, ranging from a few weeks to several thousand years. Annuals (e.g. Arabidopsis [Arabidopsis thaliana]), biennials (e.g. wheat [Triticum aestivum]), and some perennials (e.g. bamboo [Leleba oldhami]) possess a monocarpic lifestyle, which is characterized by only a single reproductive event in the life cycle. After flowering (and setting seeds or fruits), the whole plant will senesce and die (Gan, 2003). The monocarpic senescence includes three coordinated processes: (1) senescence of somatic organs and tissues such as leaves; (2) arrest of shoot apical meristems (SAM); and (3) suppression of axillary buds to prevent the formation of new shoots/branches. The longevity of an Arabidopsis plant could be prolonged by mutations leading to long-lived leaves, prolonged SAM activity, or continued generation of new shoots as a result of axillary bud outgrowth (Noodén and Penney, 2001; Gan, 2007).
Axillary buds are groups of meristematic cells located in leaf axils. Outgrowth of the buds leads to branching. The axillary buds are generally suppressed by shoot apex-produced auxin, a mechanism known as apical dominance (Thimann and Skoog, 1933; Napoli et al., 1999). Removal of the shoot apex (i.e. decapitation) alleviates this inhibition, allowing for the outgrowth of lateral buds. Since auxin appears not to enter axillary buds (Hall and Hillman, 1975; Morris, 1977) and exogenous auxin applied directly to buds does not inhibit their growth (Cline, 1996), it is believed that auxin is transported down the stem to inhibit axillary bud outgrowth indirectly (Leyser, 2009).
Another class of hormones that has an important role in regulating apical dominance and axillary bud outgrowth are cytokinins. Direct application of cytokinins to axillary buds could reverse the inhibitory effect of auxin (Sachs and Thimann, 1964). The expression of cytokinin biosynthetic genes correlates with bud outgrowth (Ferguson and Beveridge, 2009). It has been shown that the synthesis of cytokinins is regulated by auxin (Li et al., 1995; Nordström et al., 2004) and that auxin negatively regulates local biosynthesis of cytokinins by controlling the expression of isopentenyltransferase (IPT) genes (Tanaka et al., 2006). IPT catalyzes the first and rate-limiting step in the biosynthesis of cytokinins. This suggests that auxin could act by reducing the supply of cytokinins to axillary buds, thereby inhibiting their growth.
Strigolactone has been recently identified as a novel branch-inhibiting hormone (Gomez-Roldan et al., 2008; Umehara et al., 2008). Plant mutants deficient in the biosynthesis of or response to strigolactone show specific increases in bud outgrowth (for review, see Beveridge and Kyozuka, 2010; Stirnberg et al., 2010). Auxin positively regulates the expression of strigolactone biosynthetic genes (Sorefan et al., 2003; Arite et al., 2007), suggesting that strigolactone also functions downstream of auxin. While cytokinins can directly promote cell proliferation via the up-regulation of cell cycle components (Riou-Khamlichi et al., 1999; Rashotte et al., 2003), it is not clear how strigolactone regulates axillary bud outgrowth. It has been suggested that strigolactone may function by repressing the cytokinin pathway (Dun et al., 2009).
In some plants, apical dominance becomes weakened when the main shoot has grown beyond the suppression range of the apex, resulting in the outgrowth of the axillary buds to form bushy plants. In unbranched or less-branched plants, the suppression of the axillary buds is believed to be “permanent” (Napoli et al., 1999; Beveridge, 2006). This study on AtMYB2, however, indicates that in the less-branched monocarpic Arabidopsis, an AtMYB2-regulated post apical dominance mechanism functions at late stages of plant development to reduce cytokinin production and inhibit axillary bud outgrowth, which facilitates whole plant senescence.
RESULTS
AtMYB2 Transcript Accumulates in the Compressed Basal Internodes at Late Stages of Development and Is Not Detectable in Two T-DNA Insertion Lines
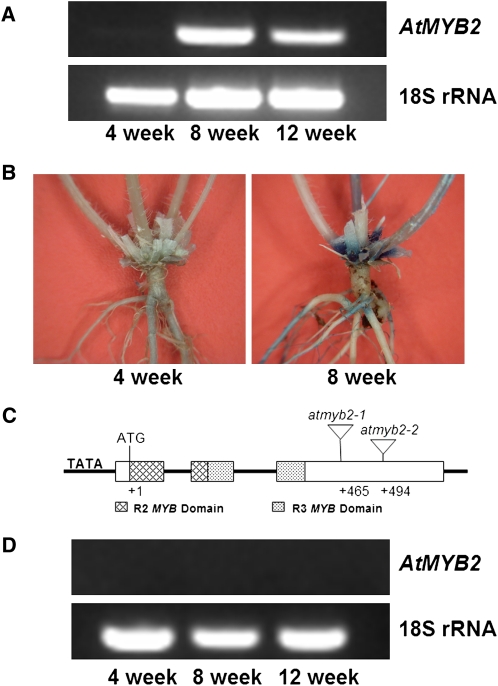
AtMYB2 encodes a transcription factor containing R2R3 MYB domains. We previously found that this gene was highly represented in an Arabidopsis senescence cDNA library (Guo et al., 2004). RNA gel-blot and reverse transcription (RT)-PCR analyses confirmed the high expression of this gene during leaf senescence (Supplemental Fig. S1). Expression of AtMYB2 in the compressed basal internodes (rosette area) is also induced by age. mRNA of this gene was detected in the compressed internodes region of 8- and 12-week-old plants but not in this region of 4-week-old plants (Fig. 1A). Consistent with the RT-PCR results, GUS staining was detected only at late stages of development in the compressed internodes region of transgenic plants harboring the AtMYB2 promoter (hereafter PAtMYB2)-GUS reporter construct (Fig. 1B).
Figure 1.
