Abstract
Background and objectives:
The potential of soy isoflavones to interfere with thyroid function has been reported. However, there are limited data regarding their effect on thyroid function and autoimmunity in surgical menopausal women. The present study aimed to evaluate the effect of isoflavones on thyroid function and autoimmunity, menopausal symptoms, serum follicle stimulating hormone (FSH) and estradiol levels in oophorectomised women.
Methods:
A randomized, double blind, placebo-controlled trial was conducted in 43 oophorectomised women to evaluate the effect of soy isoflavones (75 mg/day for 12 wk) on serum thyroid profile (free T3 , free T4 , TSH, TBG and anti-TPO antibody titres) assessed at baseline, 6 and 12 wk after randomization. Assessment was also done for menopause symptom score (MSS) three weekly, and FSH and estradiol levels at baseline and at study completion.
Results:
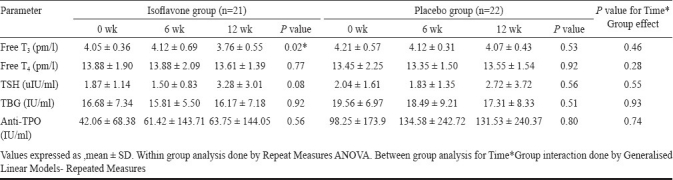
There was a significant alteration in free T 3 levels in the group receiving isoflavones (4.05 ± 0.36, 4.12 ± 0.69 and 3.76 ± 0.55 pmol/l at baseline, 6 and 12 wk, respectively; P=0.02). However, the mean change in various thyroid parameters at 12 wk from baseline was not significantly different between the two groups. MSS was also significantly decreased at 9 and 12 wk from baseline with isoflavones (12.47 ± 8.15, 9.35 ± 5.23 and 9 ± 5.14 at baseline, 9 and 12 wk respectively; P=0.004) with significant improvement in urogenital symptoms compared to placebo. Isoflavones did not significantly affect other parameters during study period. There were no serious adverse events reported and the proportion of patients experiencing adverse events was similar between the two groups.
Interpretation and conclusions:
Modest reduction in serum free T3 levels in the isoflavone group in the absence of any effect on other thyroid parameters might be considered clinically unimportant.
Keywords: Isoflavones, menopausal symptoms, oophorectomised women, phytoestrogens, thyroid autoimmunity, thyroid function
Women's Health Initiative study in 20021 and Heart and Estrogen/progestin replacement study (HERS)2,3 have indicated that the risks of hormone replacement therapy (HRT) for post-menopausal women may outweigh the benefits. In order to address the health concerns with HRT, there is a growing interest in the natural alternatives to HRT. Phytoestrogens, a class of several different chemicals produced by a variety of plants, possess demonstrable estrogenic activity. They can act as selective estrogen response modulators (SERMs) since they have measurable affinity for estrogen receptors4. Of these, the soy isoflavones have received considerable attention for development as dietary supplements for post-menopausal women due to absence of adverse effect profile of HRT.
There is a concern, however, regarding the harmful effects of soy isoflavones on thyroid function. Earlier studies had reported development of goiter in rats fed with soy5. It was earlier reported that soy isoflavones caused increase in serum thyroxine (T4)6 and triiodothyronine (T3)7 levels in rodents. Schmutzler et al8 demonstrated inhibition of iodide uptake and irreversible inactivation of thyroid peroxidase (TPO) in an in vitro study employing rodent tissue thereby indicating interference with thyroid hormone biosynthesis at the level of iodide uptake and organification by genistein, a soy isoflavone. Subsequently, in 1950s and 1960s, cases of goiter without overt signs of hypothyroidism were reported in infants consuming soy based infant formula9. Alterations in thyroid function with modified dietary soy or isoflavones have also been reported in a few studies in post-menopausal women10,11, who are in particular prescribed isoflavones as an alternative to HRT. However, there is no prospective human study to indicate the effect of soy isoflavones on thyroid autoimmune status. Women with natural menopause continue to synthesize and secrete small quantities of estrone from the ovaries and estrogen is known to cause elevation of thyroid binding globulin (TBG)12 hence the true interaction of isoflavones with thyroid function needs evaluation in women with surgical menopause.
