Summary
NalC is a TetR type regulator that represses the multidrug efflux pump MexAB-OprM in Pseudomonas aeruginosa. Here we explain the mechanism of NalC mediated regulation of MexAB-OprM. We show that NalC non-covalently binds chlorinated phenols and chemicals containing chlorophenol sidechains such as triclosan. NalC-chlorinated phenol binding results in its dissociation from promoter DNA and up-regulation of NalC’s downstream targets, including the MexR antirepressor ArmR. ArmR up-regulation and MexR-ArmR complex formation have previously been shown to upregulate MexAB-OprM. In vivo mexB and armR expression analyses were used to corroborate in vitro NalC chlorinated phenol binding. We also show that the interaction between chlorinated phenols and NalC is reversible, such that removal of these chemicals restored NalC promoter DNA binding. Thus, the NalC-chlorinated phenol interaction is likely a pertinent physiological mechanism that P. aeruginosa uses to control expression of the MexAB-OprM efflux pump.
Keywords: Pseudomonas aeruginosa, MexAB-OprM, NalC ligand, chlorinated phenol, triclosan
Introduction
Pseudomonas aeruginosa is an opportunistic pathogen associated with a wide range of community acquired and nosocomial infections (Mesaros et al., 2007). P. aeruginosa infections are responsible for a significant rise in morbidity and mortality in intensive care units (Kerr and Snelling, 2009). Intrinsically resistant to multiple antibiotics, P. aeruginosa is a versatile adversary, having the ability to modify and acquire new traits and adapt to diverse environments (Hocquet et al., 2007).
P. aeruginosa harbors several chromosomal multi-drug resistance (MDR) efflux pumps conferring resistance to a variety of antibiotics (Alekshun and Levy, 2007, Lister et al., 2009). The MexAB-OprM efflux pump has the widest spectrum among these MDR pumps. It mediates the efflux of diverse antibiotics, such as tetracyclines, fluoroquinolones, β-lactams, chloramphenicol, macrolides, novobiocin, trimethoprim and sulfonamides, and biocides such as triclosan (Lister et al., 2009). MexAB-OprM also effluxes quorum sensing molecules (Juhas et al., 2005) and virulence factors (Piddock, 2006). The apparent lack of specificity of MexAB-OprM is intriguing and its natural physiological role unclear (Neyfakh, 1997, Martinez, 2009).
MexAB-OprM has three known transcriptional regulators: MexR, NalD and NalC (Daigle et al., 2007). Among the three regulators, MexR is by far the best studied (Figure 1(a)). MexR, which is autoregulated, is transcribed divergently from the same intergenic promoter region as mexAB-oprM (Daigle et al., 2007) (Figure 1(a)). MexR’s binding to this intergenic region overlaps with promoters for mexR and mexAB-oprM and represses their expression. NalD, another repressor, binds a secondary promoter region of MexAB-OprM and down-regulates MexAB-OprM expression as well (Morita et al., 2006). The autoregulator NalC (Figure 1(a)) exerts indirect negative control over MexAB-OprM expression by repressing ArmR, an anti-repressor of MexR (Daigle et al., 2007). MexR, complexed with ArmR (Figure 1(b)), fails to attach to the intergenic promoter region, which results in the overexpression of MexAB-OprM (Wilke et al., 2008). Thus, absence of NalC binding to the promoter region will relieve ArmR repression, thereby promoting ArmR’s complex formation with MexR and increasing MexAB-OprM expression. In addition to the intricate transcriptional control that MexR expression is subjected to, MexR activity is also regulated on the posttranslational level. Oxidation of two cysteines in MexR has been shown to cause conformational changes in the protein, preventing it from binding to the promoter DNA region. This redox-mediated control of MexR function leads to the up-regulation of the MexAB-OprM operon during oxidative stress conditions both in vitro and in vivo (Chen et al., 2008).
Figure 1.
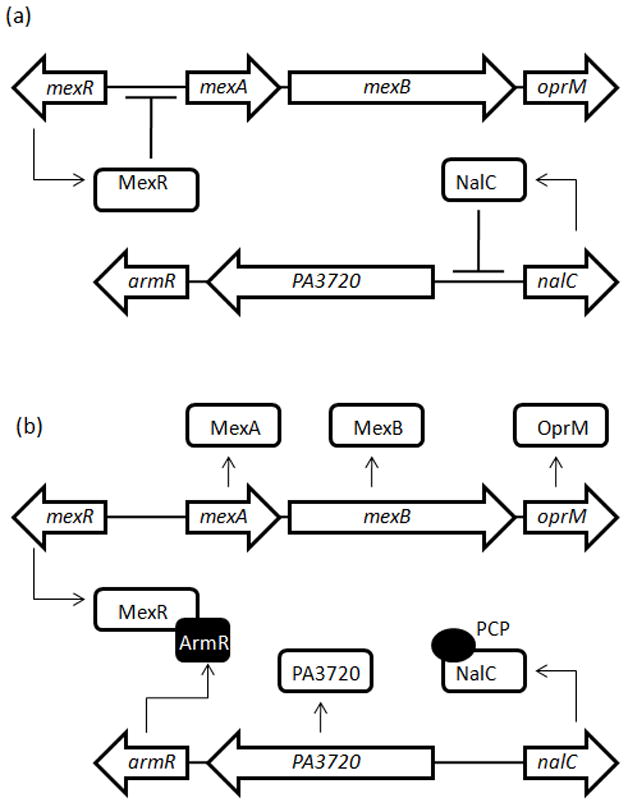
Regulation of the MexAB-OprM efflux pump in P. aeruginosa
(a) PCP not present, (b) PCP present (⊥ denotes binding to the promoter and inactivation of transcription, → denotes production of protein).
