Abstract
Background & objectives:
Rheumatic fever (RF)/rheumatic heart disease (RHD) caused by Group A streptococcus (GAS) are more prevalent in north India as compared to the western world, where invasive diseases are common. This could be due to variation in the virulence of GAS in different geographic locations. Hence, we studied the virulence potential of GAS isolated from the throat of children from north India.
Methods:
Fifty GAS isolated consecutively, from children with mild pharyngitis (20), severe pharyngitis (24) and asymptomatic pharyngeal carriers (6), were characterized by emm typing and opacity factor (OF). Adherence and internalization of GAS in HEp-2 cells and opsonophagocytosis in convalescent serum samples were studied.
Results:
Twenty emm types, six sequence types, and one non-typeable GAS were circulating in the community. emm type 74, 11, 68, StI129 and NS292 were most prevalent. Twenty seven (54%) GAS isolates were OF negative. Sixty five per cent of the most prevalent emm types were OF negative indicating their rheumatogenic potential. Adhesion of GAS ranged from 0.1 to 100 per cent. Forty eight per cent of GAS were highly adherent. Invasion of GAS in HEp-2 cells ranged between 0 to 30 per cent. Only 20 per cent isolates exhibited highest invasion. GAS were opsonophagocytosed with highly divergent efficiency ranging from 0 to 91.7 per cent. Nineteen GAS were not opsonophagocytosed and 15 multiplied during the assay. Isolates of the same emm type also varied in their virulence potential.
Interpretation & conclusions:
GAS isolates from the throat of children from north India belonged to several emm types, majority were OF negative, excellent adherents but poor invaders. This explains why throat infections in these children tend to lead to ARF/RHD rather than invasive diseases. A few isolates exhibiting high invasion efficiency indicate that GAS throat cultures can also lead to invasive diseases.
Keywords: Adherence, Group A streptococci, invasion, pharyngitis, virulence
Group A streptococci (GAS) show an incredible history of changing disease pattern1. Humans are the natural host and sole reservoirs. These are spread through droplets and infect upper respiratory tract, i.e., affecting the mucous membrane, tonsils, and also skin and deeper tissues. GAS cause about 500,000 deaths every year2. Streptococcus pyogenes possesses considerable virulence factors (surface associated, secretary factors or toxins), for causing infection3,4.
Adherence of GAS to host pharyngeal epithelium is considered as the basic step in colonization4. Though designated as an extracellular pathogen in literature, its ability to invade non-phagocytic cells and persistence in infected humans has become a matter of concern5,6. Internalization may lead to carriage and persistence of streptococci and/or to invasion of deeper tissues, depending on virulence of the invading bacterium and the site of infection. GAS also possess antiphagocytic properties. Generally, protection against GAS infection has been correlated with presence of type specific opsonic antibodies against M protein7. GAS seems to sense its microenvironment and deploys only those factors that are advantageous in a particular niche3.
GAS are quite heterogeneous in their geographical distribution8,9. The epidemiological picture of streptococcal infections in India is quite different than the western world. Acute rheumatic fever (ARF)/rheumatic heart disease (RHD) continue to be a major public health problem as compared to the invasive diseases which are rarely reported in India10. GAS pharyngitis is common in the north India8,11. The GAS may vary in their virulence in different geographic locations which could account for the variable epidemiological pattern of streptococcal diseases in different areas. We, therefore, studied the virulence potential of GAS isolated from the throats of children in a locality from north India.
Material & Methods
One hundred six GAS specimen were available at Postgraduate Institute of Medical Education and Research (PGIMER) Chandigarh. Of these, 36 GAS had been isolated from 640 consecutive throat swabs collected from Government Medical College Hospital (GMCH), Chandigarh, and 70 were isolated at PGIMER, Chandigarh from 3038 throat swabs collected from children (5-15 yr old) studying in class I to X in six randomly selected government schools of Raipur Rani Block in Haryana. Of the 106 GAS isolates, first 50 were used for the present study (36 from 640 throat swabs collected at GMCH & 14 from 1199 swabs from School Survey). Samples were collected after obtaining informed consent from teachers/parents by a physician from both tonsils (tonsillar fossae) and posterior wall of pharynx from patients who had symptoms of sore throat as well those who did not have sore throat (asymptomatic). Symptoms and signs of sore throat were recorded on a proforma. Clinical scoring system validated by Nandi et al12 was followed for categorization of clinical isolates of GAS. The isolation rate of GAS from throat was 2.7 per cent (4%, 44/1087 among symptomatic and 0.8%, 6/752 in asymptomatic children). The study was approved by the Ethics Committee of PGIMER, Chandigarh, and it was carried out from November 2000 to July 2003.