Keeping in view the safety concerns related to the widespread use of soy extracts as dietary supplements or as prescription only drugs in different parts of the world13, it was thought prudent to investigate in a randomized, double blind, placebo controlled trial whether soy isoflavones extract caused any significant alterations in the thyroid profile of women undergone oophorectomy.
Material & Methods
Subjects: All women who reported to the Obstetrics and Gynaecology out patient department of Post- graduate Institute of Medical Education and Research (PGIMER), Chandigarh, India, from March 2007 to January 2008 were screened for inclusion in the study. Those who had undergone bilateral oophorectomy, were less than 55 yr of age had baseline thyroid stimulating hormone (TSH) values within the normal range and were willing to comply with the protocol were included in the study. They were excluded if any of the following criteria were present: (1) suffering from any thyroid disease, (2) suffering from any autoimmune disorder, (3) already on isoflavones, (4) taken HRT/estrogen replacement therapy (ERT) within previous 8 wk, (5) presence of renal and/or hepatic disease, (6) active major psychiatric disorders, (7) history of thrombophlebitis or thromboembolism or cerebrovascular disorders, (8) uncontrolled hypertension with blood pressure > 180/100 mm Hg, (9) uncontrolled diabetes, (10) were on any drug which can affect thyroid function, (11) presence of active infection or malignancy, and (12) present or past history of soy or nut related food allergies.
The study protocol was approved by the Institutional Ethics Committee and all the participating women signed a written informed consent prior to enrollment to the study. Eligible women were randomized so that they had an equal probability of assignment to either of the two groups. The randomization code was developed using random number table to select random permuted blocks of length four. The identity of the drugs was hidden by packing in numbered opaque envelopes to ensure concealment of the sequence until assigned. Randomization, allocation sequence generation and packing of envelopes were done by investigators not directly involved in dispensing and evaluating the treatments so that it was double blind - both the patient and evaluating clinician not aware of the assigned treatment. The participants remained on the same allocation throughout the study period if they continued. The randomization code was revealed to the investigators once recruitment, data collection, and laboratory analyses were complete.
The participants attended the clinic at the time of randomization (baseline) and at three weekly intervals for 12 wk.
Study interventions: The participants were randomized to either placebo or study drug to be administered once a day orally for 12 wk. The study drug tablet contained 75 mg of soy isoflavones standardized to provide isoflavones 40 per cent (Genistein and Genistin 25%; Daidzein and Daidzin 15%) along with 200 mg elemental calcium, 100 mg elemental phosphorus, and 100 IU of vitamin D3. Placebo tablet matching in colour, size, shape and taste and containing identical quantity of calcium, phosphorus and vitamin D3 was used as control treatment. Patients were advised to take one tablet daily at bedtime with 150 ml of plain water for a total 12 wk period. All the patients were instructed to avoid dietary phytoestrogen consumption by avoiding soy, seeds and sprouts, beans and legumes during the study period. Other treatments which might interfere with thyroid function were also avoided during the study. However, concomitant treatments for other associated illnesses were allowed. Since the drug is available in the market at the required dose, drug product label instruction was followed for storing and dispensing of the drug. Appropriate treatment was provided for any observed adverse event. The patients were provided with a contact phone number to call in case of emergency. The patients were asked to bring the drug envelopes during subsequent visits and compliance was assessed by pill counting. More than 80 per cent compliance was considered as adequate.
In the present study the hypothesis tested was that isoflavones cause a significant alteration in thyroid economy and autoimmunity in comparison to placebo in euthyroid, surgical menopausal women. The primary outcome of the study was complete serum thyroid profile (TSH, free T3, free T4, Thyroid binding globulin and anti – TPO antibodies). The secondary outcomes included assessment of menopausal symptoms, serum follicle stimulating hormone (FSH) and estradiol levels.