Structure and expression of AtMYB2 in the compressed basal internode region in Arabidopsis. A, RT-PCR analysis of AtMYB2 transcripts in the basal internode region of 8- and 12-week-old plants. B, GUS staining in the compressed internode regions of 4- and 8-week-old transgenic plants harboring the PATMYB2-GUS construct. C, Structure of AtMYB2 and T-DNA insertion sites in two T-DNA lines, atmyb2-1 and atmyb2-2. D, No detectable AtMYB2 transcripts in both T-DNA lines, suggesting that both lines are null mutants (RT-PCR analysis of only the atmyb2-1 line is shown). [See online article for color version of this figure.]
The atmyb2 Plants Show a Bushy Phenotype at Late Stages of Development and Have a Prolonged Life Span
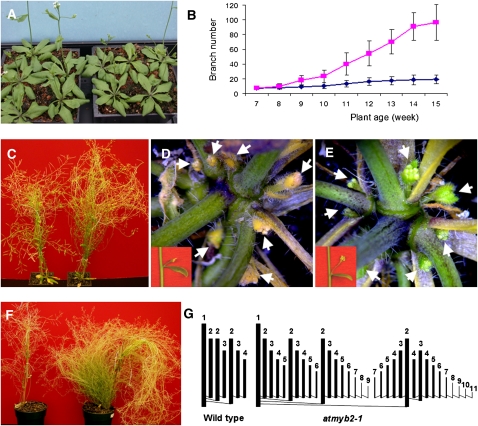
To investigate the role of AtMYB2 in plant senescence, we obtained two T-DNA insertion lines by screening the Wisconsin Arabidopsis (accession Wassilewskija) T-DNA insertion collection (Sussman et al., 2000). In both lines, the T-DNA was inserted in the third exon of AtMYB2 (Fig. 1C) and resulted in no detectable AtMYB2 transcript (Fig. 1D; Supplemental Fig. S2). The two lines were very similar to the wild type at early stages of development phenotypically (Fig. 2A); the onset and progression of leaf senescence in these mutants were also the same as those in the wild type (Fig. 2C). Up to the age of 8 weeks, the number of branches did not differ between mutant lines and the wild type (Fig. 2B). But after about 8 weeks of growth (a time when the main meristem of the wild type was arrested), the axillary buds in the mutant plants started to grow out (Fig. 2E) while the axillary buds in the wild type remained suppressed (Fig. 2D). The suppression of these buds in the wild type was further confirmed by the fact that these buds expressed bud dormancy-associated genes AtDRM1 (At1g28330) and AtDRM1 homolog (At2g33830; Tatematsu et al., 2005; Supplemental Fig. S3). By approximately 12 weeks of growth, the null plants became very bushy and continued to produce new branches (Fig. 2, B and F). A 12-week-old null mutant plant had approximately 1,150 branches (n = 3) compared with 80 branches in the wild type (n = 3).
Figure 2.
Phenotypic characterization of atmyb2-1 plants. Both atmyb2-1 and atmyb2-2 null lines display an identical phenotype, and only atmyb2-1 is shown. A, The atmyb2-1 null plants (left tray) and wild-type plants (right tray) at an early stage of development. Shown are approximately 4-week-old plants. B, Number of branches counted from 3 inches above the ground from wild-type (n = 8; blue) and atmyb2-1 (n = 9; fuchsia) plants at different developmental stages. Shown are averages with sd. C, Wild-type (left) and atmyb2-1 plants that are approximately 8 weeks old. Rosette leaves of the wild type and the null mutant plant were all senescent. D, Suppression of axillary buds of the compressed basal internodes region and of aerial stem (inset) of the wild-type plant shown in C. E, Outgrowth of axillary buds of the atmyb2-1 plant shown in C. An axillary bud associated with a cauline leaf is shown in the inset. F, Approximately 12-week-old wild-type plant (left) and atmyb2-1 null plant (right). G, Schematic diagrams of branching patterns of a typical 1° branch from a wild-type plant and an atmyb2-1 plant. A 1° branch is generated directly from the primary florescence, a 2° branch from the 1° branch, and so on. [See online article for color version of this figure.]
We further studied the branching patterns in these plants. Branches generated directly from the primary inflorescence are designated first order (1°) branches, those generated from the 1° branches are referred to as second order (2°) branches, and so on. An atmyb2 null plant had not only more branch orders (up to 14 branch orders versus four in the wild type) but also more branches at each order than the wild type. The typical branching patterns of 1° branches of atmyb2 and the wild type are shown in Figure 2G. A null plant has 34 1° branches on average compared with 12 in the wild type. In addition to the bushy phenotype, the atmyb2 null plants have reduced seed set (Supplemental Fig. S4). The reduced seed setting, the basis of which has not been further investigated in this study, however, was not the cause for the bushy phenotype, because manual removal of fruits from the wild type did not phenocopy atmyb2 (Supplemental Fig. S5).
Because both T-DNA insertion lines, atmyb2-1 and atmyb2-2, displayed the same phenotype, only atmyb2-1 was used for further analyses.
AtMYB2 Restored atmyb2 to the Wild Type
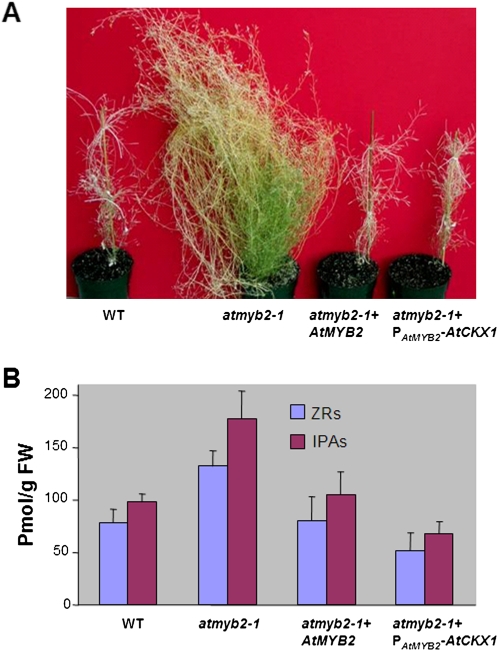
To confirm that the knockout of AtMYB2 was responsible for the bushy phenotype described above, we performed a complementation experiment by introducing the intact ATMYB2 (including its promoter) into the null plants. The complementation lines were resistant to both gentamycin (rendered by the complementation construct) and kanamycin (rendered by the original T-DNA construct) and displayed a wild-type phenotype (Fig. 3A).
