Abstract
l-Glutamate elicits the umami taste sensation, now recognized as a fifth distinct taste quality. A characteristic feature of umami taste is its potentiation by 5′-ribonucleotides such as guanosine-5'-monophosphate and inosine 5′-monophosphate, which also elicit the umami taste on their own. Recent data suggest that multiple G protein–coupled receptors contribute to umami taste. This review will focus on events downstream of the umami taste receptors. Ligand binding leads to Gβγ activation of phospholipase C β2, which produces the second messengers inositol trisphosphate and diacylglycerol. Inositol trisphosphate binds to the type III inositol trisphosphate receptor, which causes the release of Ca2+ from intracellular stores and Ca2+-dependent activation of a monovalent-selective cation channel, TRPM5. TRPM5 is believed to depolarize taste cells, which leads to the release of ATP, which activates ionotropic purinergic receptors on gustatory afferent nerve fibers. This model is supported by knockout of the relevant signaling effectors as well as physiologic studies of isolated taste cells. Concomitant with the molecular studies, physiologic studies show that l-glutamate elicits increases in intracellular Ca2+ in isolated taste cells and that the source of the Ca2+ is release from intracellular stores. Both Gα gustducin and Gα transducin are involved in umami signaling, because the knockout of either subunit compromises responses to umami stimuli. Both α-gustducin and α-transducin activate phosphodiesterases to decrease intracellular cAMP. The target of cAMP in umami transduction is not known, but membrane-permeant analogs of cAMP antagonize electrophysiologic responses to umami stimuli in isolated taste cells, which suggests that cAMP may have a modulatory role in umami signaling.
INTRODUCTION
One hundred years ago, Kikunae Ikeda isolated l-glutamate from dried konbu and identified it as a unique taste, different from the tastes of bitter, sweet, salty, and sour. Ikeda called this taste “umami,” from the Japanese word umai, meaning delicious. A characteristic feature of umami taste is its potentiation by ribonucleotides such as inosine 5′-monophosphate (IMP) and guanosine-5'-monophosphate (GMP), which also elicit umami taste on their own. Despite Ikeda's seminal discovery, umami taste was not completely accepted as a unique taste quality until the recent molecular identification of specific G protein–coupled receptors for glutamate taste that exhibited nucleotide potentiation when expressed in heterologous cells. In this review, I will briefly describe the receptors that have been identified, although these will be covered in more detail in other chapters of this volume (1, 2). This review will focus instead on physiologic responses of taste cells to umami stimuli and describe the intracellular signaling events downstream of the umami taste receptors.
UMAMI TASTE RECEPTORS
Several receptors that bind glutamate and/or nucleotides have been identified in taste cells, including the heterodimer T1R1/T1R3 (3, 4), the taste-specific isoforms of metabotropic glutamate receptors mGluR4 (5) and mGluR1 (2, 6), and mGluR2 and mGluR3 (7), and several ionotropic glutamate receptors, including both NMDA and kainate receptors (8). One problem with identifying the potential role of these receptors in taste transduction is that glutamate also serves as a neurotransmitter, and for receptors to be considered taste receptors they must be expressed on the apical membrane of taste cells where they will encounter glutamate in the oral cavity. In this regard, both NMDA and kainate receptors have been identified on basolateral membranes of taste cells, where they likely respond to glutamate as a neurotransmitter (9). The definitive role of a particular receptor in umami transduction requires that the taste is modified when the receptor is genetically ablated. In this regard, the only receptor for which there is genetic data is the T1R1/T1R3 heterodimer. In one study, knockout of either T1R1 or T1R3 completely eliminated the responses to oral glutamate, which suggests that the heterodimer is the only umami receptor (10). However, in another study, knockout of T1R3 only eliminated nucleotide potentiation of glutamate taste responses, with little effect on responses to glutamate alone (11). Although the reason for these discrepancies is not known, the latter data strongly suggest the existence of multiple receptors for umami taste. The metabotropic glutamate receptors are the likely candidates, but knockout will be necessary to confirm a role in taste transduction.