For each patient, the serum thyroid profile was assessed at baseline, six wk and at study completion (12 wk) while presence of any goiter was ruled out by palpation of thyroid gland during each visit. The assessment for serum FSH and estradiol was done at baseline and 12 wk while menopausal symptoms were assessed during each three weekly visit.
For the assessment of various parameters, about 10 ml blood was withdrawn aseptically by venipuncture from each patient at baseline (0 wk), six and 12 wk after initiation of treatment. The blood samples were allowed to clot at room temperature and centrifuged at 1260 g for 10 min. The serum thus separated was stored at -20°C for further estimations. To reduce the effects of interassay variability, all samples from each subject were analysed in duplicate in the same batch. Free T3 and free T4 were determined by single antibody coated tube Radio-immuno assay (RIA) using 125I labelled hormone (Roche Diagnostics, Indianapolis, IN, USA). TSH was determined by double antibody RIA using 125I labelled hormone (Roche Diagnostics, Indianapolis, IN, USA). Thyroid binding globulin (TBG) and anti-TPO antibodies were determined by electrochemiluminescence immunoassay using specific antibodies labelled with a ruthenium complex (Roche Diagnostics). Serum FSH was determined quantitatively by chemiluminescence immunoassay in vitro which employs two different monoclonal antibodies specifically directed against human FSH. Radioimmunoassay was used to estimate serum estradiol levels (reference range in postmenopausal women 70-190 pmol/l).
The assessment of menopausal symptoms was done by calculating the menopause symptom score for each patient using Menopause rating scale (MRS)14. The total score of MRS ranges between 0 (asymptomatic) to 44 (highest degree of severity).The score increases point by point with increasing severity of subjectively perceived complaints in each of the 11 items (severity expressed as 0-4 points in each). The various symptoms included in questionnaire were - psychological symptoms - 0 to 16 points (4 symptoms: depressed, irritable, anxious, exhausted); somato-vegetative symptoms – 0 to 16 points (4 symptoms: sweating/flash, cardiac complaints, sleeping disorders, joint and muscle complaints); and urogenital symptoms – 0 to 12 points (3 symptoms: sexual problems, urinary complaints, vaginal dryness). The composite scores for each of the dimensions (sub-scales) is based on adding up the scores of the items of the respective dimensions. The composite score (total score) is the sum of the dimension scores.
The participants were also assessed for general health status by routine clinical examination and appearance of any adverse events during each visit.
Sample size: The normal range of TSH is 0.5-5.5 micro IU/ml (3± 2.5). Considering a change of 2.5 micro IU/ml from baseline to the end of follow up to be clinically significant in isoflavone arm over placebo and assuming a SD of 2.5 at alpha of 5 per cent and power of 80 per cent, 16 patients were required in each group; thus needing a total of 32 patients. Expecting 60 per cent compliance, it was decided to include up to 54 patients in the study.
Statistical analysis: Data were expressed as mean ± SD unless specified, numbers (percentages) and median (interquartile range). Proportions were compared using chi-square tests with continuity correction or Fisher's exact test when appropriate. The between and within groups repeated measurements of outcomes were analysed using Generalized linear models- Repeat measures and Repeated measures analysis of variance, respectively. Also, mean change from baseline in various parameters between the two groups was compared using unpaired Student's t test with Welch correction for parametric data or Mann whitney U test for non parametric data. Two sided significance tests were used throughout. P<0.05 was considered significant.
Results
A total of 77 women with bilateral oophorectomy underwent screening. Of these, 43 underwent randomization, with 21 and 22 assigned to the isoflavone and placebo groups respectively (Fig.). In the isoflavone group, one patient withdrew consent later on due to non specific reasons and one discontinued treatment because of adverse effects. In the placebo group, two patients discontinued treatment due to adverse effects. Two patients in isoflavone group and three patients in placebo group were lost to follow up and could not be contacted. Hence, 17 patients in each group completed the study.
Fig.