Figure 3.
Restoration of the atmyb2-1 plants to the wild type (WT), morphologically and in terms of levels of endogenous cytokinins, by intact AtMYB2 or by PAtMYB2-AtCKX1 (a chimeric gene in which the Arabidopsis cytokinin oxidase 1 gene is under the control of the AtMYB2 promoter). A, Phenotypes. B, Endogenous levels of the ZR and IPA cytokinins in the basal internodes regions of approximately 8-week-old plants. The cytokinin levels were determined using ELISA. Data shown are averages with sd from three biological replicates. FW, Fresh weight. [See online article for color version of this figure.]
The atmyb2 Null Mutant Had Elevated Levels of Cytokinins
We hypothesized that the branchy phenotype in atmyb2 was caused by elevated levels of cytokinins in atmyb2. We determined the endogenous levels of cytokinins in compressed internodes of 8-week-old atmyb2 and wild-type plants using an indirect competitive ELISA (Setter et al., 2001) on the compressed internode tissues of 8-week-old plants. Anti-ZR (for transzeatin riboside) monoclonal antibody and anti-IPA monoclonal antibody were used to quantify all the ZR-type and IPA-type cytokinins. The levels of both total ZR-type and IPA-type cytokinins extracted from atmyb2 were more than 90% higher than those in the wild type (Fig. 3B).
PAtMYB2-Directed Expression of a Cytokinin Oxidase Restored atmyb2 to the Wild Type
To further investigate whether the increased level of cytokinins caused the bushy phenotype in the atmyb2 null plants, we used PAtMYB2 to direct AtCKX1 (encoding an Arabidopsis cytokinin oxidase that destroys cytokinins [Schmülling et al., 2003]) in the atmyb2 plants. The PAtMYB2-AtCKX1 transgene reduced the higher levels of cytokinins in atmyb2 to levels that were even lower than those in the wild type (Fig. 3B) and restored the atmyb2 bushy plants to the wild type (Fig. 3A). The PAtMYB2-AtCKX1 transgenic plants (atmyb2 null background) had almost the same number of branches as the wild type. These data strongly suggest that the increased cytokinin levels are responsible for the bushy phenotype of the atmyb2 plants.
The Overaccumulation of Cytokinins in the atmyb2 Plants Likely Resulted from Enhanced Local Biosynthesis in the Stem
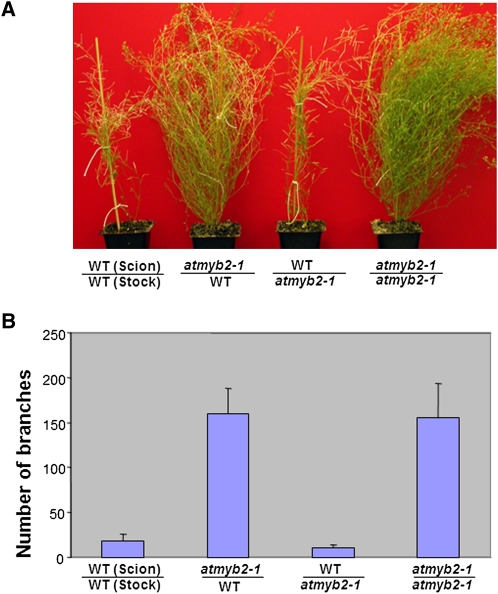
The high levels of cytokinins in the compressed internodes region of atmyb2 could result from (1) increased transport from roots (cytokinins are generally believed to be synthesized mainly in roots [Sakakibara, 2005]) or (2) locally enhanced cytokinin biosynthesis. To distinguish these possibilities, we performed a reciprocal grafting experiment between the atmyb2 null plants and the wild type. As shown in Figure 4, the graft with the wild type as stock (bottom) and atmyb2 as scion (shoot) displayed the bushy phenotype, whereas the graft with atmyb2 stock developed as the wild type. Thus, the root system was not responsible for the elevated levels of cytokinins in the compressed basal internodes.
Figure 4.
Branching patterns in plants resulting from reciprocal grafts between atmyb2-1 and wild-type (WT) plants. A, Phenotypes of various grafts. B, Numbers of branches of the grafts. The grafts were cut at 3 inches above the soil, and the branches at the cut were counted. Four plants were counted for each type of grafting. Averages with sd are shown. [See online article for color version of this figure.]
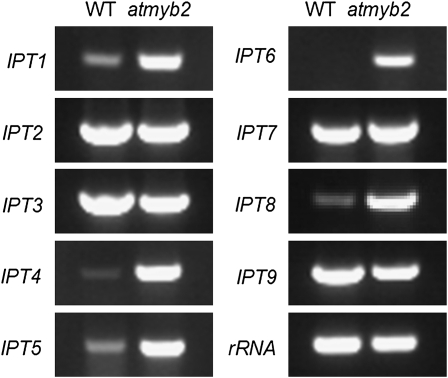
We further performed RT-PCR analysis of the expression patterns of IPTs in the compressed internode tissues of 8-week-old atmyb2 and wild-type plants. IPT catalyzes the first step in cytokinin biosynthesis (Gan and Amasino, 1995). There exist nine IPTs (Miyawaki et al., 2004) in the Arabidopsis genome. As shown in Figure 5, IPT1, 4, 5, 6, and 8 were up-regulated in atmyb2 relative to the wild type, suggesting that locally enhanced cytokinin biosynthesis is responsible for the elevated cytokinin levels and that AtMYB2 is likely a negative regulator of some IPTs.