DOWNSTREAM SIGNALING EFFECTORS
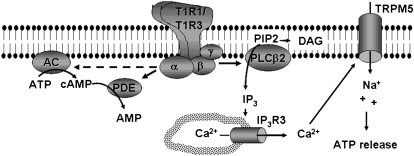
The T1R1/T1R3 heterodimer is coupled to a heteromeric G protein, where the Gβγ subunit appears to mediate the predominant leg of the signaling pathway. Ligand-binding activates Gβ3γ13, which results in activation of phospholipase C β2 (PLCβ2) (12, 13), which produces inositol trisphosphate (IP3) and diacylglycerol. IP3 activates the type III IP3 receptor (IP3R3) (14, 15),which results in the release of Ca2+ from intracellular stores and Ca2+-dependent activation of a monovalent-selective cation channel, TRPM5 (16–18). TRPM5 is expected to depolarize taste cells, which results in action potential generation and release of ATP, which activates ionotropic purinergic receptors on gustatory afferent nerve fibers (19–21) (Figure 1). Evidence of involvement of this pathway in umami taste transduction comes from several studies. First, all of these signaling effectors are co-localized with the T1R1/T1R3 heterodimer in the type II (receptor) taste cells (17). Second, knockout of PLCβ2 (17), IP3R3 (22), and TRPM5 (23) all reduce umami taste responses in a manner similar to that of the knockout of T1R3 (11). Third, pharmacologic inhibitors of PLCβ2 and Ca2+ ATPase, which maintain intracellular Ca2+ stores, virtually eliminate responses to glutamate and nucleotides applied selectively to the taste pore in Ca2+ imaging studies of a lingual slice preparation (24).
FIGURE 1.
Model illustrating the signaling effectors downstream of the umami receptor T1R1/T1R3. Receptor binding activates Gβ3γ13, which in turn activates phospholipase C β2 (PLCβ2), to produce the second messengers inositol trisphosphate (IP3) and diacylglycerol (DAG). IP3 binds type III IP3 receptor (IP3R3) to elicit release of Ca2+ from intracellular stores and subsequent Ca2+ activation of TRPM5, which results in taste cell depolarization and release of ATP onto gustatory afferent fibers. In fungiform and palate taste buds, Gα gustducin or Gα transducin activates phosphodiesterase (PDE) to decrease intracellular cAMP concentrations. In circumvallate and foliate taste buds, cAMP is also decreased by activation of Gα, but likely by inhibition of adenylyl cyclase (AC) (dashed line) rather than by activation of PDE. The target of the decreased cAMP is not known, but cAMP antagonizes responses to umami stimuli in physiologic studies, which suggests that it may modulate the sensitivity of the PLC signaling pathway.
The Gα subunit that mediates umami transduction varies according to taste field. In fungiform and palatal taste buds, T1R1/T1R3 is almost completely co-localized with α-gustducin, whereas T1R1/T1R3 in circumvallate and foliate taste buds is expressed with a different and as yet unidentified Gα (25, 26). Gα-gustducin is related to Gα-transducin, which is also expressed in taste buds. Both α-gustducin and α-transducin activate phosphodiesterases (PDEs), which results in decreases in intracellular cAMP concentrations (Figure 1). Knockout of either α-gustducin or α-transducin compromises umami taste, which suggests that both Gα-gustducin and Gα-transducin participate in umami transduction (27). Physiologic studies also support a role of cAMP in umami taste. Because the activation of PDEs suppresses cAMP concentrations, cAMP should antagonize responses to umami stimuli. This has been shown in whole-cell patch clamp studies of rat fungiform taste cells, where responses to glutamate, GMP, and the synergistic response to glutamate and GMP were suppressed by membrane-permeant cAMP (28). Furthermore, biochemical studies have shown that glutamate decreases cAMP concentrations in taste buds, and the response is potentiated by 5′-nucleotides (29, 30). These latter experiments were performed on rat circumvallate taste buds, which suggests that cAMP modulates umami signaling in posterior taste fields as well, likely mediated by a Gα subunit other than α gustducin. Gαi-2 is abundantly expressed in taste buds, so this Gα may couple to T1R1/T1R3 in circumvallate and foliate taste buds (31, 32). Alternatively, a different umami receptor, such as taste-mGluR4, may mediate the responses to umami stimuli in posterior taste buds.
The role of cAMP in umami signaling is unclear. Cyclic nucleotide-gated cation channels have been identified in mammalian taste buds (33), but physiologic studies have failed to show conductance changes in response to membrane permeant cAMP analogs in taste cells. It is more likely that cAMP modulates the efficacy of Ca2+ signaling. Both IP3R3 (34) and PLCβ2 (35) are modulated in other tissues by cAMP-dependent phosphorylation, and, in both cases, phosphorylation decreases the Ca2+ released from intracellular stores. Additional studies will be required to determine whether decreases in cAMP mediated by α-gustducin, α-transducin, or αi-2 modulate Ca2+ signaling in taste buds. (Other articles in this supplement to the Journal include references 1, 2, and 36–62.)
Acknowledgments
I thank Aurelie Vandenbeuch for comments on the manuscript.
