Abstract
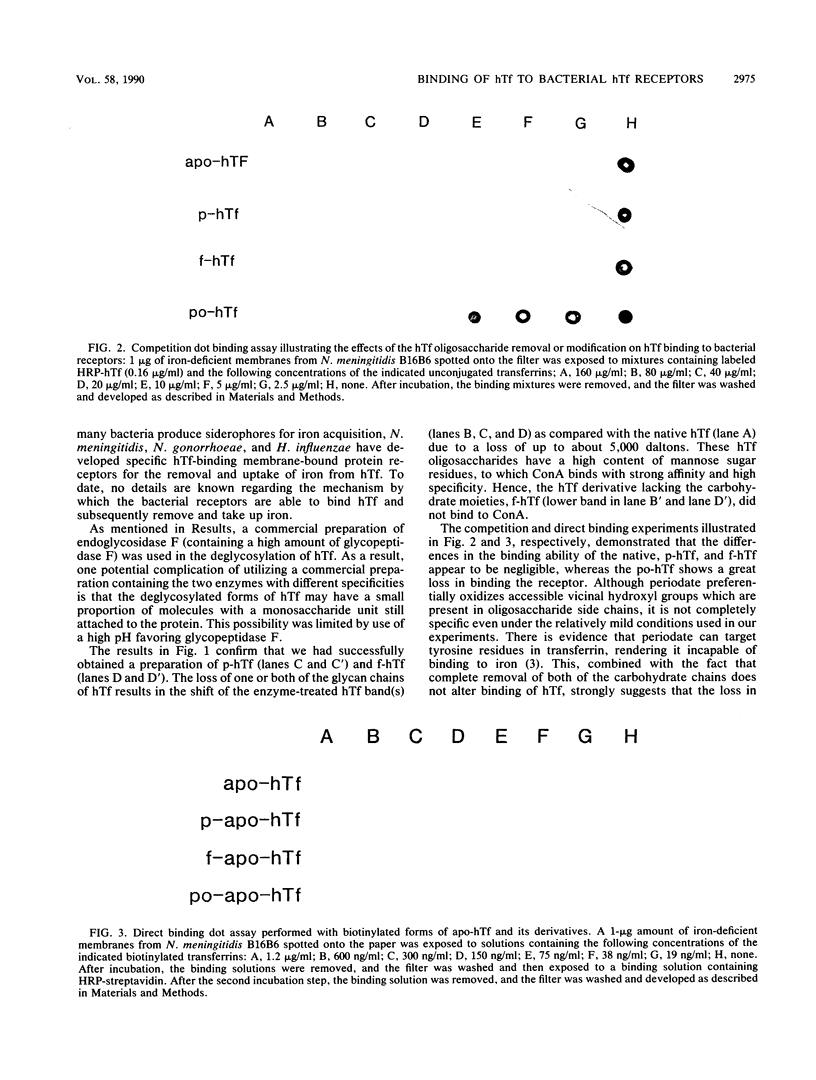
Derivatives of human transferrin (hTf) with removed or modified N-linked oligosaccharides were compared with native hTf with respect to their binding to bacterial hTf receptors from Neisseria meningitidis, N. gonorrhoeae, and Haemophilus influenzae. Partially and fully deglycosylated hTf were prepared by enzymatic deglycosylation with glycopeptidase F and isolated by concanavalin A-Sepharose affinity chromatography. Oligosaccharide-modified hTf was prepared via mild periodate oxidation. Competition and direct binding experiments with the hTf derivatives demonstrated that the hTf oligosaccharides are not essential for binding to the bacterial hTf receptors.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Glass W. F., 2nd, Briggs R. C., Hnilica L. S. Use of lectins for detection of electrophoretically separated glycoproteins transferred onto nitrocellulose sheets. Anal Biochem. 1981 Jul 15;115(1):219–224. doi: 10.1016/0003-2697(81)90549-2. [DOI] [PubMed] [Google Scholar]
- Hawkes R. Identification of concanavalin A-binding proteins after sodium dodecyl sulfate--gel electrophoresis and protein blotting. Anal Biochem. 1982 Jun;123(1):143–146. doi: 10.1016/0003-2697(82)90634-0. [DOI] [PubMed] [Google Scholar]
- Herrington D. A., Sparling P. F. Haemophilus influenzae can use human transferrin as a sole source for required iron. Infect Immun. 1985 Apr;48(1):248–251. doi: 10.1128/iai.48.1.248-251.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll H. P., Bhakdi S., Taylor P. W. Membrane changes induced by exposure of Escherichia coli to human serum. Infect Immun. 1983 Dec;42(3):1055–1066. doi: 10.1128/iai.42.3.1055-1066.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee B. C., Schryvers A. B. Specificity of the lactoferrin and transferrin receptors in Neisseria gonorrhoeae. Mol Microbiol. 1988 Nov;2(6):827–829. doi: 10.1111/j.1365-2958.1988.tb00095.x. [DOI] [PubMed] [Google Scholar]
- MacGillivray R. T., Mendez E., Shewale J. G., Sinha S. K., Lineback-Zins J., Brew K. The primary structure of human serum transferrin. The structures of seven cyanogen bromide fragments and the assembly of the complete structure. J Biol Chem. 1983 Mar 25;258(6):3543–3553. [PubMed] [Google Scholar]
- McKenna W. R., Mickelsen P. A., Sparling P. F., Dyer D. W. Iron uptake from lactoferrin and transferrin by Neisseria gonorrhoeae. Infect Immun. 1988 Apr;56(4):785–791. doi: 10.1128/iai.56.4.785-791.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melancon J., Murgita R. A., Devoe I. W. Activation of murine B lymphocytes by Neisseria meningitidis and isolated meningococcal surface antigens. Infect Immun. 