Figure 5.
RT-RCR analysis of expression patterns of IPT1 to 9 genes in the basal internode regions of approximately 8-week-old wild-type (WT) and atmyb2-1 plants.
DISCUSSION
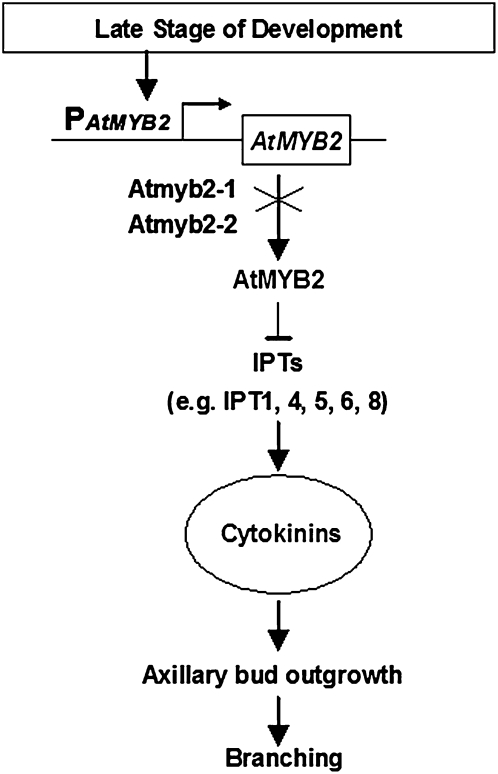
The data reported here reveal a new mechanism of AtMYB2 controlling branching at late stages of development in Arabidopsis (Fig. 6). AtMYB2 is expressed at late developmental stages in the compressed basal internodes to suppress cytokinin biosynthesis and prevent axillary buds from outgrowth. In atmyb2 null plants, the suppression is removed, resulting in elevated levels of cytokinins that promote the outgrowth of the axillary buds, leading to a bushy phenotype. The expression of AtCKX1 under the direction of PAtMYB2 destroys cytokinins in the mutant plants and restores the bushy phenotype to the wild type. We propose that as part of the whole plant senescence program, AtMYB2 functions to suppress axillary bud outgrowth at late developmental stages when the auxin-mediated apical dominance mechanism is no longer active.
Figure 6.
Model of AtMYB2 action. At late stages of plant development, AtMYB2 is expressed to inhibit the expression of the IPT genes to prevent cytokinin production. In atmyb2 mutant plants, the inhibition is released, leading to the increased production of cytokinins, which promotes axillary bud outgrowth.
Recently, much progress has been made in unraveling the regulation of axillary bud outgrowth at early stages of development in various plant species. Many plant mutants have been isolated to have impaired apical dominance and increased axillary bud outgrowth. This includes mutants with defects in the auxin signal transduction pathway, such as axr1 in Arabidopsis (Lincoln et al., 1990), cytokinin-overproducing mutants such as altered meristem program1 (Chaudhury et al., 1993; Nogue et al., 2000), and supershoot (Tantikanjana et al., 2001) and high organogenic capacity (Catterou et al., 2002) in Arabidopsis. There are also mutants defective in biosynthesis or the response to the branch-inhibiting hormone strigolactone, including the more axillary growth mutants in Arabidopsis (Stirnberg et al., 2002, 2007; Booker et al., 2004, 2005), the decreased apical dominance mutants in petunia (Petunia hybrida; Snowden et al., 2005; Simons et al., 2007), the ramosus mutants in pea (Pisum sativum; Sorefan et al., 2003), and the dwarf mutants in rice (Oryza sativa; Arite et al., 2007, 2009).
Unlike atmyb2 plants, which do not show a massive outgrowth of axillary buds until a very late stage of development, all of the above-mentioned branching mutants start exhibiting a bushy phenotype at vegetative growth stages or early reproductive stages. One important hypothesis of the apical dominance mechanism is that auxin (indole-3-acetic acid [IAA]) is produced at the growing shoot apex. It has been demonstrated that IAA is produced in tissues with rapid cell division and/or cell expansion, such as apical meristems, young leaves, young stem tissues, and developing fruits and seeds (Marumo et al., 1968; White et al., 1975). In this study, the massive outgrowth of axillary buds in atmyb2 plants was not observed until the plants were 8 weeks old, a stage when the SAM was arrested, all leaves had started senescing, and the majority of the siliques were mature (Fig. 2C). It is unlikely that the shoot apex at this stage is still actively producing IAA. Then how are the axillary buds continuously suppressed without the auxin-mediated apical dominance? This study revealed a new mechanism that is designated the “AtMYB2-mediated post apical dominance mechanism.”
Although distinct from the traditional auxin-mediated apical dominance, the AtMYB2-mediated branching control mechanism appears to have cross talk with at least one class of the branching-regulating hormones: several cytokinin biosynthetic IPT genes were up-regulated in the atmyb2 mutant (Fig. 5). It is interesting that auxin also regulates branching by suppressing the biosynthesis of cytokinins. In addition to polar auxin transport, which is responsible for apical dominance and many other important developmental processes, it has become clear that highly localized de novo auxin biosynthesis is also important in regulating auxin functions (Zhao, 2010). Therefore, we cannot exclude the possibility of cross talk between the AtMYB2-mediated post apical dominance mechanism and auxin signaling as the result of local biosynthesis. Further investigation on how AtMYB2 regulates the transcription of IPTs, and how the AtMYB2-cytokinin pathway is related to local synthesis of auxin and the third branching-regulating hormone strigolactone, will be necessary to unravel the mechanisms of AtMYB2-mediated branching control and whole plant senescence.