In this paper we show that chlorinated phenols function as NalC effector molecules by reversibly binding to NalC and decreasing its DNA binding affinity, resulting in de-repression of MexAB-OprM expression (Figure 1(b)). NalC is a TetR type regulator with a helix-turn-helix DNA binding domain and a ligand binding domain (Ramos et al., 2005). The interaction between NalC and chlorinated phenols explains the overexpression of MexAB-OprM and up-regulation of mexR, nalC and armR observed by Muller et al. (2007) in P. aeruginosa chemostat cultures in response to treatment with pentachlorophenol (PCP) (Muller et al., 2007). By using in vitro binding studies, we demonstrate that unlike in response to other organic solvents (Li and Poole, 1999), the PCP-mediated upregulation of the mexAB-oprM operon is not linked to mutations in MexR but is due to the reversible binding of PCPs to NalC. While chlorinated aromatics including chlorophenols can be produced by natural bacterial and fungal activity (Bengtson et al., 2009), chlorophenols are also commercially produced and are widely present in biocides and disinfectants (Weber et al., 2007). We address the significance of chlorinated phenol mediated MexAB-OprM regulation in the light of its environmental occurrences.
Results and Discussion
MexAB-OprM regulators are overexpressed in the presence of PCP
In order to corroborate the results of Muller et al.(2007), who demonstrated PCP-mediated up-regulation of mexAB-oprM and its regulators in chemostat cultures, we used quantitative RT-PCR to analyze the expression of mexB, mexR, armR and nalC in log phase cultures (OD600 ~ 0.3) with and without 120 μM PCP. We observed ~1.5 fold increases in both mexB and mexR expression in the presence of PCP compared to cultures grown in the absence of PCP (SI Figure 1). Expression of armR, a direct target of NalC and MexR’s anti-repressor increased greater than 100-fold, while nalC expression increased 3 to 4 fold in the presence of PCP. All increases were statistically significant (p values < 0.05) based on analysis of variance using MacAnova 5.03. While the fold-changes were numerically different, increased expression of these genes in the presence of PCP is consistent with microarray-derived expression data by Muller et al, who reported a 3–4 fold up-regulation of mexAB-oprM and mexR and 9 and 15 fold increases in armR, PA3720 and nalC in the presence of ~ 150 μM PCP.
PCP does not select for mexR and nalC mutants
Up-regulation of the mexAB-oprM operon in the presence of organic solvents has been previously shown to be connected to mutations in the mexR gene (Li and Poole, 1999). To investigate whether a similar mechanism is responsible for our observed increases in the expression of MexR and NalC-regulated genes, we cultured P. aeruginosa PAO1 in the presence of PCP and selected colonies on plates containing 150 μM, 1.5 mM and 3.75 mM PCP. None of the colonies analyzed from these PCP plates had mutations in either mexR or nalC. This result suggests that up-regulation of the mexR and nalC mRNA in the presence of PCP does not require genetic changes in these regulators but functions as a regulated transcriptional response to PCP treatment.
PCP causes dissociation of NalC from its promoter DNA
Up-regulation of both nalC and armR coupled with the lack of detectable mutations in nalC led us to hypothesize that PCP might be directly binding NalC, thus causing the de-repression of its own expression and that of the downstream target armR. ArmR, in turn, would then interact with the repressor MexR, preventing its binding to DNA, and subsequently causing the observed up-regulation of mexR and mexAB-oprM. To test our hypothesis we analyzed the binding between NalC and promoter DNA in the presence and absence of PCP using electrophoretic mobility shift assay (EMSA) (Figure 2(a)). A 262 bp DNA segment encompassing the nalC/PA3720 intergenic region including the start sites of both these genes and the respective promoters, as identified by Cao et al., was used in EMSA (Cao et al., 2004). We found that NalC binding to the promoter DNA region reduced the mobility of the 262 bp DNA segment (Figure 2(a)). DNA binding was nearly saturated when 2.3 μM of NalC and 1.2 μM promoter DNA were combined.
Figure 2.
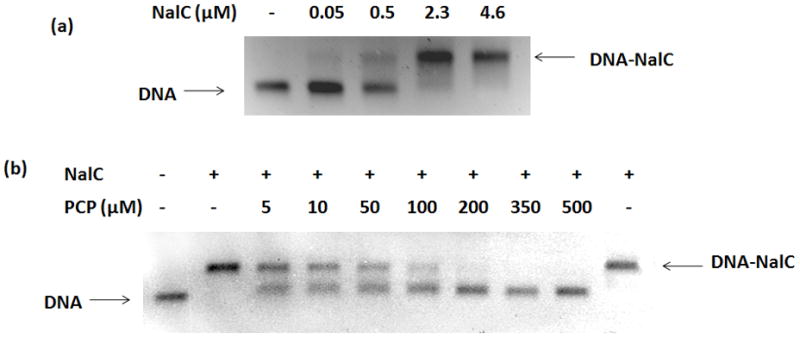
In-vitro analysis of interaction between NalC, promoter DNA and PCP
(a) EMSA with 1.2 μM promoter DNA and varying concentrations of NalC, (b) EMSA with 1.2 μM promoter DNA, 4.6 μM NalC and varying concentrations of PCP. Last lane: 70 μM NalC and 7.6 mM PCP incubated followed by PCP removal by gel filtration and incubation with DNA. (+): present, (−): absent.
To assess the effects of PCP on NalC’s DNA binding affinity, we tested increasing concentrations of PCP in the presence of 4.6 μM of NalC and 1.2 μM of promoter DNA. As shown in Figure 2(b), the presence of low micromolar concentrations of PCP was sufficient to significantly decrease the binding affinity of NalC to DNA. At concentrations of PCP above 200 μM, no binding of NalC to DNA was observed (Figure 2(b)). These results are consistent with the conclusion that PCP acts as a ligand of NalC and prevents NalC from binding DNA.
To exclude the possibility that PCP caused any irreversible modifications of NalC, thus inactivating the DNA binding protein, we incubated 70 μM NalC with 7.6 mM of PCP for 1 hour. This PCP concentration was sufficient to prevent NalC binding to DNA. Following this incubation, the PCP-NalC mixture was filtered through an Illustra NAP-5 column. The Sephadex gel filtration matrix allows for re-equilibration of protein and ligand and has been used to study reversible protein-ligand binding in the Hummel and Dreyer method (Cann and Hinman, 1976). Following gel filtration, binding between NalC and DNA was completely restored (Figure 2(b), last lane), demonstrating that exposure of NalC to PCP did not permanently change NalC’s DNA binding affinity. We concluded from these results that PCP reversibly interacts with NalC to prevent it from binding DNA.