Standard microbiological procedures were employed to identify β-haemolysis of streptococci (BHS) on sheep blood agar plates13. Bacitracin sensitivity test was performed on BHS14 which were further confirmed as Group A streptococci by latex agglutination test using a Streptex Murex kit (Remel Biotech Ltd, UK). Initial 50 GAS isolates in a defined period and one M1 GAS strain were included in the virulence study.
Opacity factor was determined by taking 10 μl of overnight culture supernatant, added to the wells of microplate having 100 μl of inactivated horse serum15. The results were read at 450 nm with an ELISA microplate reader (Tecan Austria, GmbH). To determine emm type, genomic DNA was isolated and emm typing was done using PCR and sequencing9. The emm gene sequence was subjected to homology search against CDC reference strains by depositing the sequences to CDC website (http://www.cdc.gov/ncidod/biotech/strep/strepblast.htm).
Adherence assay: HEp-2 cell line (ATCC No. CCL-23) procured from National Center for Cell Science (NCCS), Pune, India, was grown to confluency in 25cm2 plastic tissue culture flasks (Cell Star, Greiner Bio-one, Germany) in Dulbecco’s Minimal Essential Medium (DMEM, Gibco, BRL, USA) containing 10 per cent heat inactivated foetal calf serum (FCS), penicillin (100 IU/ml) and streptomycin (100 μg/ml) at 37°C and 5 per cent CO2 atmosphere. Cells were trypsinized, checked for viability and then seeded in wells with DMEM without antibiotics (~106cells/ml) for adherence and internalization assays.
A previously described flow cytometric method was followed16. Briefly, 100 μl aliquots of fluorescein isothiocyanate (FITC, Sigma c0 hemicals Co, USA) labelled streptococcal suspension (108bacteria) was added in each well of multi-well plate containing confluent monolayers of cell line (1:100). After 30 min at 37°C under 5 per cent CO2 atmosphere, the wells were washed thrice with 0.15M NaCl to remove non-adherent bacteria. The fluorescence associated with adherent streptococci was measured in a flowcytometer (Becton Dickinson, FACS Calibur, USA) and recorded as mean fluorescence intensity (green channel) for each isolate. The adherent streptococci were calculated from the absolute mean fluorescence value of the total number of streptococci obtained at the end in each assay from the plots constructed with serial dilutions of fluorescent streptococci.
Actually the FITC labelling did not alter streptococcal adherence properties; however, it was observed that all bacteria did not take FITC equally. Hence, four statistical curves were generated for different bacteria by plotting their number vs fluorescence. Accordingly from the slope, the numbers of test bacteria were determined corresponding to the observed test fluorescence intensity. Finally, percentage adherence was calculated from the initial total inoculum used. Isolates were grouped into ones with adherence from 0 - 30, >30 - 60 and >60 - 100 per cent. The threshold criteria used by Sela et al17 originally proposed by Jadoun et al18, were followed to categorize adherence levels.
Internalization assay: GAS isolates were analyzed for invasiveness based on the inability of penicillin and gentamicin to enter epithelial cells, thereby allowing quantification of internalized bacteria. The original method described by Isberg and Falkow19 and modified by Rubens et al20 was followed to determine streptococcal invasion to HEp-2 cells. Confluent monolayers of HEp-2 cells in the 12 well plates were infected with 5×105 cfu of streptococci for 2 h, at 37°C in 5 per cent CO2 and 100 per cent relative humidity. Cells were washed thrice with phosphate buffered saline (PBS). DMEM (1 ml) containing gentamicin (100 μg/ml) and penicillin (5 μg/ml) was added to kill unbound bacteria. Streptococci were released from disrupted monolayers by addition of 400 μl of 0.025 per cent Triton X-100. Lysate was washed with PBS. Bacteria added at zero minutes and the viable intracellular streptococci were determined by plating appropriate serial dilutions of cell lysate onto blood agar plates and the cfu was determined. The data were expressed as mean number of cfu recovered per ml for three independent determinations in a single assay and, then finally as the per cent invasion. The isolates were categorized into four groups with internalization percentages between 0 - <0.01, 0.01 - <0.1, 0.1 - <1 and 1 - 100. Molinari and Chhatwal criteria21 was followed to compare internalization.