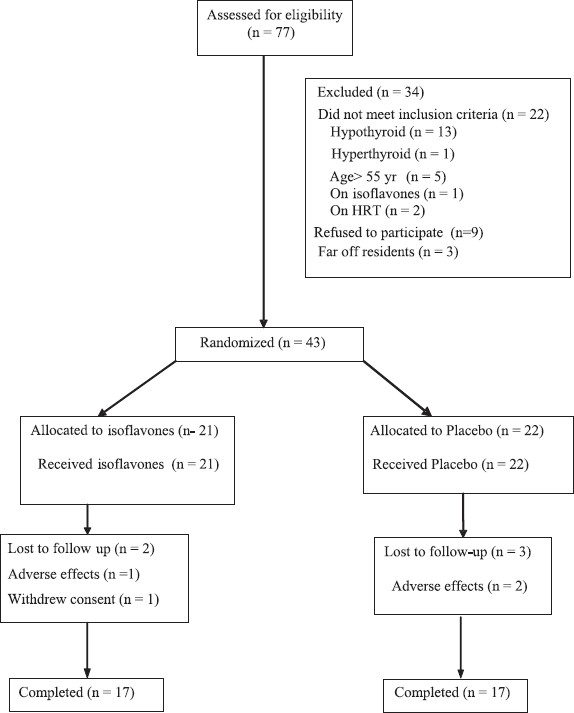
The CONSORT diagram for flow of participants.
The primary analysis was done by modified intention to treat (ITT) principle and all patients for whom at least one blood sample for estimation was available after treatment allocation were included in the analysis. For thyroid parameters, the total number of missing values were three in isoflavones group and five in placebo group. The primary analysis for all outcomes was done by last observation carried forward principle. For primary outcomes, an additional sensitivity analysis was carried out by replacing the missing values with least and highest values for the respective patient and results compared.
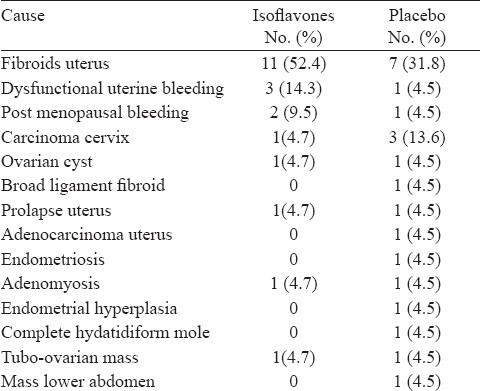
At baseline, the two study groups were similar in terms of age, body mass index, time since surgery (bilateral oophorectomy), past use of HRT, co-existing and past medical history (Table I). The most common cause of surgery in both the groups was fibroid uterus (Table II). Three patients in the isoflavones group and one patient in the placebo group had taken HRT in the past on account of post-hysterectomy or complaints of menorrhagia. The duration of HRT ranged from two months to five years but all the patients had stopped the intake of HRT between 6 to 48 months prior to enrolment to the present study.
Table I.
Demographic profile and clinical characteristics of patients in isoflavone and placebo groups at baseline
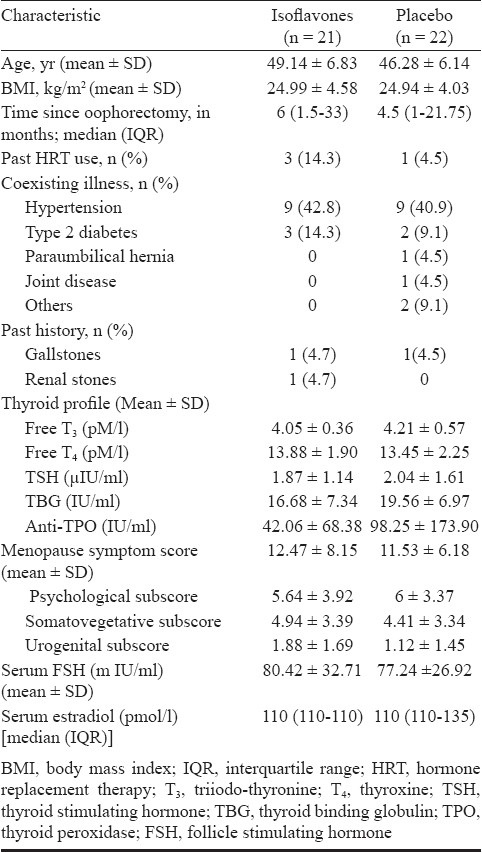
Table II.