Dichlorophenol, trichlorophenol and triclosan bind NalC
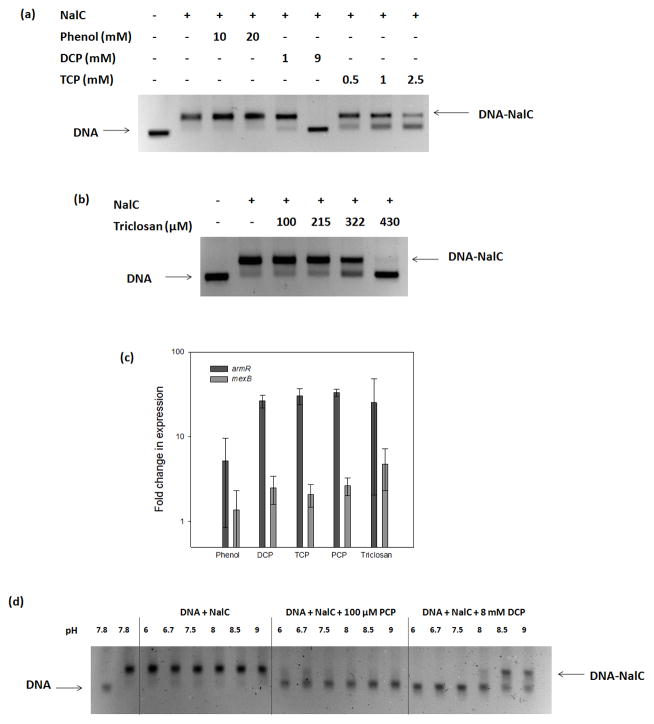
To assess the ligand specificity of NalC, we examined chemicals structurally similar to PCP for their ability to bind NalC and prevent DNA binding. We tested phenol, 2,4-dichlorophenol (DCP) and 2,4,6-trichlorophenol (TCP) and the phenol based disinfectant triclosan, which has a monochlorophenol group (Table 1). As before, we assessed binding of 4.6 μM of NalC to 1.2 μM of promoter DNA in the presence of these chemicals. While phenol, even at 20 mM, did not affect NalC-DNA binding (Figure 3(a)), we found that both DCP and TCP diminished the apparent binding affinity of NalC to DNA, similar to the results observed with PCP. In contrast to PCP, however, significantly higher concentrations of DCP and TCP were required to prevent NalC binding to DNA. At 1 mM, DCP had minimal effects on NalC’s DNA binding while TCP at this concentration partially interfered with NalC-DNA binding. Triclosan also required higher concentrations than PCP to abolish NalC-DNA binding. At 215 μM, triclosan did not affect NalC-DNA binding, while presence of 430 μM triclosan nearly completely prevented NalC’s binding to DNA (Figure 3(b)). As shown for PCP, DNA binding of NalC was largely restored upon removal of the ligands by dialysis (data not shown). Our findings that the chlorinated phenol derivatives prevent NalC’s binding to the promoter region in vitro were in excellent agreement with in vivo expression studies of armR and mexB (Figure 3(c)). We found that armR and mexB transcription levels were reproducibly increased in the presence of DCP, TCP and triclosan, but not in the presence of phenol. These results strongly suggest that chlorination of the phenol is an important characteristic of NalC ligands.
Table 1.
Chemicals tested for binding to NalC using EMSA
| Chemical | pKa | log Kow | Binding to 4.6 μM NalC |
|---|---|---|---|
|
|
9.95 | 1.46 | No binding with 20 mM |
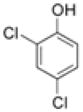 2,4-DCP 2,4-DCP
|
7.8 | 3.06 | Yes (complete at 9 mM) |
 2,4,6-TCP 2,4,6-TCP
|
6.0 | 3.72 | Yes (partial at 2.5 mM) |
 PCP PCP
|
4.74 | 5.12 | Yes (complete at 125 μM) |
 Triclosan Triclosan
|
7.9 | 4.76 | Yes (partial at 430 μM) |
pKa and log Kow values are from ChemIDplus (United States National Library of Medicine)
Kow Octanol-water partition coefficient. It is the ratio of the concentration of thechemical in octanol and in water.
Octanol is used as a surrogate for natural organic matter.
Figure 3.
Interaction of phenolics with NalC
(a) EMSA with 1.2 μM promoter DNA, 4.6 μM NalC and varying concentrations of phenol, DCP and TCP (b) EMSA with 1.2 μM promoter DNA, 4.6 μM NalC and varying concentrations of triclosan, (c) fold change in expression of armR and mexB normalized to rpsL after addition of 5 mM Phenol, 300 μM of DCP, 400 μM TCP, 120 μM PCP or 100 μM triclosan to log phase batch cultures. Bars represent means from 3 separate batch cultures and error bars represent standard deviations about the mean, (d) EMSA with 1.2 μM promoter DNA, 4.6 μM NalC. PCP and DCP were used as indicated. pH was varied between 6 and 9. (+): present, (−): absent.
Chlorophenols are weak acids. While PCP shows a pKa of 4.74 indicating that it is almost completely de-protonated under our assay conditions (pH 7.8), DCP shows a pKa of 7.8 and is predicted to be about 50% de-protonated. We were curious about the relative strength of the protonated versus the de-protonated forms in interacting with NalC and abolishing DNA binding, which might explain the observed differences in relative affinity of the ligands. We found that binding of NalC to DNA was relatively unaffected in the pH range of 6 to 9 (Figure 3(d)). Similarly, the effects of PCP on NalC’s binding affinity were pH-independent in the chosen pH range where PCP is largely deprotonated. In stark contrast, however, DCP at pH below 8 (primarily protonated) completely prevented NalC-DNA binding (Figure 3(d)). NalC-DNA binding increased in the presence of DCP with increasing pH values, indicating that the protonated form has higher affinity for NalC. These results suggest that the protonation state of the phenol alone cannot explain NalC binding affinities.