The author's travel expenses associated with participation in the symposium and an honorarium were paid by the conference sponsor, the International Glutamate Technical Committee, a nongovernmental organization funded by industrial producers and users of glutamate in food. There were no conflicts of interest related to the material presented in this article.
REFERENCES
- 1.Chaudhari N, Pereira E, Roper SD. Taste receptors for umami: the case for multiple receptors. Am J Clin Nutr 2009;90(suppl):738S–42S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.San Gabriel A, Maekawa T, Uneyama H, Torii K. Metabotropic glutamate receptor type 1 in taste tissue. Am J Clin Nutr 2009;90(suppl):743S–6S [DOI] [PubMed] [Google Scholar]
- 3.Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E. Human receptors for sweet and umami taste. Proc Natl Acad Sci USA 2002;99:4692–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson G, Chandrashekar J, Hoon MA, et al. An amino-acid taste receptor. Nature 2002;416:199–202 [DOI] [PubMed] [Google Scholar]
- 5.Chaudhari N, Landin AM, Roper SD. A metabotropic glutamate receptor variant functions as a taste receptor. Nat Neurosci 2000;3:113–9 [DOI] [PubMed] [Google Scholar]
- 6.Toyono T, Seta Y, Kataoka S, Kawano S, Shigemoto R, Toyoshima K. Expression of metabotropic glutamate receptor group I in rat gustatory papillae. Cell Tissue Res 2003;313:29–35 [DOI] [PubMed] [Google Scholar]
- 7.Toyono T, Kataoka S, Seta Y, Shigemoto R, Toyoshima K. Expression of group II metabotropic glutamate receptors in rat gustatory papillae. Cell Tissue Res 2007;328:57–63 [DOI] [PubMed] [Google Scholar]
- 8.Brand JG. Receptor and transduction processes for umami taste. J Nutr 2000;130:942S–5S [DOI] [PubMed] [Google Scholar]
- 9.Caicedo A, Jafri MS, Roper SD. In situ Ca2+ imaging reveals neurotransmitter receptors for glutamate in taste receptor cells. J Neurosci 2000;20:7978–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao GQ, Zhang Y, Hoon MA, et al. The receptors for mammalian sweet and umami taste. Cell 2003;115:255–66 [DOI] [PubMed] [Google Scholar]
- 11.Damak S, Rong M, Yasumatsu K, et al. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science 2003;301:850–3 [DOI] [PubMed] [Google Scholar]
- 12.Rossler P, Kroner C, Freitag J, Noe J, Breer H. Identification of a phospholipase C beta subtype in rat taste cells. Eur J Cell Biol 1998;77:253–61 [DOI] [PubMed] [Google Scholar]
- 13.Huang L, Shanker YG, Dubauskaite J, et al. Ggamma13 colocalizes with gustducin in taste receptor cells and mediates IP3 responses to bitter denatonium. Nat Neurosci 1999;2:1055–62 [DOI] [PubMed] [Google Scholar]
- 14.Clapp TR, Stone LM, Margolskee RF, Kinnamon SC. Immunocytochemical evidence for co-expression of Type III IP3 receptor with signaling components of bitter taste transduction. BMC Neurosci 2001;2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyoshi MA, Abe K, Emori Y. IP(3) receptor type 3 and PLCbeta2 are co-expressed with taste receptors T1R and T2R in rat taste bud cells. Chem Senses 2001;26:259–65 [DOI] [PubMed] [Google Scholar]
- 16.Perez CA, Huang L, Rong M, et al. A transient receptor potential channel expressed in taste receptor cells. Nat Neurosci 2002;5:1169–76 [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Hoon MA, Chandrashekar J, et al. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell 2003;112:293–301 [DOI] [PubMed] [Google Scholar]
- 18.Zhang Z, Zhao Z, Margolskee RF, Liman ER. The transduction channel TRPM5 is gated by intracellular calcium in taste cells. J Neurosci 2007;27:5777–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finger TE, Danilova V, Barrows J, et al. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science 2005;310:1495–9 [DOI] [PubMed] [Google Scholar]