Comparison of causes of bilateral oophorectomy in isoflavones and placebo groups
The alterations in thyroid profile with time between isoflavone and placebo groups is depicted in Table III. None of the parameters showed significant alteration with time in isoflavone group compared to placebo. However, there was a statistically significant change in free T3 levels within isoflavone group. At baseline, four patients in the isoflavone group and five in the placebo group had levels of anti-TPO antibodies higher than the normal range (>34 IU/ml), of whom three patients in each group exhibited further increase in antibodies levels at 12 wk. There was a decrease in antibody levels from baseline in one patient in each group though the levels remained above the normal range and in one patient in placebo group, antibody levels declined to normal (6.5 IU/ml) from baseline value of 36.1 IU/ml. Of the patients having normal antibodies levels at baseline, one patient in placebo group showed an increase in levels (40.19 IU/ml at 12 wk) from baseline value (9 IU/ml) while in the remaining the levels remained well in the normal range during the study period (Fisher's exact test for between groups, P=0.99). The results of the sensitivity analysis for all the parameters were similar to the primary analysis.
Table III.
Comparison of changes in thyroid profile with time between isoflavone and placebo groups
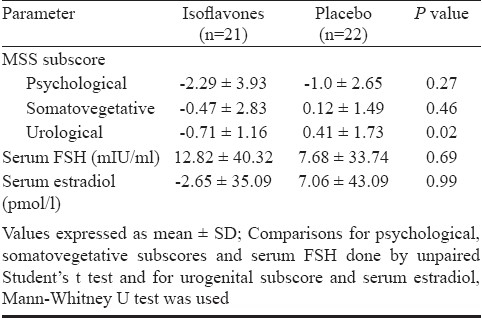
MSS for each patient at baseline, 3, 6, 9 and 12 wk was recorded and change in the score with time between two groups compared (Table IV). There was no significant change in the score with time in isoflavones group compared to placebo. When the mean change at 12 wk in various subscores was compared separately between two groups, a significant improvement in urogenital symptoms was seen with isoflavones as compared to placebo (Table V). The mean change in serum FSH and estradiol levels at 12 wk from baseline in two groups was not statistically significant from each other (Table V).
Table IV.
Comparison of change in Menopause Symptom Score (MSS) with time between isoflavone and placebo groups
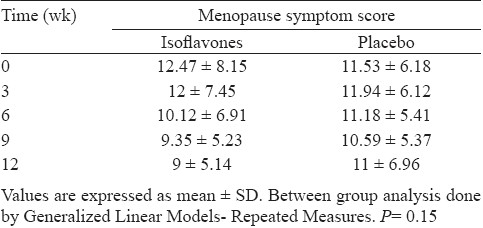
Table V.
Comparison for mean change in various subscores of Menopause Symptom Score (MSS), serum FSH and estradiol at 12 wk from baseline between isoflavone and placebo groups
One of the 21 women enrolled (2%) in the isoflavone group discontinued treatment after 4 wk because of disturbed sleep, anxiety and restlessness. In the placebo group, two of the 22 patients (5%) experienced adverse effects in the form of generalized bodyaches and increased fatiguability and they discontinued treatment at 2 wk and 2 days respectively. The proportion of patients experiencing adverse events was similar between two groups.
Discussion
In the present study, a significant change in free T3 levels was observed in isoflavone group. Isoflavone group also demonstrated an improvement in urogenital symptoms compared to placebo.
Increased prevalence of subclinical hypothyroidism has been seen in women due to increase in TSH levels with advancing age15. In this study, at the time of screening, about 18 per cent of women were suffering from thyroid disease. Different studies in rodents have also documented the effect of soy isoflavones on thyroid function7,16.