Thermodynamic stability of NalC
The regulatory mechanism of most TetR family members involves the binding of ligands to their ligand binding domain (LBD), which in turn induces the dissociation of DNA from their DNA binding domain (DBD). A widely accepted explanation for TetR’s allosteric mechanism is that the protein assumes two distinct structures in the ligand-free and ligand-bound states, one which binds DNA and the other which does not (Orth et al., 2000, Ramos et al., 2005). Recently, a different mechanism was proposed by Reichheld et al. (2009), who suggested that the ligand-free TetR has a flexible DBD, arising from a lack of interaction between the DBD and the LBD, and that this flexibility of the DBD is important for DNA binding. The authors then showed that the ligand-free TetR unfolds in a three-state manner, with the unfolding of the DNA binding domain preceding the unfolding of the ligand binding domain. Binding to the ligand tetracycline increased cooperativity between the two domains of TetR, resulting in rigidification of the DBD and a single cooperative unfolding transition. Based on these data, Reichheld et al.suggested that lack of flexibility of the DBD in the ligand-bound state is responsible for its failure to bind DNA.
To monitor the conformational rearrangements of NalC upon ligand binding and its stability in the absence and presence of PCP, we conducted Far-UV circular dichroism (CD) measurements of NalC as read-out for changes in its secondary structure. We found that in the presence of PCP, NalC gained helicity, suggesting significant structural rearrangements in the LBD upon PCP binding (Figure 4(a)). Thermal transition data fitted with sigmoidal curves in SigmaPlot 10 show that the apparent median melting temperature of NalC also increased from 59°C in the absence of PCP to 78°C in the presence of PCP, confirming that PCP binds to NalC, hence the increased stability (Figure 4(b)). Upon removal of PCP, NalC behaved like the ligand-free protein, providing further support for the reversibility of NalC-PCP binding. Incubation of NalC in presence of 1.25 mM phenol, a ligand that does not bind NalC, did not affect the structure of NalC (SI Figure 2). To further exclude that non-specific effects of PCP might be responsible for the observed changes in NalC’s structure and thermal stability, we analyzed the CD spectrum and thermal transition of the redox-sensitive chaperone Hsp33 in the presence of 400 PCP (SI Figure 3). Hsp33 is a two-domain protein containing a meta-stable linker region, and is not known to have ligand binding properties (Graf et al., 2004). As shown in SI Figure 3, we were unable to detect any effect of PCP on the structure or thermal stability of Hsp33. These results strongly suggest that the binding of PCP to NalC is specific and increases the stability of the regulator. In contrast to the reported results on TetR, however, ligand-free NalC showed a classical two-state transition whereas the unfolding of PCP-bound NalC was non-cooperative (Figure 4(b)), suggesting that the effects of ligand binding might differ among the members of this large protein family. However, neither one of the transitions was fully reversible, precluding us from the possibility to precisely assess the role of ligand binding on the stability of NalC.
Figure 4.
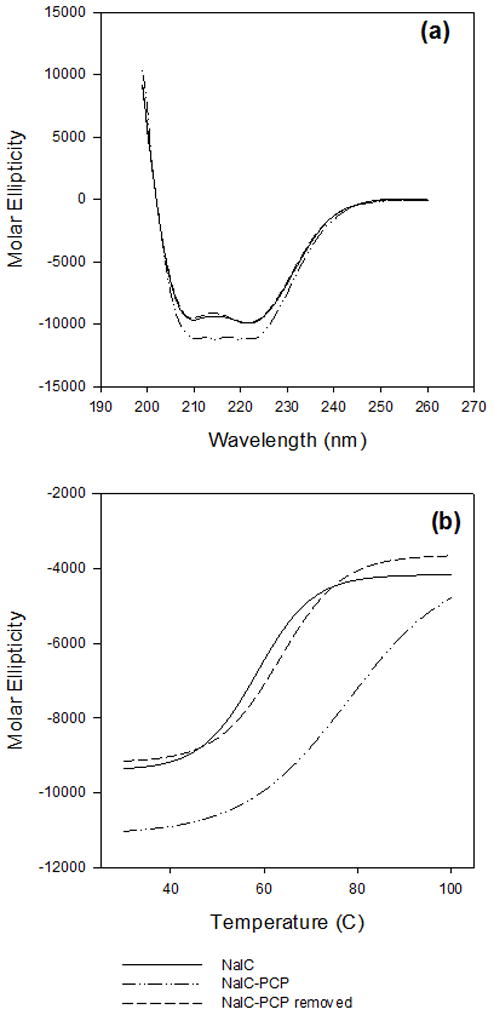
Effect of PCP on the thermodynamic stability of NalC
(a) CD spectra of NalC (straight line), NalC bound to PCP (dashed and dotted line) and NalC incubated with PCP followed by PCP removal by gel filtration (dashed line). (b) Thermal transitions curves for NalC (straight line), NalC bound to PCP (dashed and dotted line) and NalC incubated with PCP followed by PCP removal by gel filtration (dashed line). The samples were heated with a rate of 1°C/min and the CD signal at 222 nm was monitored. The thermal transitions are not completely reversible.