Opsonophagocytosis assay: Convalescent serum samples from pharyngitis cases were compared for their ability to opsonize GAS7. The overnight bacterial cultures were serially diluted in saline to have 30-300 cfu. Three sets of conditions were taken, i.e., 50 μl bacterial dilution was mixed with 50 μl heat inactivated normal serum (NS) and/or convalescent serum (CS) and 400 μl of non opsonic heparinized donor blood, as a source of neutrophils and complement. Whole blood from normal donor lacking significant opsonic antibodies against M1 was screened pre-assay, to support GAS growth at least 32 times the inoculum level in 3 h incubation at 37°C and used within 5-30 min in the assay. Normal serum was obtained from 23 children below 3 yr age (antitreptolyin O ASO <200, agglutination absent) from the same locality and pooled. Pooled convalescent serum was also taken from 11 (5-10 yr old) children from the same locality having history of pharyngitis about 2-3 wk ago and had not received antibiotics within 2 wk (ASO titre >800). The mixture was incubated end over end rotation at 37°C for 3 h; 50 μl from each tube was pour plated in duplicate, on sheep blood agar plates and incubated overnight. Colonies were counted and opsonic activity (% killing) and per cent activity inhibition was calculated. The strains were categorized into three groups- non-opsonized, 1-50 per cent opsonized and 51-100 per cent opsonophagocytosed.
Statistical analysis: Chi square test was applied to statistically test adherence, internalization and opsonophagocytosis in different clinical categories. P<0.05 was considered statistically significant using EPI-6 (Epi Info Version 6) statcalc software (Center for Disease Control and Prevention, Atlanta, USA & World Health Organization, Geneva, Switzerland).
Results
Based on the clinical categorization, 50 GAS isolates were grouped into severe pharyngitis (24), mild pharyngitis (20), and asymptomatic (6). Twenty-seven isolates (54%) were OF negative. The proportion of OF negative isolates was almost equal among the three clinical categories. The PCR product of emm gene for OF negative and positive isolates was between 1.0-1.6 kb and 0.9-1.1 kb in size respectively.
Significant diversity of emm types was observed (Fig. 1). A total of 27 emm types; 20 known emm types, 6 sequence types and a novel M non typeable isolate were obtained. emm 74 (12%), 11 and StI129 (8%), and emm 68 and NS292 (3 times) constituted 40 per cent of our isolates. Of these prevalent types, all the four isolates of emm11; five out of the six emm74 isolates and three out of the four StI129 isolates were OF negative, hence majority of the isolates (65%, 13/20) belonging to the above most common emm types were OF negative. Amongst the less prevalent emm types some (emm 3, 28, 77, 49 and 74) were OF negative, and a few (emm 2.1, 60, 68, 75, 81, 109) were OF positive.
Fig. 1.
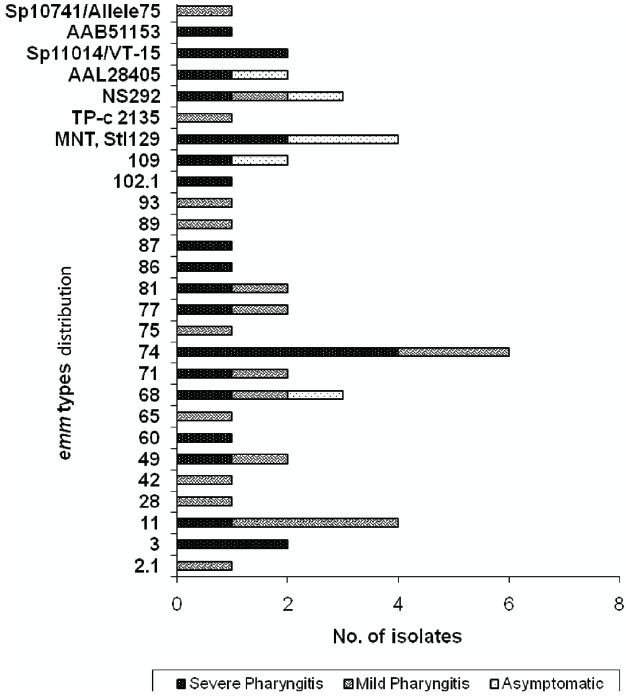
Distribution of emm types of GAS isolates according to their clinical status (n=50).