A number of potential mechanisms by which isoflavones interact with thyroid gland have been hypothesized. These include interference with enterohepatic circulation of thyroxine17, competition with thyroid hormones for thyroid sulfotransferase enzymes18, inhibition of thyroid peroxidase19 and interference with thyroid hormone synthesis by inhibition of iodide uptake and organification8. Besides, isoflavones lead to immune dysfunction by causing potent stimulation of T cell and B cell mediated immunity due to induced structural changes in thyroid peroxidase20.
A study in premenopausal women21 demonstrated a decline in free T3 levels with high iso diet. Another study conducted in post-menopausal women demonstrated a rise in T4 with 56 mg isoflavones/day and a rise in T3 and TSH with 90 mg isoflavones/day at 6 months compared with controls but the alterations were considered clinically non-significant10.
Several other studies revealed non significant changes in thyroid profile with isoflavones in menopausal women11,22,23. The precise reason for such reported variations is unclear although differences in doses, dosage forms, isoflavone composition or the duration of treatment might be an important factor.
In the present study, the effect of isoflavones on Menopause rating scale was also assessed, which is a validated scale to detect the severity of menopausal symptoms24,25. A significant reduction in urogenital subscore was observed with isoflavones. No observed benefit on hot flushes might partially be explained by a rise in FSH levels with isoflavones. Previous studies also report variable results26–28.
Isoflavones cause a reduction in estradiol levels by decreasing peripheral aromatization of androgens10,11 and inhibiting aromatase activity in preadipocytes29. An absence of such a finding in the present study might be due to inadequate sample size as the study was not powered to detect the same.
In the present study, women with surgical menopause were taken and not natural menopausal women as some amount of estrone is produced by ovary even after menopause and they are not estrogen null in true sense and hence the need to ameliorate the possible interaction between endogenous estrogen and thyroid function, as estrogen causes an elevation of thyroid binding globulin levels12. Also, we studied the effect of isoflavones on thyroid autoimmunity by quantitative estimation of anti-TPO antibodies, which was not done in previous clinical studies.
There were a few limitations in the present study. The study did not measure isoflavonoids in blood or in urine. Pharmacokinetic data for isoflavones would have provided an opportunity to ascertain the compliance and bioavailability and understand the kinetic – dynamic correlation for the agents. Secondly, it was a fixed dose study for a limited duration, whereas the postmenopausal women consume soy products over prolonged periods of time. Currently, there are no specific guidelines available to justify a particular dose or a particular composition of soy isoflavones to offer greater benefit as compared to the risk to the postmenopausal women. The use of active comparator in the form of synthetic HRT would have been more justified but in view of risks associated with HRT as highlighted in Women's Health Initiative report, 20021 and consequent declining trend towards HRT prescription, we preferred to use placebo control. The dietary intake of soy in the participants prior to enrolment in the study was not estimated which could have a possible influence on the results. Also, iodine status was not evaluated in study subjects as there remains a theoretical concern based on in vitro and animal data that soy may increase the risk of clinical hypothyroidism particularly in individuals whose iodine intake is marginal30.
In conclusion, it might be inferred from the present study that at 75 mg per day, isoflavones are relatively innocuous in terms of thyroid functional status. However, one needs to exercise caution in advocating these agents to women who might be iodine deficient. Also, isoflavones can be beneficial for relief of menopausal symptoms with variable effect on different components of this symptom complex.
Acknowledgments
Authors thank Dr Reddy's Laboratories Ltd, Hyderabad, India for providing the study drugs.