Significance of NalC activation by chlorinated phenols
Our observation that NalC expression is induced by chlorinated phenols leads us to speculate on its natural physiological significance. Halogenated organics are naturally present in soil (Myneni, 2002). Different chlorinated phenols, particularly chlorinated methoxyphenols, have been detected in pristine river waters in the ppm (μg/L) concentration range (Michalowicz et al., 2008). We looked at armR expression in presence of varying concentrations of PCP, and found that concentrations as low as 40 ppm of PCP significantly increased levels of expression of armR in P. aeruginosa (data not shown). Many soil microorganisms, including bacteria and fungi, harbor halogenases and haloperoxidases and potentially generate organohalogens (Wagener et al., 2009, Bengtson et al., 2009). These observations suggest that P. aeruginosa, which is also found in soil (Schobert and Tielen, 2010), is likely to encounter chlorinated organics including chlorophenols in its natural habitat and may have evolved NalC mediated efflux to protect itself. While addition of 150 μM PCP to logarithmic phase batch cultures of P. aeruginosa PAO1 did not affect its growth rate (SI Figure 4(a)), strains lacking the functional mexB or armR gene showed reduced growth in presence of PCP (SI Figure 4(b) and 4(c)). Curiously enough, P. aeruginosa also harbors a functional chloroperoxidase (Song et al., 2006).
Apart from naturally produced chlorinated phenols, P. aeruginosa is likely to be exposed to these chemicals also due to human activities. Chlorophenols, such as 2-chlorophenol, 2,4-DCP, 2,4,6-TCP and PCP have long been used as biocides and wood preservatives and can contaminate soil (McLellan et al., 2007). Chlorination of drinking water also results in the production of minor quantities of chlorophenols (Ge et al., 2008). Both are environments that harbor P. aeruginosa (Mena and Gerba, 2009, Schobert and Tielen, 2010). Another environment where P. aeruginosa may be exposed to chlorophenols is health-care units (Weber et al., 2007). Triclosan is an antimicrobial used both in health-care units and in a wide variety of household products (Fiss et al., 2007). Other examples of chlorophenol-based disinfectants include chloroxylenol and ortho-benzyl-para-chlorophenol (Rutala, 2008). The effect of low doses of different halogenated phenols and chlorinated phenicols in these environments on P. aeruginosa survival, proliferation and other characteristics such as resistance and virulence remains a pertinent question.
Conclusion
We show here that chlorinated phenols interact with the transcriptional regulator NalC of the MexAB-OprM MDR efflux pump to control its expression. NalC binding to chlorinated phenols results in de-repression of NalC and ArmR. Increased expression of ArmR, a MexR anti-repressor, results in MexR-ArmR complex formation (Wilke et al., 2008) and alleviates MexR-mediated repression of MexAB-OprM. We demonstrated that NalC-chlorinated phenol binding is fully reversible as NalC regained its DNA binding activity once chlorophenols were removed. We found that triclosan also reversibly binds to NalC. This observation expands the range of chemicals that potentially induce NalC to include chemicals with chlorophenol side chains. It will now be interesting to determine the precise ligand recognition mechanism that is used by NalC.
As we continue to use and release various purportedly toxic or benign chemicals, it is important for us to understand their full potential in impacting the biosphere. Now we know that antibiotics do not only have antagonistic roles (Linares et al., 2006) and that mechanisms of resistance to antibiotics did not necessarily evolve in response to antibiotics (Piddock, 2006). Similarly, transcriptional regulators are not simple on/off switches, and responses are modulated by the intensity of signaling molecules as well as the different regulatory pathways that intersect to produce a functional organism (Cases and de Lorenzo, 2005). Our observation that chlorinated phenols induce a MDR efflux pump regulator fits these paradigms.
Experimental Procedures
Bacterial strains and growth conditions
Wild type P. aeruginosa strain PAO1 was obtained from H. Schweizer (Schweizer, 1998). PAO1 was grown in Luria-Burtani (LB) medium at 37°C unless otherwise stated. E. coli BL21(DE3) (B834 derivative, F−ompThsdSB(rB−mB−) gal dcm (DE3), Novagen, EMD Biosciences, San Diego, CA) with the pet-15b plasmid (Expression vector with N-terminal His • Tag® Apr, Novagen) was grown on LB with 50 μg/mL of ampicillin at 37°C.
Chemicals
Pentachlorophenol (PCP), 2,4-dichlorophenol (DCP) and 2,4,6-triclorophenol (TCP), and triclosan were purchased from Sigma-Aldrich (Table 1). PCP stock was made at 10 mg/mL in 36 mM NaOH. DCP stock was made at a concentration of 5 mg/mL in Tris buffer (pH 7.8). TCP stock was made at 5 mg/mL in 36 mM NaOH. Triclosan stock was made at 1.25 mg/mL in 50% solution of 36 mM NaOH. Concentrations of DCP and TCP used in experiments were verified using HPLC.
Gene expression analysis using qRT-PCR
P. aeruginosa PAO1 was grown in minimal medium (Muller et al., 2007) with and without 120 μM PCP. Triplicate batch cultures were used for both growth conditions. Samples were collected in duplicate during logarithmic growth phase (OD600 ~ 0.3), immediately treated with RNAprotect Bacterial Reagent (Qiagen, Valencia, CA) and stored at −80°C for RNA extraction. Gene expression was also analyzed in presence of 300 μM DCP, 400 μM TCP, and 100 μM triclosan. PCP (120 μM) and phenol (5 mM) were used as positive and negative control respectively. Each condition was tested in triplicate. For this experiment P. aeruginosa PAO1 was grown in LB to early logarithmic growth phase (OD600 ~ 0.2), when chemicals were added. Cells were harvested at OD600 ~ 0.6 (still in logarithmic growth phase), immediately treated with RNAprotect Bacteria Reagent and stored at −80°C. RNA was extracted using the RNeasy Mini Kit (Qiagen). DNase I, Amplification Grade (Invitrogen Life Science, Carlsbad, CA) was used for DNase digestion prior to cDNA synthesis using the SuperScript II RT kit (Invitrogen Life Science). Random hexamer primers were used for cDNA synthesis. Multiple aliquots of cDNA were stored at −20°C for RT-PCR analysis.
Primers used in qRT-PCR for mexR, mexB, armR and nalC genes are listed in Table 2. Housekeeping genes, nadB and rpsL were used as controls. Quantitative PCR was performed in a Mastercycler ep realplex thermocycler (Eppendorf, Hauppauge, NY) using the Power SYBR Green Master Mix (Applied Biosystems, Foster City, CA). PCR conditions and primer concentrations were optimized to eliminate the formation of primer-dimers and non-specific products. All qPCR analyses were performed in triplicate.