The AtMYB2-mediated branching regulation is of evolutionary significance. AtMYB2 has been shown to be induced by stresses such as drought and salinity (Urao et al., 1993; Abe et al., 1997), and plants under such stresses are often unbranched or much less branched (Frederick et al., 2001; Doust, 2007). As an evolutionary adaptation, plants under unfavorable environmental conditions minimize vegetative growth (e.g. branching) and finish their life cycle by producing seeds earlier with the limited resources. A change in phenotype in response to environmental factors such as this represents an example of phenotypic plasticity, which is controlled by gene expression (Sultan, 2000). The transgenic plants overexpressing AtMYB2 showed a phenotype of early flowering, less branching, and small stature (Supplemental Fig. S6), as if the plants were stressed, suggesting that AtMYB2 might be a regulator of plant phenotypic plasticity under stressed conditions. Since stresses induce senescence (Guo and Gan, 2005), it will be interesting to find out whether under stressed conditions, AtMYB2 functions directly in response to stress or functions after senescence is induced. Functions of AtMYB2 might be different in different developmental processes and in response to different environmental stimuli. Although originally identified as a leaf senescence-associated gene (Supplemental Fig. S1), knockout of AtMYB2 does not seem to affect the progression of leaf senescence (Fig. 2C), suggesting the involvement of other factors that may function redundantly with AtMYB2 in regulating leaf senescence.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
The Arabidopsis (Arabidopsis thaliana) T-DNA insertion lines and various transgenic lines were grown side by side with the wild type for comparison. Seeds were sown on petri dishes containing half-strength Murashige and Skoog salts, 0.8% (w/v) phytoagar, and appropriate antibiotics. Seeds were imbibed at 4°C overnight. Two-week-old seedlings were transplanted to 4-inch standard pots filled with Cornell mix soils (3:2:1 peat moss:vermiculite:perlite). Plants were grown at 23°C with 60% relative humidity under constant light (150 μmol m−2 s−1 light from a mixture of fluorescent and incandescent bulbs). Arabidopsis accession Wassilewskija was used unless otherwise stated.
Isolation of T-DNA Insertion Lines
The α-population of T-DNA insertion lines of the University of Wisconsin Arabidopsis Knockout Facility (Sussman et al., 2000) was screened using a pair of AtMYB2-specific primers, G468 and G484, and the T-DNA left border primer JL-202 (Supplemental Table S1), according to the protocol available at http://www.biotech.wisc.edu/Arabidopsis/. Two T-DNA insertion lines, designated as atmyb2-1 and atmyb2-2, were obtained. Both lines displayed an identical phenotype, and atmyb2-1 was used for further analyses unless otherwise indicated.
Transgenic Studies
The genomic sequence of AtMYB2, including a 2.5-kb promoter region, was PCR amplified with primers G910 (5′-ATCCTAGGATAGTACACGTTTTGGCATC-3′; the underlined section is an engineered AvrII site) and G911 (5′-AATTCGAAAACGTGACAAGGAAGAGAAT-3′) using Pfu DNA polymerase (Stratagene). The 4.3-kb PCR product, upon an A-tailing procedure described by the manufacturer, was cloned into pGEM-T (Promega) to form pGL1805. The pGL1805 plasmid DNA was sequenced. pGL1805 was digested with AvrII and PstI, and the released AtMYB2 genomic fragment was cloned into the binary vector pPZP221 (Hajdukiewicz et al., 1994) at XbaI and PstI sites, resulting in pGL1810. pGL1810 was used for a complementation test involving the atmyb2-1 null plants.
The chimeric gene PATMYB2-AtCKX1, which is AtCKX1 directed by the AtMYB2 promoter, was constructed as follows. The coding region of AtCKX1 (At2g41510) was PCR amplified with primers G1401 (5′-ATGGATCCGCCTCTTATTCTTTGC-3′; the underlined section is an engineered BamHI site) and G1402 (5′-CGCCTAGGATTTTTATGGAGATCCT-3′; the underlined section is an engineered AvrII site) using the Pfu DNA polymerase. The 2.4-kb PCR product was cloned into pGEM-T to form pGL1183. The promoter region of AtMYB2 was similarly cloned into pGL1187 by using primers G1469 (5′-ATCCTAGGTCCCATGTATCGGGCTTT-3′; the underlined section is an engineered AvrII site) and G1550 (5′-TAGGATCCCTACTCTACCACACTTAC-3′; the underlined section is an engineered BamHI site). Both pGL1183 and pGL1187 were sequenced. The AtCKX1 coding region was released from pGL1183 by BamHI and SphI and was subsequently cloned into pGL1187 to form pGL1802. The PATMYB2-AtCKX1 fusion in pGL1802 was released upon AvrII digestion and subcloned into pGL1804, a binary vector containing the NOS terminator, to form pGL1808.
To make the PATMYB2-GUS reporter construct, the AtMYB2 promoter was amplified using primers G618 (5′-GACTAGTGTTTCTTAATTATTATTCCCAAC-3′) and G619 (5′-TCCATGGCTTATAAAGAAACTTTAAATGA-3′) with engineered SpeI and NcoI sites, respectively (underlined). The PCR product was cloned into pSG506 at SpeI and NcoI to generate pGL1131. The PATMYB2-GUS-MAS terminator chimeric gene was released from pGL1131 with SpeI and BamHI and cloned into binary vector PZP221 by the XbaI and BamHI sites to create pGL1138.
To overexpress AtMYB2, the AtMYB2 coding sequence (1,070 bp) was amplified with a pair of primers, G853 (5′-AAGCTTCAAATCTAATCCACAAAACC-3′; the underlined section is an engineered HindIII site) and G854 (5′-CTGCAGGGGATTAAAACAAGAGAGGA-3′; the underlined section is an engineered PstI site) using the Pfu DNA polymerase. The PCR product was digested with HindIII and PstI and cloned into the binary vector pGL800 in the sense orientation to form pGL1149. pGL800 contains the 35S promoter-msc-RBS terminator.