- 20.Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc Natl Acad Sci USA 2007;10:6436–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romanov RA, Rogachevskaja OA, Bystrova MF, Jiang P, Margolskee RF, Kolesnikov SS. Afferent neurotransmission mediated by hemichannels in mammalian taste cells. EMBO J 2007;26:657–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hisatsune C, Yasumatsu K, Takahashi-Iwanaga H, et al. Abnormal taste perception in mice lacking the type 3 inositol 1,4,5-trisphosphate receptor. J Biol Chem 2007;282:37225–31 [DOI] [PubMed] [Google Scholar]
- 23.Damak S, Rong M, Yasumatsu K, et al. Trpm5 null mice respond to bitter, sweet, and umami compounds. Chem Senses 2006;31:253–64 [DOI] [PubMed] [Google Scholar]
- 24.Maruyama Y, Pereira E, Margolskee RF, Chaudhari N, Roper SD. Umami responses in mouse taste cells indicate more than one receptor. J Neurosci 2006;26:2227–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim MR, Kusakabe Y, Miura H, Shindo Y, Ninomiya Y, Hino A. Regional expression patterns of taste receptors and gustducin in the mouse tongue. Biochem Biophys Res Commun 2003;312:500–6 [DOI] [PubMed] [Google Scholar]
- 26.Stone LM, Barrows J, Finger TE, Kinnamon SC. Expression of T1Rs and Gustducin in Palatal Taste Buds of Mice. Chem Senses 2007;32:255–62 [DOI] [PubMed] [Google Scholar]
- 27.He W, Yasumatsu K, Varadarajan V, et al. Umami taste responses are mediated by alpha-transducin and alpha-gustducin. J Neurosci 2004;24:7674–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin W, Ogura T, Kinnamon SC. Responses to di-sodium guanosine 5′-monophosphate and monosodium L-glutamate in taste receptor cells of rat fungiform papillae. J Neurophysiol 2003;89:1434–9 [DOI] [PubMed] [Google Scholar]
- 29.Abaffy T, Trubey KR, Chaudhari N. Adenylyl cyclase expression and modulation of cAMP in rat taste cells. Am J Physiol 2003;284:C1420–8 [DOI] [PubMed] [Google Scholar]
- 30.Trubey KR, Culpepper S, Maruyama Y, Kinnamon SC, Chaudhari N. Tastants evoke cAMP signal in taste buds that is independent of calcium signaling. Am J Physiol 2006;291:C237–44 [DOI] [PubMed] [Google Scholar]
- 31.Kusakabe Y, Yamaguchi E, Tanemura K, et al. Identification of two alpha-subunit species of GTP-binding proteins, Galpha15 and Galphaq, expressed in rat taste buds. Biochim Biophys Acta 1998;1403:265–72 [DOI] [PubMed] [Google Scholar]
- 32.Sainz E, Cavenagh MM, Lopez Jimenez ND, et al. The G-protein coupling properties of the human sweet and amino acid taste receptors. Dev Neurobiol 2007;67:948–59 [DOI] [PubMed] [Google Scholar]
- 33.Misaka T, Kusakabe Y, Emori Y, Gonoi T, Arai S, Abe K. Taste buds have a cyclic nucleotide-activated channel, CNGgust. J Biol Chem 1997;272:22623–9 [DOI] [PubMed] [Google Scholar]
- 34.Giovannucci DR, Groblewski GE, Sneyd J, Yule DI. Targeted phosphorylation of inositol 1,4,5-trisphosphate receptors selectively inhibits localized Ca2+ release and shapes oscillatory Ca2+ signals. J Biol Chem 2000;275:33704–11 [DOI] [PubMed] [Google Scholar]
- 35.Liu M, Simon MI. Regulation by cAMP-dependent protein kinease of a G-protein-mediated phospholipase C. Nature 1996;382:83–7 [DOI] [PubMed] [Google Scholar]
- 36.Fernstrom JD. Introduction to the symposium. Am J Clin Nutr 2009;90(suppl):705S–6S [DOI] [PubMed] [Google Scholar]
- 37.Krebs JR. The gourmet ape: evolution and human food preferences. Am J Clin Nutr 2009;90(suppl):707S–11S [DOI] [PubMed] [Google Scholar]
- 38.Curtis RI. Umami and the foods of classical antiquity. Am J Clin Nutr 2009;90(suppl):712S–8S [DOI] [PubMed] [Google Scholar]
- 39.Kurihara K. Glutamate: from discovery as a food flavor to role as a basic taste (umami). Am J Clin Nutr 2009;90(suppl):719S–22S [DOI] [PubMed] [Google Scholar]
- 40.Beauchamp GK. Sensory and receptor responses to umami: an overview of pioneering work. Am J Clin Nutr 2009;90(suppl):723S–7S [DOI] [PubMed] [Google Scholar]
- 41.Sano C. History of glutamate production. Am J Clin Nutr 2009;90(suppl):728S–32S [DOI] [PubMed] [Google Scholar]