Table 2.
Primers for RT-qPCR
| Name | Sequence (5′→3′) | Reference |
|---|---|---|
| nalC | F: CCT CAC ATG GAC GAG GAA AC R: AGG TAG CAG GCG ATG ATG TC |
This study |
| armR | F: CCT GAA CAC TCC GCG CAA C R: GTG CTC GCC GTA GAG GTC C |
(Cao et al., 2004) |
| mexR | F: GAG CTG GAG GGA AGA AAC CT R: AGG CAC TGG TCG AGG AGA T |
This study |
| mexB | F: GTG TTC GGC TCG CAG TAC TC R: AAC CGT CGG GAT TGA CCT TG |
(Muller et al., 2007) |
| nadB | F: CTTCACCGTGGAGCATAGC R: GCCTTCCTCGTGGTTGTG |
(Muller et al., 2007) |
| rpsL | F: TAC ATC GGT GGT GAA GGT CA R: TAC TTC GAA CGA CCC TGC TT |
This study |
Selection of colonies with high PCP tolerance
To test if PCP selected for P. aeruginosa PAO1 with mutations in regulatory genes, colonies grown in medium containing PCP were screened for mutations. Briefly, cultures were grown overnight with 120 μM PCP in LB broth, transferred into fresh PCP-containing LB broth and grown to mid-log phase. Logarithmic phase cultures were 10-fold serially diluted in 10 mM phosphate buffer saline (pH ~ 7.5) and 100 μL cultures were plated onto LB agar containing PCP at concentration of 150 μM, 1.5 mM, 3.75 mM and 6 mM. No growth was observed at 6 mM PCP. Colonies were randomly picked from plates containing 150 μM, 1.5 mM and 3.75 mM PCP (10 colonies per PCP concentration) and analyzed for mutations in mexR and nalC. Colony PCP was used for amplification of the entire length of these genes. Primers used for amplifying mexR were forward 5′-CATTAGGTTTACTCGGCCAAACC-3′ and reverse 5′-CGCCAGTAAGCGGATACCTG-3′ (Daigle et al., 2007) and nalC were forward 5′-GAATGAAGCGGAAGTGCTTGC-3′ and reverse 5′-CGAGATCCACCTCACCGAAC-3′(Cao et al., 2004). Amplicons were sequenced at the DNA Sequencing Core facility at the University of Michigan (Ann Arbor).
Expression and purification of NalC
Wild type nalC was PCR amplified from P. aeruginosa PAO1 using forward primer 5′-GTGTGTAAGGCATATGAACGATGCTTCTCC-3′ (NdeI site underlined) and reverse primer 5′-TTCGTATTGGATCCACCTCACCGAACTGC-3′(BamHI site underlined), cloned into pET-15b vector containing the 6xHis tag (EMD Biosciences) and transformed into E. coli BL21(DE3) (EMD Biosciences). BL21(DE3) with nalC was grown to OD600 of ~0.6 and induced with 1 mM IPTG for 3 hours. Cells were pelleted, resuspended in phosphate buffer (40 mM KH2PO4, 200 mM KCl, pH 7.5) and treated with Halt Protease Inhibitor Cocktail (Thermo Fisher Scientific, Rockford, IL). Cells were lysed in the French press. 6xHis-NalC was purified using a bench-top process using the HisPur Cobalt Resin (Thermo Fisher Scientific) following the manufacturer’s protocol. NalC was eluted with a 100 mM imidazole buffer (50 mM KH2PO4, 300 mM NaCl, pH 7.4). Purity of 6xHis-NalC was assessed using SDS-PAGE analysis on a 14% Tris-glycine gel (Invitrogen). 6xHis tag removal was performed using Restriction Grade Thrombin (EMD Chemicals Inc.) followed by elution through the HisPur Cobalt Resin. The cleaved protein was concentrated and loaded onto a HiLoad 26/60 Superdex 75 Prep grade column (GE Healthcare, Piscataway, NJ) equilibrated with HEPES buffer (40 mM HEPES, 100 mM KCl, pH 7.8). Peak fractions were analyzed for the presence of pure NalC using SDS-PAGE. NalC containing fractions were pooled and concentrated to 2 mg/mL. Protein purity was >97% as assessed by SDS-PAGE analysis.
Electrophoretic mobility shift assay (EMSA)
The nalC/PA3720 intergenic promoter region (Cao et al., 2004) was amplified using primers 5′-AGGCATCGATATCCAACAGG-3′ and 5′-GGGAGAAGCATCGTTCAT-3′ and amplification products were purified using QIAquick PCR Purification Kit (Qiagen, Valencia CA). EMSA was set up with purified NalC, promoter DNA and varying concentrations of chemicals in a 10 mM Tris-HCl binding buffer (pH 7.8) containing 1 mM EDTA, 100 mM KCl, 5% v/vglycerol, 0.1 mM DTT, 0.01 mg/mL BSA (Hellman and Fried, 2007). The NalC protein was pre-incubated with the respective chemicals in the 10 mM Tris-HCl buffer for 1 hour. Then, DNA was added and incubation was continued for another 45 minutes. Samples were run on 0.8% agarose gels in 1× TAE at 100V at room temperature and stained with SYBR Safe DNA Gel Stain (Invitrogen, Carlsbad, CA). DNA bands were visualized using a Dark Reader Transilluminator (Clare Chemical Research).
NAP-5 filtration and dialysis
PCP-NalC mixtures were filtered through an Illustra NAP-5 column (GE Healthcare Lifesciences, Piscataway, NJ) packed with Sephadex™ G-25 and equilibrated with PCP-free 10 mM Tris-HCl binding buffer to remove any non-covalently bound PCP. Similarly, DCP, TCP or triclosan were removed from NalC by dialysis using a regenerated cellulose Spectra/Por membrane with an 8 kDa cutoff (Spectrum Laboratories, Inc.). TCP and triclosan were dialyzed overnight, while DCP was dialyzed for 2 days.