1983 Nov;42(2):471–479. doi: 10.1128/iai.42.2.471-479.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickelsen P. A., Blackman E., Sparling P. F. Ability of Neisseria gonorrhoeae, Neisseria meningitidis, and commensal Neisseria species to obtain iron from lactoferrin. Infect Immun. 1982 Mar;35(3):915–920. doi: 10.1128/iai.35.3.915-920.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Rylatt D. B., Parish C. R. Protein determination on an automatic spectrophotometer. Anal Biochem. 1982 Mar 15;121(1):213–214. doi: 10.1016/0003-2697(82)90578-4. [DOI] [PubMed] [Google Scholar]
- Schryvers A. B. Characterization of the human transferrin and lactoferrin receptors in Haemophilus influenzae. Mol Microbiol. 1988 Jul;2(4):467–472. doi: 10.1111/j.1365-2958.1988.tb00052.x. [DOI] [PubMed] [Google Scholar]
- Schryvers A. B. Identification of the transferrin- and lactoferrin-binding proteins in Haemophilus influenzae. J Med Microbiol. 1989 Jun;29(2):121–130. doi: 10.1099/00222615-29-2-121. [DOI] [PubMed] [Google Scholar]
- Schryvers A. B., Lee B. C. Comparative analysis of the transferrin and lactoferrin binding proteins in the family Neisseriaceae. Can J Microbiol. 1989 Mar;35(3):409–415. doi: 10.1139/m89-063. [DOI] [PubMed] [Google Scholar]
- Schryvers A. B., Morris L. J. Identification and characterization of the human lactoferrin-binding protein from Neisseria meningitidis. Infect Immun. 1988 May;56(5):1144–1149. doi: 10.1128/iai.56.5.1144-1149.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schryvers A. B., Morris L. J. Identification and characterization of the transferrin receptor from Neisseria meningitidis. Mol Microbiol. 1988 Mar;2(2):281–288. doi: 10.1111/j.1365-2958.1988.tb00029.x. [DOI] [PubMed] [Google Scholar]
- Tarentino A. L., Gómez C. M., Plummer T. H., Jr Deglycosylation of asparagine-linked glycans by peptide:N-glycosidase F. Biochemistry. 1985 Aug 13;24(17):4665–4671. doi: 10.1021/bi00338a028. [DOI] [PubMed] [Google Scholar]
- Uzan G., Frain M., Park I., Besmond C., Maessen G., Trépat J. S., Zakin M. M., Kahn A. Molecular cloning and sequence analysis of cDNA for human transferrin. Biochem Biophys Res Commun. 1984 Feb 29;119(1):273–281. doi: 10.1016/0006-291x(84)91648-6. [DOI] [PubMed] [Google Scholar]
- Woodward M. P., Young W. W., Jr, Bloodgood R. A. Detection of monoclonal antibodies specific for carbohydrate epitopes using periodate oxidation. J Immunol Methods. 1985 Apr 8;78(1):143–153. doi: 10.1016/0022-1759(85)90337-0. [DOI] [PubMed] [Google Scholar]
- Yancey R. J., Finkelstein R. A. Siderophore production by pathogenic Neisseria spp. Infect Immun. 1981 May;32(2):600–608. doi: 10.1128/iai.32.2.600-608.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]