Fig. 2 shows the bacterial adherence, internalization and opsonophagocytosis among various clinical isolates of GAS. The bacterial adherence ranged from 0.1 to 100 per cent of the original inoculum . Internalization frequency ranged between 0 to 30 per cent. Considerable variations were observed in opsonophagocytosis which ranged from negative to 92 per cent. Forty eight per cent (24/50) of the isolates were highly adherent. Only 20 per cent (10/50) isolates exhibited high invasion . Nineteen (38%) GAS isolates were not opsonophagocytosed, of which 15 multiplied during the assay.
Fig. 2.
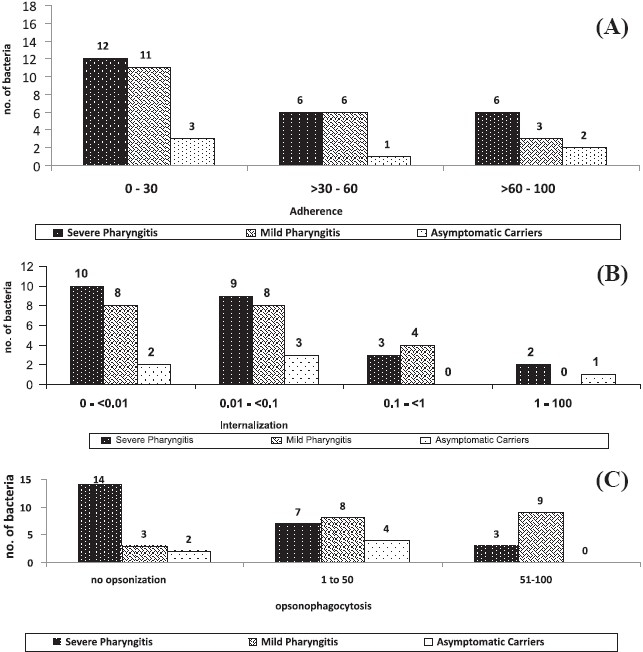
Virulence potential of S. pyogenes isolated from children. (A) Adherence of GAS to HEp-2 cell line. (B) Internalization of S. pyogenesin HEp-2 cell line. (C) Opsonophagocytosis of S. pyogenes.
Highest adherence was seen in three isolates cultured from severe pharyngitis patients and one each from mild pharyngitis and asymptomatic respectively. Highest internalization was seen for GAS from severe pharyngitis patient followed by one from an asymptomatic case. A few GAS isolates invaded, persisted and survived intracellularly within human epithelial cells, even after 24 h of infection (data not shown). None of the asymptomatic isolates showed more than 50 per cent opsonization. Majority of the isolates that belonged to severe pharyngitis cases multiplied during the assay. Streptococcal isolates of the same emm type -74, 71, 11, NS292 and StI129, but different clinical status also varied considerably in their ability to adhere, invade HEp-2 cell line as well being opsonophagocytosed by polymorphonuclear cells. The virulence potential of GAS isolates cultured from throats of pharyngitis cases was not found to be statistically significant compared to the asymptomatic carriers.
GAS isolates with high adherence but low invasion or vice versa were identified. Hence, isolates could be categorized into different phenotypic groups, of the isolates showing high adherence (n=24), high internalization was seen only in 14 isolates; and in those with low adherence (n=26), 16 showed high internalization. Further, these isolates could be classified according to the level of opsonophagocytosis. Importantly, these three phenotypic characteristics were found to exist independent of each other.
Discussion
Considerable heterogeneity has been observed amongst the isolates in the same community. A number of emm types found during the present study have also been isolated in India in the past few years8,9,11. Half of our isolates were OF negative. It is reported that generally rheumatogenic strains are OF negative22.