Circular Dichroism (CD) spectroscopy
NalC was diluted to 8.7 μM (0.2 mg/mL) in 20 mM KH2PO4 buffer (pH 7.8). For monitoring the molar ellipticity of NalC bound to PCP, 8.7 μM of NalC was pre-incubated with 400 μM PCP for 1 hour at room temperature. This PCP concentration was sufficient to completely prevent NalC’s binding to DNA. To assess reversibility of PCP binding, NalC-PCP was gel filtered through an Illustra NAP-5 column as described previously and re-tested. Far UV CD scans (199–260 nm) for NalC, NalC-PCP and NalC after PCP removal were performed using a Jasco J-810 CD spectrophotometer (Jasco Analytical Instruments, Easton, MD). Six scans were accumulated. The spectra of buffer alone or buffer with PCP were subtracted from the protein spectra. Thermal transitions of NalC, NalC-PCP and NalC after PCP removal were analyzed between 30°C and 100°C (temperature was controlled by a Jasco PTC-423S) and readings were taken at 222 nm. The rate of temperature increase was 1°C/min. Thermal transition data were fitted with sigmoidal curves in SigmaPlot 10 to determine apparent median melting temperatures.
Supplementary Material
Acknowledgments
This work was supported by funding from the College of Engineering, University of Michigan for S.G. and supplies and, in part, by The National Institute of Health Grant GM065318 to U.J. We would like to thank H. Schweizer and K. Poole for P. aeruginosa strains.
References
- Alekshun MN, Levy SB. Molecular mechanisms of antibacterial multidrug resistance. Cell. 2007;128:1037–1050. doi: 10.1016/j.cell.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Bengtson P, Bastviken D, de Boer W, Oberg G. Possible role of reactive chlorine in microbial antagonism and organic matter chlorination in terrestrial environments. Environ Microbiol. 2009;11:1330–1339. doi: 10.1111/j.1462-2920.2009.01915.x. [DOI] [PubMed] [Google Scholar]
- Cann JR, Hinman ND. Hummel-Dreyer gel chromatographic procedure as applied to ligand-mediated association. Biochemistry. 1976;15:4614–4622. doi: 10.1021/bi00666a011. [DOI] [PubMed] [Google Scholar]
- Cao L, Srikumar R, Poole K. MexAB-OprM hyperexpression in NalC-type multidrug-resistant Pseudomonas aeruginosa: identification and characterization of the nalC gene encoding a repressor of PA3720-PA3719. Mol Microbiol. 2004;53:1423–1436. doi: 10.1111/j.1365-2958.2004.04210.x. [DOI] [PubMed] [Google Scholar]
- Cases I, de Lorenzo V. Promoters in the environment: Transcriptional regulation in its natural context. Nat Rev Microbiol. 2005;3:105–118. doi: 10.1038/nrmicro1084. [DOI] [PubMed] [Google Scholar]
- Chen H, Hu J, Chen PR, Lan LF, Li ZL, Hicks LM, et al. The Pseudomonas aeruginosa multidrug efflux regulator MexR uses an oxidation-sensing mechanism. Proc Natl Acad Sci U S A. 2008;105:13586–13591. doi: 10.1073/pnas.0803391105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daigle DM, Cao L, Fraud S, Wilke MS, Pacey A, Klinoski R, et al. Protein modulator of multidrug efflux gene expression in Pseudomonas aeruginosa. J Bacteriol. 2007;189:5441–5451. doi: 10.1128/JB.00543-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiss EM, Rule KL, Vikesland PJ. Formation of chloroform and other chlorinated byproducts by chlorination of triclosan-containing antibacterial products. Environ Sci Technol. 2007;41:2387–2394. doi: 10.1021/es062227l. [DOI] [PubMed] [Google Scholar]
- Ge F, Zhu LZ, Wang J. Distribution of chlorination products of phenols under various pHs in water disinfection. Desalination. 2008;225:156–166. [Google Scholar]
- Graf PCF, Martinez-Yamout M, VanHaerents S, Lilie H, Dyson HJ, Jakob U. Activation of the redox-regulated chaperone Hsp33 by domain unfolding. J Biol Chem. 2004;279:20529–20538. doi: 10.1074/jbc.M401764200. [DOI] [PubMed] [Google Scholar]
- Hellman LM, Fried MG. Electrophoretic mobility shift assay (EMSA) for detecting protein-nucleic acid interactions. Nat Protoc. 2:1849–1861. doi: 10.1038/nprot.2007.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocquet D, Berthelot P, Roussel-Delvallez M, Favre R, Jeannot K, Bajolet O, et al. Pseudomonas aeruginosa may accumulate drug resistance mechanisms without losing its ability to cause bloodstream infections. Antimicrob Agents Chemother. 2007;51:3531–3536. doi: 10.1128/AAC.00503-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs MA, Alwood A, Thaipisuttikul I, Spencer D, Haugen E, Ernst S, et al. Comprehensive transposon mutant library of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 2003;100:14339–14344. doi: 10.1073/pnas.2036282100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhas M, Eberl L, Tummler B. Quorum sensing: the power of cooperation in the world of Pseudomonas. Environ Microbiol. 2005;7:459–471. doi: 10.1111/j.1462-2920.2005.00769.x. [DOI] [PubMed] [Google Scholar]
- Kerr KG, Snelling AM. Pseudomonas aeruginosa: a formidable and ever-present adversary. J Hosp Infect. 2009;73:338–344. doi: 10.1016/j.jhin.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Li XZ, Poole K. Organic solvent-tolerant mutants of Pseudomonas aeruginosa display multiple antibiotic resistance. Can J Microbiol. 1999;45:18–22. doi: 10.1139/cjm-45-1-18. [DOI] [PubMed] [Google Scholar]