References
- 1.Lawton B, Rose S, McLeod D, Dowell A. Changes in use of hormone replacement therapy after the report from the Women's Health Initiative: cross sectional survey of users. BMJ. 2003;327:845–6. doi: 10.1136/bmj.327.7419.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grady D, Herrington D, Bittner V, Blumenthal R, Davidson M, Hlatky M, et al. HERS Research Group. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/ progestin Replacement Study follow-up (HERS II) JAMA. 2002;288:49–57. doi: 10.1001/jama.288.1.49. [DOI] [PubMed] [Google Scholar]
- 3.Hulley S, Furberg C, Barrett-Connor E, Cauley J, Grady D, Haskell W, et al. HERS Research Group. Noncardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II) JAMA. 2002;288:58–66. doi: 10.1001/jama.288.1.58. [DOI] [PubMed] [Google Scholar]
- 4.Murkies A, Wilcox G, Davis SR. Clinical review 92: Phytoestrogens. J Clin Endocrinol Metab. 1998;83:297–303. doi: 10.1210/jcem.83.2.4577. [DOI] [PubMed] [Google Scholar]
- 5.McCarrison R. The goitrogenic action of soybean and ground-nut. Indian J Med Res. 1933;21:179–81. [Google Scholar]
- 6.Balmir F, Staack R, Jeffrey E, Jimenez MD, Wang L, Potter SM. An extract of soy flour influences serum cholesterol and thyroid hormones in rats and hamsters. J Nutr. 1996;126:3046–53. doi: 10.1093/jn/126.12.3046. [DOI] [PubMed] [Google Scholar]
- 7.Madej A, Persson E, Lundh T, Ridderstrale Y. Thyroid gland function in ovariectomized ewes exposed to phytoestrogens. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;777:281–7. doi: 10.1016/s1570-0232(02)00082-x. [DOI] [PubMed] [Google Scholar]
- 8.Schmutzler C, Gotthardt I, Hofmann PJ, Radovic B, Kovacs G, StemmLer L, et al. Endocrine disruptors and the thyroid gland - a combined in vitro and in vivo analysis of potential new biomarkers. Environ Health Perspect. 2007;115(Suppl 1):77–83. doi: 10.1289/ehp.9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hydovitz JD. Occurence of goiter in an infant soy diet. N Engl J Med. 1960;262:351–3. doi: 10.1056/NEJM196002182620707. [DOI] [PubMed] [Google Scholar]
- 10.Persky VW, Turyk ME, Wang L, Freels S, Chatterton R, Barnes S, et al. Effect of soy protein on endogenous hormones in postmenopausal women. Am J Clin Nutr. 2002;75:145–53. doi: 10.1093/ajcn/75.1.145. [DOI] [PubMed] [Google Scholar]
- 11.Duncan AM, Underhill KE, Xu X, Lavalleur J, Phipps WR, Kurzer MS. Modest hormonal effects of soy isoflavones in postmenopausal women. J Clin Endocrinol Metab. 1999;84:3479–84. doi: 10.1210/jcem.84.10.6067. [DOI] [PubMed] [Google Scholar]
- 12.Ain KB, Mori Y, Refetoff S. Reduced clearance rate of thyroxine binding globulin (TBG) with increased sialylation: a mechanism for estrogen induced elevation of serum TBG concentration. J Clin Endocrinol Metab. 1987;65:689–96. doi: 10.1210/jcem-65-4-689. [DOI] [PubMed] [Google Scholar]
- 13.Doerge DR, Sheehan DM. Goitrogenic and estrogenic activity of soy isoflavones. Environ Health Perspect. 2002;110(Suppl 3):349–53. doi: 10.1289/ehp.02110s3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heinemann K, Ruebig A, Potthoff P, Schneider HP, Strelow F, Heinemann LA, et al. The Menopause Rating Scale (MRS) scale: a methodological review. Health Qual Life Outcomes. 2004;2:45. doi: 10.1186/1477-7525-2-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sowers MF, Luborsky J, Perdue C, Araujo KL, Goldman MB, Harlow SD. SWAN. Thyroid stimulating hormone (TSH) concentrations and menopausal status in women at the mid-life: SWAN. Clin Endocrinol (oxf) 2003;58:340–7. doi: 10.1046/j.1365-2265.2003.01718.x. [DOI] [PubMed] [Google Scholar]