Substantial adherence of streptococci with epithelial cells has been observed. According to selected criteria18,19 half of the isolates showed high adherence and 68 per cent showed adherence more than the reference M1 strain. Multiple adhesions, even the anchorless adhesions and different mechanisms involved in adherence have been documented23. It seems that bacterial adherence occurs in a selective manner in different niches24, and is isolate specific.
Recurrence of GAS pharyngotonsillitis after assumingly adequate antibiotic treatment has puzzled scientists for many years25. In our study, 20 per cent isolates were highly invasive and 62 per cent showed internalization more than M1. GAS can be invasive (normally sterile sites/deep wounds) or non invasive (pharyngeal or uncomplicated skin infection) according to isolation site26. Some of our GAS isolates, though isolated from non-invasive site (pharynx), invaded, persisted and survived intracellularly within human epithelial cells, thus confirming earlier findings5,27. Throat and/or skin isolates exhibiting highest invasion efficiency than blood isolates have been reported28, however, GAS from invasive and non invasive diseases adhered to and penetrated equally well into HEp-2 cells29. Many researchers did not observe multiplication of intracellular streptococci, and showed infected cell lines eventually get cleared of bacteria. All the streptococci studied, survived in the pharyngeal cells beyond 24 h (data not shown). It would have been more appropriate if GAS isolates from deeper tissues or blood were compared along with those from the pharynx, but due to limited reporting of invasive diseases from north India, such isolates could not be included in this study.
Different theories have been proposed for opsonophagocytosis of streptococci and how they evade the immune system. Opsonization is suggested to be strain specific30. In the present study, individual or pooled normal and convalescent sera were used irrespective of particular virulence factor or M type, simply to quantify the immune response against bacteria in the community. Though the pooled convalescent serum obtained from a few patients might not contain antibodies against the specific antigenic determinant of the tested strains, considerable variations were observed. In some isolates no opsonization occurred, just as in a murine model, GAS avoided killing by polymorphonuclear cells, survived, divided, underwent phenotypic switching and became more virulent. How GAS breach vacuolar membrane has still not been elucidated31.
Experimental data regarding the contribution of M protein to GAS pathogenesis are conflicting. Genetically distinct population exists within an M-type32. Individual isolates of GAS varied considerably in their virulence potential. Hence the M types and virulence efficiency could not be correlated as even isolates from same types like emm 74, 11, 68, etc., did not show a definite pattern, confirming its isolate dependence.
Ideally isolates with high invasiveness should exhibit high attachment. However, the isolates showing high attachment but poor invasion or vice versa were observed. Reduced in vitro invasiveness of strains that adhered well by itself is unable to promote internalization and hence not sufficient to cause infection. Certain less adherant strains invaded well, suggesting they might express different invasins that mediate rapid internalization. Also it seems internalization may lead to invasive diseases or just containment of the organism, proving the existence of asymptomatic strains. Surprisingly, some isolates survived in convalescent serum, raising the possibility that specific antibodies were either absent, not sufficient to elicit a response or surface proteins of GAS like immunoglobulin binding proteins and other factors than M type bound antibodies non-type specifically and rendered them ineffective for opsonophagocytosis. Different combinations of pathogenic traits were observed, but the reasons for these differences are incomprehensible. It seems streptococci sense multiple signals in the environment and different regulatory mechanisms controlling expression of virulent determinants, eukaryotic cell attachment and internalization are activated.
In summary, high diversity in emm types was encountered among GAS isolated from the throat of children from an area in north India. Nearly half of the isolates were opacity factor negative indicating their rheumatogenic potential. The virulence potential varied substantially showing excellent adherence but poor invasion properties. This explains why throat infections tend to lead to ARF/RHD rather than invasive diseases in this population. However, a few isolates exhibiting high invasion efficiency indicate that GAS throat cultures can also lead to invasive diseases.
Acknowledgments
Authors acknowledge the Indian Council of Medical Research (ICMR), New Delhi for financial support.