- Linares JF, Gustafsson I, Baquero F, Martinez JL. Antibiotics as intermicrobial signaling agents instead of weapons. Proc Natl Acad Sci U S A. 2006;103:19484–19489. doi: 10.1073/pnas.0608949103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister PD, Wolter DJ, Hanson ND. Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin Microbiol Rev. 2009;22:582–610. doi: 10.1128/CMR.00040-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez JL. The role of natural environments in the evolution of resistance traits in pathogenic bacteria. Proc R Soc B-Biol Sci. 2009;276:2521–2530. doi: 10.1098/rspb.2009.0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan L, Carvalho M, Pereira CS, Hursthouse A, Morrison C, Tatner P, et al. The environmental behaviour of polychlorinated phenols and its relevance to cork forest ecosystems: a review. J Environ Monit. 2007;9:1055–1063. doi: 10.1039/b701436h. [DOI] [PubMed] [Google Scholar]
- Mena KD, Gerba CP. Reviews of Environmental Contamination and Toxicology. Vol. 201. New York: Springer; 2009. Risk Assessment of Pseudomonas aeruginosa in Water; pp. 71–115. [DOI] [PubMed] [Google Scholar]
- Mesaros N, Nordmann P, Plesiat P, Roussel-Delvallez M, Van Eldere J, Glupczynski Y, et al. Pseudomonas aeruginosa: resistance and therapeutic options at the turn of the new millennium. Clin Microbiol Infect. 2007;13:560–578. doi: 10.1111/j.1469-0691.2007.01681.x. [DOI] [PubMed] [Google Scholar]
- Michalowicz J, Bukowska B, Duda W. The differences in phenolic content in rivers exposed and non-exposed to anthropogenic contamination. Chemosphere. 2008;71:735–741. doi: 10.1016/j.chemosphere.2007.10.017. [DOI] [PubMed] [Google Scholar]
- Morita Y, Cao L, Gould VC, Avison MB, Poole K. nalD encodes a second repressor of the mexAB-oprM multidrug efflux operon of Pseudomonas aeruginosa. J Bacteriol. 2006;188:8649–8654. doi: 10.1128/JB.01342-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JF, Stevens AM, Craig J, Love NG. Transcriptome analysis reveals that multidrug efflux genes are upregulated to protect Pseudomonas aeruginosa from pentachlorophenol stress. Appl Environ Microbiol. 2007;73:4550–4558. doi: 10.1128/AEM.00169-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myneni SCB. Formation of stable chlorinated hydrocarbons in weathering plant material. Science. 2002;295:1039–1041. doi: 10.1126/science.1067153. [DOI] [PubMed] [Google Scholar]
- Neyfakh AA. Natural functions of bacterial multidrug transporters. Trends Microbiol. 1997;5:309–313. doi: 10.1016/S0966-842X(97)01064-0. [DOI] [PubMed] [Google Scholar]
- Orth P, Schnappinger D, Hillen W, Saenger W, Hinrichs W. Structural basis of gene regulation by the tetracycline inducible Tet repressor-operator system. Nat Struct Biol. 2000;7:215–219. doi: 10.1038/73324. [DOI] [PubMed] [Google Scholar]
- Piddock LJV. Multidrug-resistance efflux pumps - not just for resistance. Nat Rev Microbiol. 2006;4:629–636. doi: 10.1038/nrmicro1464. [DOI] [PubMed] [Google Scholar]
- Poole K, Tetro K, Zhao QX, Neshat S, Heinrichs DE, Bianco N. Expression of the multidrug resistance operon mexA-mexB-oprM in Pseudomonas aeruginosa: mexR encodes a regulator of operon expression. Antimicrob Agents Chemother. 1996;40:2021–2028. doi: 10.1128/aac.40.9.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos JL, Martinez-Bueno M, Molina-Henares AJ, Teran W, Watanabe K, Zhang XD, et al. The TetR family of transcriptional repressors. Microbiol Mol Biol Rev. 2005;69:326–356. doi: 10.1128/MMBR.69.2.326-356.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichheld SE, Yu Z, Davidson AR. The induction of folding cooperativity by ligand binding drives the allosteric response of tetracycline repressor. Proc Natl Acad Sci U S A. 2009;106:22263–22268. doi: 10.1073/pnas.0911566106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutala WAR, Weber DJ the Healthcare Infection Control Practices Advisory Committee (HICPAC) Guideline for disinfection and sterilization in healthcare facilities. Center for Disease Control and Prevention; 2008. [Google Scholar]
- Schobert M, Tielen P. Contribution of oxygen-limiting conditions to persistent infection of Pseudomonas aeruginosa. Future Microbiol. 2010;5:603–621. doi: 10.2217/fmb.10.16. [DOI] [PubMed] [Google Scholar]
- Schweizer HP. Intrinsic resistance to inhibitors of fatty acid biosynthesis in Pseudomonas aeruginosa is due to efflux: Application of a novel technique for generation of unmarked chromosomal mutations for the study of efflux systems. Antimicrob Agents Chemother. 1998;42:394–398. doi: 10.1128/aac.42.2.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JK, Ahn HJ, Kim HS, Song BK. Molecular cloning and expression of perhydrolase genes from Pseudomonas aeruginosa and Burkholderia cepacia in Escherichia coli. Biotechnol Lett. 2006;28:849–856. doi: 10.1007/s10529-006-9016-8. [DOI] [PubMed] [Google Scholar]
- Wagner C, El Omari M, Konig GM. Biohalogenation: Nature’s Way to Synthesize Halogenated Metabolites. J Nat Prod. 2009;72:540–553. doi: 10.1021/np800651m. [DOI] [PubMed] [Google Scholar]
- Weber DJ, Rutala WA, Sickbert-Bennett EE. Outbreaks associated with contaminated antiseptics and disinfectants. Antimicrob Agents Chemother. 2007;51:4217–4224. doi: 10.1128/AAC.00138-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke MS, Heller M, Creagh AL, Haynes CA, McIntosh LP, Poole K, Strynadka NCJ. The crystal structure of MexR from Pseudomonas aeruginosa in complex with its antirepressor ArmR. Proc Natl Acad Sci U S A. 2008;105:14832–14837. doi: 10.1073/pnas.0805489105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.