- 42.Li X. T1R receptors mediate mammalian sweet and umami taste. Am J Clin Nutr 2009;90(suppl):733S–7S [DOI] [PubMed] [Google Scholar]
- 43.Yasumatsu K, Horio N, Murata Y, et al. Multiple receptors underlie glutamate taste responses in mice. Am J Clin Nutr 2009;90(suppl):747S–52S [DOI] [PubMed] [Google Scholar]
- 44.Bachmanov AA, Inoue M, Ji H, Murata Y, Tordoff MG, Beauchamp GK. Glutamate taste and appetite in laboratory mice: physiologic and genetic analyses. Am J Clin Nutr 2009;90(suppl):756S–63S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shigemura N, Shirosaki S, Ohkuri T, et al. Variation in umami perception and in candidate genes for the umami receptor in mice and humans. Am J Clin Nutr 2009;90(suppl):764S–9S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen Q-Y, Alarcon S, Tharp A, et al. Perceptual variation in umami taste and polymorphisms in TAS1R taste receptor genes. Am J Clin Nutr 2009;90(suppl):770S–9S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mennella JA, Forestell CA, Morgan LK, Beauchamp GK. Early milk feeding influences taste acceptance and liking during infancy. Am J Clin Nutr 2009;90(suppl):780S–8S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raliou M, Wiencis A, Pillias A-M, et al. Nonsynonymous single nucleotide polymorphisms in human tas1r1, tas1r3, and mGluR1 and individual taste sensitivity to glutamate. Am J Clin Nutr 2009;90(suppl):789S–99S [DOI] [PubMed] [Google Scholar]
- 49.Donaldson LF, Bennett L, Baic S, Melichar JK. Taste and weight: is there a link? Am J Clin Nutr 2009;90(suppl):800S–3S [DOI] [PubMed] [Google Scholar]
- 50.Rolls ET. Functional neuroimaging of umami taste: what makes umami pleasant? Am J Clin Nutr 2009;90(suppl):804S–13S [DOI] [PubMed] [Google Scholar]
- 51.Blachier F, Boutry C, Bos C, Tomé D. Metabolism and functions of l-glutamate in the epithelial cells of the small and large intestines. Am J Clin Nutr 2009;90(suppl):814S–21S [DOI] [PubMed] [Google Scholar]
- 52.Kokrashvili Z, Mosinger B, Margolskee RF. Taste signaling elements expressed in gut enteroendocrine cells regulate nutrient-responsive secretion of gut hormones. Am J Clin Nutr 2009;90(suppl):822S–5S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Akiba Y, Kaunitz JD. Luminal chemosensing and upper gastrointestinal mucosal defenses. Am J Clin Nutr 2009;90(suppl):826S–31S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kondoh T, Mallick HN, Torii K. Activation of the gut-brain axis by dietary glutamate and physiologic significance in energy homeostasis. Am J Clin Nutr 2009;90(suppl):832S–7S [DOI] [PubMed] [Google Scholar]
- 55.Tomé D, Schwarz J, Darcel N, Fromentin G. Protein, amino acids, vagus nerve signaling, and the brain. Am J Clin Nutr 2009;90(suppl):838S–43S [DOI] [PubMed] [Google Scholar]
- 56.Yamamoto S, Tomoe M, Toyama K, Kawai M, Uneyama H. Can dietary supplementation of monosodium glutamate improve the health of the elderly? Am J Clin Nutr 2009;90(suppl):844S–9S [DOI] [PubMed] [Google Scholar]
- 57.Burrin DG, Stoll B. Metabolic fate and function of dietary glutamate in the gut. Am J Clin Nutr 2009;90(suppl):850S–6S [DOI] [PubMed] [Google Scholar]
- 58.Brosnan ME, Brosnan JT. Hepatic glutamate metabolism: a tale of 2 hepatocytes. Am J Clin Nutr 2009;90(suppl):857S–61S [DOI] [PubMed] [Google Scholar]
- 59.Stanley CA. Regulation of glutamate metabolism and insulin secretion by glutamate dehydrogenase in hypoglycemic children. Am J Clin Nutr 2009;90(suppl):862S–6S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hawkins RA. The blood-brain barrier and glutamate. Am J Clin Nutr 2009;90(suppl):867S–74S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Magistretti PJ. Role of glutamate in neuron-glia metabolic coupling. Am J Clin Nutr 2009;90(suppl):875S–80S [DOI] [PubMed] [Google Scholar]
- 62.Fernstrom JD. Symposium summary. Am J Clin Nutr 2009;90(suppl):881S–5S [DOI] [PubMed] [Google Scholar]