References
- 1.Cunningham MW. Pathogenesis of group A streptococcal infections. Clin Microbiol Rev. 2000;13:470–511. doi: 10.1128/cmr.13.3.470-511.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carapetis JR, Steer AC, Mulholand EK, Weber M. The global burben of group A streptococcal diseases. Lancet Infect Dis. 2005;5:685–94. doi: 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- 3.Heath A, Di Diita VJ, Barg NL, Engleberg NC. A two- component regulatory system, CsrR-CsrS, represses expression of three Streptococcus pyogenes virulence factors, hyaluronic acid capsule, streptolysin s0 and pyrogenic exotoxin B. Infect Immun. 1999;67:5298–305. doi: 10.1128/iai.67.10.5298-5305.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hynes W. Virulence factors of the group A streptococci and genes that regulate their expression. Front Biosci. 2004;9:3399–433. doi: 10.2741/1491. [DOI] [PubMed] [Google Scholar]
- 5.LaPenta D, Rubens C, Chi E, Cleary PP. Group A streptococci efficiently invade human respiratory epithelial cells. Proc Natl Acad Sci USA. 1994;91:12115–9. doi: 10.1073/pnas.91.25.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osterlund A, Popa R, Nikkilä T, Scheynius A, Engstrand L. Intracellular reservoir of Streptococcus pyogenes in vivo: a possible explanation for recurrent pharyngotonsillitis. Laryngoscope. 1997;107:640–7. doi: 10.1097/00005537-199705000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Lancefield RC. Differentiation of group A streptococci with a common R antigen into three serological types, with a special reference to the bactericidal test. J Exp Med. 1957;106:525–44. doi: 10.1084/jem.106.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma M, Shah B, Dhaliwal RS, Kumar R, Brahmadathan KN, Vohra H, et al. Heterogeneity of community based pediatric GAS isolates from India: Challenges to the multivalent vaccine approach. In: Sriprakash KS, editor. Streptococci - New insights into an old enemy. Netherlands: Elsevier International Congress Series, 1289; 2006. pp. 49–53. [Google Scholar]
- 9.Dey N, McMillan DJ, Yarwood PJ, Joshi RM, Kumar R, Good MF, et al. High diversity of group A streptococcal emm types in an Indian community: the need to tailor multivalent vaccines. Clin Infect Dis. 2005;40:46–51. doi: 10.1086/426443. [DOI] [PubMed] [Google Scholar]
- 10.Krause RM. A half-century of streptococcal research: then & now. Indian J Med Res. 2002;115:215–41. [PubMed] [Google Scholar]
- 11.Kumar R, Vohra H, Chakraborty A, Sharma YP, Bandhopadhya S, Dhanda V, et al. Epidemiology of group A streptococcal pharyngitis & impetigo: a cross sectional and follow-up study in a rural community of northern India. Indian J Med Res. 2009;130:765–71. [PubMed] [Google Scholar]
- 12.Nandi S, Kumar R, Ray P, Vohra H, Ganguly NK. Clinical score card for diagnosis of group A streptococcal sore throat. Indian J Pediatr. 2002;69:471–5. doi: 10.1007/BF02722644. [DOI] [PubMed] [Google Scholar]
- 13.Johnson DR, Kaplan EL. Geneva: World Health Organization; 1996. Laboratory diagnosis of group A streptococcal infections: a laboratory manual; pp. 16–9. [Google Scholar]
- 14.Maxted WR. The use of bacitracin for identifying group A haemolytic streptococci. J Clin Pathol. 1953;6:224–6. doi: 10.1136/jcp.6.3.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson DR, Kaplan EL. Microtechnique for serum opacity factor characterization of group A streptococci adaptable to the use of human sera. J Clin Microbiol. 1988;26:2025–30. doi: 10.1128/jcm.26.10.2025-2030.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sethman CR, Doyle RJ, Cowan MM. Flow cytometric evaluation of adhesion of Streptococcus pyogenes to epithelial cells. J Microbiol Methods. 2002;51:35–42. doi: 10.1016/s0167-7012(02)00054-4. [DOI] [PubMed] [Google Scholar]