The constructs pGL1810, pGL1808, pGL1138, and pGL1149 were transferred into Agrobacterium tumefaciens (strain ABI) using the freeze-thaw method as described previously (Adachi and Lieber, 2002). The Agrobacterium cells containing the respective construct were then used to transform heterozygous atmyb2 or wild-type plants via vacuum infiltration (Bechtold et al., 1993). The atmyb2 plants are resistant to kanamycin. Transgenic plants harboring pGL1810 or pGL1808 were selected on plates containing both 50 mg L−1 kanamycin and 80 mg L−1 gentamycin and were allowed to self. Progeny homozygous for atmyb2 were selected using PCR for further analysis.
RT-PCR Analyses
Total RNA extraction was performed as described (Guo and Gan, 2006). RT-PCR was carried out with the RetroScript Kit (Ambion) according to the manufacturer’s instruction. The QuantumRNA Universal 18S Internal Standard Kit (Ambion) was used as an internal standard. Primers used for RT-PCR and/or for amplification of RNA gel-blot probes are listed in Supplemental Table S1.
Counting and Pattern of Branches
The numbers of branches was counted using two methods: whole plant approach and partial counting approach. In the whole plant approach, all branches of a 3-month-old plant were counted. In the partial counting approach, a 3-month-old plant was cut at 3 inches above the soil and the number of branches at cutting was counted. For the purpose of examining branching pattern, the branch order and the number of branches of a given order of branch were individually counted. A 1° branch is one originating directly from the primary inflorescence, including those generated from the upper part of the main shoot and from the compressed internodes region at the rosette, a 2° branch is the one from the 1° branch, and so on.
Fruit Removal and Grafting Experiments
For the fruit removal experiment, newly developed fruits were removed from plants daily. Grafting was carried out as described by Turnbull et al. (2002). Arabidopsis seedlings were kept under dim light for 4 d to produce elongated hypocotyls. The seedlings were cut with a sharp surgical blade, and the stock and scion were inserted into a 5-mm-long silicone tube with 0.3 mm i.d. (VWR). The two cut ends were firmly appressed inside the tube. The grafted seedlings were kept in petri dishes for 2 weeks (by that time, two true leaves should have been produced if the grafting was successful) and were transplanted to soil. Adventitious roots from the scions (if any) were removed. The number of branches of individual grafts was assessed after an additional 10 weeks of growth.
Measurement of Cytokinin Levels
The compressed basal internode tissues for cytokinin analysis were harvested from 8-week-old Arabidopsis plants. Extraction, purification, and quantification of ZR and IPA families of cytokinins were performed according to Setter et al. (2001). For details, see Supplemental Information S1.
Microscopic Analysis
The basal internode regions including axillary buds from Arabidopsis plants at different developmental stages were examined, and images were taken with a dissecting microscope (Leica S6E).
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers AtMYB2 (AT2G47190), AtCKX1 (AT2G41510), AtDRM1 (AT1G28330), AtDRM1 homolog (AT2G33830), IPT1 (AT1G68460), IPT2 (AT2G27760), IPT3 (AT3G63110), IPT4 (AT4G24650), IPT5 (AT5G19040), IPT6 (AT1G25410), IPT7 (AT3G23630), IPT8 (AT3G19160), and IPT9 (AT5G20040).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Expression of AtMYB2 during leaf senescence.
Supplemental Figure S2. Expression of AtMYB2 in wild-type and T-DNA insertion lines.
Supplemental Figure S3. Expression of bud dormancy-associated genes in the wild type and atmyb2-1.
Supplemental Figure S4. Inflorescences from 8-week-old plants of the wild type and atmyb2-1.
Supplemental Figure S5. Effect of fruit removal on branching.
Supplemental Figure S6. Effect of the overexpression of AtMYB2 on branching and other aspects of plant development.
Supplemental Table S1. Primers used in this study.
Supplemental Information S1. Cytokinin measurement.
Supplementary Material
Acknowledgments
We thank Tim Setter (Cornell University) for help in cytokinin quantification and Richard Amasino (University of Wisconsin, Madison), June Nasrallah (Cornell University) and Steve Tanksley (Cornell University) for critical reading of an early version of the manuscript.