- 16.Chang HC, Doerge DR. Dietary genistein inactivates rat thyroid peroxidase in vivo without an apparent hypothyroid effect. Toxicol Appl Pharmacol. 2000;168:244–52. doi: 10.1006/taap.2000.9019. [DOI] [PubMed] [Google Scholar]
- 17.Van Middlesworth L. Re-evaluation of certain aspects of iodine metabolism. Recent Prog Horm Res. 1960;16:405–38. [PubMed] [Google Scholar]
- 18.Ebmeier CC, Anderson RJ. Human thyroid phenol sulfotransferase enzymes 1A1 and 1A3: activities in normal and diseased thyroid glands and inhibition by thyroid hormones and phytoestrogens. J Clin Endocrinol Metab. 2004;89:5597–605. doi: 10.1210/jc.2003-031939. [DOI] [PubMed] [Google Scholar]
- 19.Tuohy PG. Soy infant formula and phytoestrogens. J Paediatr Child Health. 2003;39:401–5. doi: 10.1046/j.1440-1754.2003.00178.x. [DOI] [PubMed] [Google Scholar]
- 20.Chen A, Rogan WJ. Isoflavones in soy infant formula: a review of evidence for endocrine and other activity in infants. Annu Rev Nutr. 2004;24:33–54. doi: 10.1146/annurev.nutr.24.101603.064950. [DOI] [PubMed] [Google Scholar]
- 21.Duncan AM, Merz BE, Xu X, Nagel TC, Phipps WR, Kurzer MS. Soy isoflavones exert modest hormonal effects in premenopausal women. J Clin Endocrinol Metab. 1999;84:192–7. doi: 10.1210/jcem.84.1.5387. [DOI] [PubMed] [Google Scholar]
- 22.Borchers TR, Chew B, Park JS, McGuire M, Fournier L, Beerman K. Effects of dietary and supplemental forms of isoflavones on thyroid function in healthy postmenopausal women. Top Clin Nutr. 2008;23:13–22. [Google Scholar]
- 23.Teas J, Braverman LE, Kurzer MS, Pino S, Hurley TG, Hebert JR. Seaweed and soy: companion foods in Asian cuisine and their effects on thyroid function in American women. J Med Food. 2007;10:90–100. doi: 10.1089/jmf.2005.056. [DOI] [PubMed] [Google Scholar]
- 24.Chedraui P, Aguirre W, Hidalgo L, Fayad L. Assessing menopausal symptoms among healthy middle aged women with the Menopause Rating Scale. Maturitas. 2007;57:271–8. doi: 10.1016/j.maturitas.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 25.Waidyasekera H, Wijewardena K, Lindmark G, Naessen T. Menopausal symptoms and quality of life during the menopausal transition in Sri Lankan women. Menopause. 2009;16:164–70. doi: 10.1097/gme.0b013e31817a8abd. [DOI] [PubMed] [Google Scholar]
- 26.Lethaby AE, Brown J, Marjoribanks J, Kronenberg F, Roberts H, Eden J. Phytoestrogens for vasomotor menopausal symptoms. Cochrane Database Syst Rev. 2007;4:CD001395. doi: 10.1002/14651858.CD001395.pub3. [DOI] [PubMed] [Google Scholar]
- 27.Nagata C, Takatsuka N, Kawakami N, Shimizu H. Soy product intake and hot flashes in Japanese women: results from a community-based prospective study. Am J Epidemiol. 2001;153:790–3. doi: 10.1093/aje/153.8.790. [DOI] [PubMed] [Google Scholar]
- 28.Upmalis DH, Lobo R, Bradley L, Warren M, Cone FL, Lamia CA. Vasomotor symptom relief by soy isoflavone extract tablets in postmenopausal women: a multicenter, double-blind, randomized, placebo-controlled study. Menopause. 2000;7:236–42. doi: 10.1097/00042192-200007040-00005. [DOI] [PubMed] [Google Scholar]
- 29.Wang C, Mäkelä T, Hase T, Adlercreutz H, Kurzer MS. Lignans and flavonoids inhibit aromatase enzyme in human preadipocytes. J Steroid Biochem Mol Biol. 1994;50:205–12. doi: 10.1016/0960-0760(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 30.Messina M, Redmond G. Effects of soy protein and soybean isoflavones on thyroid function in healthy adults and hypothyroid patients: a review of the relevant literature. Thyroid. 2006;16:249–58. doi: 10.1089/thy.2006.16.249. [DOI] [PubMed] [Google Scholar]