- 17.Sela S, Neeman R, Keller N, Barzilai A. Relationship between asymptomatic carriage of Streptococcus pyogenes and the ability of the strains to adhere to and be internalized by cultured epithelial cells. J Med Microbiol. 2000;49:499–502. doi: 10.1099/0022-1317-49-6-499. [DOI] [PubMed] [Google Scholar]
- 18.Jadoun J, Ozeri V, Burstein E, Skutelsky E, Hanski E, Sela S. Protein F1 is required for efficient entry of Streptococcus pyogenes into epithelial cells. J Infect Dis. 1998;178:147–58. doi: 10.1086/515589. [DOI] [PubMed] [Google Scholar]
- 19.Isberg RR, Falkow S. A single genetic locus encoded by Yersinia pseudotuberculosis permits invasion of cultured animal cells by Escherichia coli K-12. Nature (London) 1985;317:262–4. doi: 10.1038/317262a0. [DOI] [PubMed] [Google Scholar]
- 20.Rubens CE, Smith S, Hulse M, Chi EY, van Belle G. Respiratory epithelial cell invasion by group B streptococci. Infect Immun. 1992;60:5157–63. doi: 10.1128/iai.60.12.5157-5163.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molinari G, Chhatwal GS. Invasion and survival of Streptococcus pyogenes in eukaryotic cells correlates with the source of the clinical isolates. J Infect Dis. 1998;177:1600–7. doi: 10.1086/515310. [DOI] [PubMed] [Google Scholar]
- 22.Stollerman GH. Rheumatic fever. Lancet. 1997;349:935–42. doi: 10.1016/S0140-6736(96)06364-7. [DOI] [PubMed] [Google Scholar]
- 23.Kreikemeyer B, Oehmcke S, Nakata M, Hoffrogge R, Podbielski A. Streptococcus pyogenes fibronectin binding protein F2: expression profile, binding characteristics, and impact on eukaryotic cell interactions. J Biol Chem. 2004;279:15850–9. doi: 10.1074/jbc.M313613200. [DOI] [PubMed] [Google Scholar]
- 24.Hasty DL, Ofek I, Courtney HS, Doyle RJ. Multiple adhesins of streptococci. Infect Immun. 1992;60:2147–52. doi: 10.1128/iai.60.6.2147-2152.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaplan EL, Chhatwal GS, Rohde M. Reduced ability of penicillin to eradicate ingested group A streptococci from epithelial cells: clinical and pathogenetic implications. Clin Infect Dis. 2006;43:1398–406. doi: 10.1086/508773. [DOI] [PubMed] [Google Scholar]
- 26.Bronze MS, Dale JB. The reemergence of serious group A streptococcal infections and acute rheumatic fever. Am J Med Sci. 1996;311:41–54. doi: 10.1097/00000441-199601000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Bennet-Wood VR, Carapetis JR, Robins-Browne RM. Ability of clinical isolates of group A streptococci to adhere to and invade HEp-2 epithelial cells. J Med Microbiol. 1998;47:899–906. doi: 10.1099/00222615-47-10-899. [DOI] [PubMed] [Google Scholar]
- 28.Gladstone P, Jesudason MV, Sridharan G. Invasive properties of south Indian strains of Streptococcus pyogenes in a HEp-2 cell model. Clin Microbiol Infect. 2003;9:1031–4. doi: 10.1046/j.1469-0691.2003.00710.x. [DOI] [PubMed] [Google Scholar]
- 29.Hagman MM, Dale JB, Stevens DL. Comparison of adherence to and penetration of a human laryngeal epithelial cell line by group A streptococci of various M protein types. FEMS Immunol Med Microbiol. 1999;23:195–204. doi: 10.1111/j.1574-695X.1999.tb01239.x. [DOI] [PubMed] [Google Scholar]
- 30.de-Malmanche SA, Martin DR. Protective immunity to the group A streptococcus may be only strain specific. Med Microbiol Immunol. 1994;183:299–306. doi: 10.1007/BF00196680. [DOI] [PubMed] [Google Scholar]
- 31.Medina E, Rohde M, Chhatwal GS. Intracellular survival of Streptococcus pyogenes in polymorphonuclear cells results in increased bacterial virulence. Infect Immun. 2003;71:5376–80. doi: 10.1128/IAI.71.9.5376-5380.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Villaseñor-Sierra A, McShan WM, Salmi D, Kaplan KL, Johnson DR, Stevens DL. Variable susceptibility to opsonophagocytosis of group A streptococcus M-1 strains by human immune sera. J Infect Dis. 1999;180:1921–8. doi: 10.1086/315120. [DOI] [PubMed] [Google Scholar]