References
- Abe H, Yamaguchi-Shinozaki K, Urao T, Iwasaki T, Hosokawa D, Shinozaki K. (1997) Role of Arabidopsis MYC and MYB homologs in drought- and abscisic acid-regulated gene expression. Plant Cell 9: 1859–1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi N, Lieber MR. (2002) Bidirectional gene organization: a common architectural feature of the human genome. Cell 109: 807–809 [DOI] [PubMed] [Google Scholar]
- Arite T, Iwata H, Ohshima K, Maekawa M, Nakajima M, Kojima M, Sakakibara H, Kyozuka J. (2007) DWARF10, an RMS1/MAX4/DAD1 ortholog, controls lateral bud outgrowth in rice. Plant J 51: 1019–1029 [DOI] [PubMed] [Google Scholar]
- Arite T, Umehara M, Ishikawa S, Hanada A, Maekawa M, Yamaguchi S, Kyozuka J. (2009) d14, a strigolactone-insensitive mutant of rice, shows an accelerated outgrowth of tillers. Plant Cell Physiol 50: 1416–1424 [DOI] [PubMed] [Google Scholar]
- Bechtold N, Ellis J, Pelletier G. (1993) In planta Agrobacterium-mediated gene transfer by infiltration of adult Arabidopsis plants. C R Acad Sci Paris 316: 1194–1199 [Google Scholar]
- Beveridge CA. (2006) Axillary bud outgrowth: sending a message. Curr Opin Plant Biol 9: 35–40 [DOI] [PubMed] [Google Scholar]
- Beveridge CA, Kyozuka J. (2010) New genes in the strigolactone-related shoot branching pathway. Curr Opin Plant Biol 13: 34–39 [DOI] [PubMed] [Google Scholar]
- Booker J, Auldridge M, Wills S, McCarty D, Klee H, Leyser O. (2004) MAX3/CCD7 is a carotenoid cleavage dioxygenase required for the synthesis of a novel plant signaling molecule. Curr Biol 14: 1232–1238 [DOI] [PubMed] [Google Scholar]
- Booker J, Sieberer T, Wright W, Williamson L, Willett B, Stirnberg P, Turnbull C, Srinivasan M, Goddard P, Leyser O. (2005) MAX1 encodes a cytochrome P450 family member that acts downstream of MAX3/4 to produce a carotenoid-derived branch-inhibiting hormone. Dev Cell 8: 443–449 [DOI] [PubMed] [Google Scholar]
- Catterou M, Dubois F, Smets R, Vaniet S, Kichey T, Van Onckelen H, Sangwan-Norreel BS, Sangwan RS. (2002) hoc: an Arabidopsis mutant overproducing cytokinins and expressing high in vitro organogenic capacity. Plant J 30: 273–287 [DOI] [PubMed] [Google Scholar]
- Chaudhury AM, Letham S, Craig S, Dennis ES. (1993) Amp1: a mutant with high cytokinin levels and altered embryonic pattern, faster vegetative growth, constitutive photomorphogenesis and precocious flowering. Plant J 4: 907–916 [Google Scholar]
- Cline MG. (1996) Exogenous auxin effects on lateral bud outgrowth in decapitated shoots. Ann Bot (Lond) 78: 255–266 [Google Scholar]
- Doust AN. (2007) Grass architecture: genetic and environmental control of branching. Curr Opin Plant Biol 10: 21–25 [DOI] [PubMed] [Google Scholar]
- Dun EA, Brewer PB, Beveridge CA. (2009) Strigolactones: discovery of the elusive shoot branching hormone. Trends Plant Sci 14: 364–372 [DOI] [PubMed] [Google Scholar]
- Ferguson BJ, Beveridge CA. (2009) Roles for auxin, cytokinin, and strigolactone in regulating shoot branching. Plant Physiol 149: 1929–1944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick JR, Camp CR, Bauer PJ. (2001) Drought-stress effects on branch and mainstem seed yield and yield components of determinate soybean. Crop Sci 41: 759–763 [Google Scholar]
- Gan S. (2003) Mitotic and postmitotic senescence in plants. Sci SAGE KE 2003: RE7. [DOI] [PubMed] [Google Scholar]
- Gan S. (2007) Mitotic senescence in plants. Gan S, , Senescence Processes in Plants. Blackwell Publishing, Oxford, pp 1–11 [Google Scholar]
- Gan S, Amasino RM. (1995) Inhibition of leaf senescence by autoregulated production of cytokinin. Science 270: 1986–1988 [DOI] [PubMed] [Google Scholar]
- Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pagès V, Dun EA, Pillot JP, Letisse F, Matusova R, Danoun S, Portais JC, et al. (2008) Strigolactone inhibition of shoot branching. Nature 455: 189–194 [DOI] [PubMed] [Google Scholar]
- Guo Y, Cai Z, Gan S. (2004) Transcriptome of Arabidopsis leaf senescence. Plant Cell Environ 27: 521–549 [Google Scholar]
- Guo Y, Gan S. (2005) Leaf senescence: signals, execution, and regulation. Curr Top Dev Biol 71: 83–112 [DOI] [PubMed] [Google Scholar]
- Guo Y, Gan S. (2006) AtNAP, a NAC family transcription factor, has an important role in leaf senescence. Plant J 46: 601–612 [DOI] [PubMed] [Google Scholar]
- Hajdukiewicz P, Svab Z, Maliga P. (1994) The small, versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Mol Biol 25: 989–994 [DOI] [PubMed] [Google Scholar]
- Hall SM, Hillman JR. (1975) Correlative inhibition of lateral bud growth in Phaseolus vulgaris L: timing of bud growth following decapitation. Planta 123: 137–143 [DOI] [PubMed] [Google Scholar]
- Leyser O. (2009) The control of shoot branching: an example of plant information processing. Plant Cell Environ 32: 694–703 [DOI] [PubMed] [Google Scholar]
- Li CJ, Guevara E, Herrera J, Bangerth F. (1995) Effect of apex excision and replacement by 1-naphthylacetic acid on cytokinin concentration and apical dominance in pea plants. Physiol Plant 94: 465–469 [Google Scholar]
- Lincoln C, Britton JH, Estelle M. (1990) Growth and development of the axr1 mutants of Arabidopsis. Plant Cell 2: 1071–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marumo S, Hattori H, Abe H, Munakata K. (1968) Isolation of 4-chloroindolyl-3-acetic acid from immature seeds of Pisum sativum. Nature 219: 959–960 [DOI] [PubMed] [Google Scholar]
- Miyawaki K, Matsumoto-Kitano M, Kakimoto T. (2004) Expression of cytokinin biosynthetic isopentenyltransferase genes in Arabidopsis: tissue specificity and regulation by auxin, cytokinin, and nitrate. Plant J 37: 128–138 [DOI] [PubMed] [Google Scholar]
- Morris DA. (1977) Transport of exogenous auxin in 2-branched dwarf pea seedlings (Pisum sativum L): some implications for polarity and apical dominance. Planta 136: 91–96 [DOI] [PubMed] [Google Scholar]
- Napoli CA, Beveridge CA, Snowden KC. (1999) Reevaluating concepts of apical dominance and the control of axillary bud outgrowth. Curr Top Dev Biol 44: 127–169 [DOI] [PubMed] [Google Scholar]
- Nogue N, Hocart H, Letham DS, Dennis ES, Chaudhury AM. (2000) Cytokinin synthesis is higher in the Arabidopsis amp1 mutant. Plant Growth Regul 32: 267–273 [Google Scholar]
- Noodén LD, Penney JP. (2001) Correlative controls of senescence and plant death in Arabidopsis thaliana (Brassicaceae). J Exp Bot 52: 2151–2159 [DOI] [PubMed] [Google Scholar]
- Nordström A, Tarkowski P, Tarkowska D, Norbaek R, Astot C, Dolezal K, Sandberg G. (2004) Auxin regulation of cytokinin biosynthesis in Arabidopsis thaliana: a factor of potential importance for auxin-cytokinin-regulated development. Proc Natl Acad Sci USA 101: 8039–8044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashotte AM, Carson SDB, To JPC, Kieber JJ. (2003) Expression profiling of cytokinin action in Arabidopsis. Plant Physiol 132: 1998–2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riou-Khamlichi C, Huntley R, Jacqmard A, Murray JAH. (1999) Cytokinin activation of Arabidopsis cell division through a D-type cyclin. Science 283: 1541–1544 [DOI] [PubMed] [Google Scholar]
- Sachs T, Thimann KV. (1964) Release of lateral buds from apical dominance. Nature 201: 939–940 [Google Scholar]
- Sakakibara H. (2005) Cytokinin biosynthesis and metabolism. Davies PJ, , Plant Hormones: Biosynthesis, Signal Transduction, Action. Kluwer Academic Publishers, Boston, pp 95–114 [Google Scholar]
- Schmülling T, Werner T, Riefler M, Krupková E, Bartrina y Manns I. (2003) Structure and function of cytokinin oxidase/dehydrogenase genes of maize, rice, Arabidopsis and other species. J Plant Res 116: 241–252 [DOI] [PubMed] [Google Scholar]
- Setter TL, Flannigan BA, Melkonian J. (2001) Loss of kernel set due to water deficit and shade in maize: carbohydrate supplies, abscisic acid, and cytokinins. Crop Sci 41: 1530–1540 [Google Scholar]
- Simons JL, Napoli CA, Janssen BJ, Plummer KM, Snowden KC. (2007) Analysis of the DECREASED APICAL DOMINANCE genes of petunia in the control of axillary branching. Plant Physiol 143: 697–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden KC, Simkin AJ, Janssen BJ, Templeton KR, Loucas HM, Simons JL, Karunairetnam S, Gleave AP, Clark DG, Klee HJ. (2005) The decreased apical dominance1/Petunia hybrida CAROTENOID CLEAVAGE DIOXYGENASE8 gene affects branch production and plays a role in leaf senescence, root growth, and flower development. Plant Cell 17: 746–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorefan K, Booker J, Haurogné K, Goussot M, Bainbridge K, Foo E, Chatfield S, Ward S, Beveridge C, Rameau C, et al. (2003) MAX4 and RMS1 are orthologous dioxygenase-like genes that regulate shoot branching in Arabidopsis and pea. Genes Dev 17: 1469–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirnberg P, Furner IJ, Ottoline Leyser HM. (2007) MAX2 participates in an SCF complex which acts locally at the node to suppress shoot branching. Plant J 50: 80–94 [DOI] [PubMed] [Google Scholar]
- Stirnberg P, van De Sande K, Leyser HM. (2002) MAX1 and MAX2 control shoot lateral branching in Arabidopsis. Development 129: 1131–1141 [DOI] [PubMed] [Google Scholar]
- Stirnberg P, Ward S, Leyser O. (2010) Auxin and strigolactones in shoot branching: intimately connected? Biochem Soc Trans 38: 717–722 [DOI] [PubMed] [Google Scholar]
- Sultan SE. (2000) Phenotypic plasticity for plant development, function and life history. Trends Plant Sci 5: 537–542 [DOI] [PubMed] [Google Scholar]
- Sussman MR, Amasino RM, Young JC, Krysan PJ, Austin-Phillips S. (2000) The Arabidopsis knockout facility at the University of Wisconsin-Madison. Plant Physiol 124: 1465–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Takei K, Kojima M, Sakakibara H, Mori H. (2006) Auxin controls local cytokinin biosynthesis in the nodal stem in apical dominance. Plant J 45: 1028–1036 [DOI] [PubMed] [Google Scholar]
- Tantikanjana T, Yong JW, Letham DS, Griffith M, Hussain M, Ljung K, Sandberg G, Sundaresan V. (2001) Control of axillary bud initiation and shoot architecture in Arabidopsis through the SUPERSHOOT gene. Genes Dev 15: 1577–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatematsu K, Ward S, Leyser O, Kamiya Y, Nambara E. (2005) Identification of cis-elements that regulate gene expression during initiation of axillary bud outgrowth in Arabidopsis. Plant Physiol 138: 757–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimann KV, Skoog F. (1933) Studies on the growth hormone of plants. III. The inhibiting action of the growth substance on bud development. Proc Natl Acad Sci USA 19: 714–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull CG, Booker JP, Leyser HM. (2002) Micrografting techniques for testing long-distance signalling in Arabidopsis. Plant J 32: 255–262 [DOI] [PubMed] [Google Scholar]
- Umehara M, Hanada A, Yoshida S, Akiyama K, Arite T, Takeda-Kamiya N, Magome H, Kamiya Y, Shirasu K, Yoneyama K, et al. (2008) Inhibition of shoot branching by new terpenoid plant hormones. Nature 455: 195–200 [DOI] [PubMed] [Google Scholar]
- Urao T, Yamaguchi-Shinozaki K, Urao S, Shinozaki K. (1993) An Arabidopsis myb homolog is induced by dehydration stress and its gene product binds to the conserved MYB recognition sequence. Plant Cell 5: 1529–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JC, Medlow GC, Hillman JR, Wilkins MB. (1975) Correlative inhibition of lateral bud growth in Phaseolus vulgaris L: isolation of indoleacetic acid from inhibitory region. J Exp Bot 26: 419–424 [Google Scholar]
- Zhao Y. (2010) Auxin biosynthesis and its role in plant development. Annu Rev Plant Biol 61: 49–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.