Abstract
This article provides an overview of our studies of variation in voluntary glutamate consumption in mice. In 2-bottle preference tests, mice from the C57BL/6ByJ (B6) strain consume more monosodium l-glutamate (MSG) than do mice from the 129P3/J (129) strain. We used these mice to study physiologic and genetic mechanisms that underlie the strain differences in glutamate intake. Our genetic analyses showed that differences between B6 mice and 129 mice in MSG consumption are unrelated to strain variation in consumption of sodium or sweeteners and therefore are attributed to mechanisms specific for glutamate. These strain differences could be due to variation in responses to either taste or postingestive effects of glutamate. To examine the role of taste responsiveness, we measured MSG-evoked activity in gustatory nerves and showed that it is similar in B6 and 129 mice. On the other hand, strain-specific postingestive effects of glutamate were evident from our finding that exposure to MSG increases its consumption in B6 mice and decreases its consumption in 129 mice. We therefore examined whether B6 mice and 129 mice differ in postingestive metabolism of glutamate. We showed that, after intragastric administration of MSG, the MSG is preferentially metabolized through gluconeogenesis in B6 mice, whereas thermogenesis is the predominant process for 129 mice. We hypothesize that a process related to gluconeogenesis of the ingested glutamate generates the rewarding stimulus, which probably occurs in the liver before glucose enters the general circulation, and that the glutamate-induced postingestive thermogenesis generates an aversive stimulus. Our animal model studies raise the question of whether humans also vary in glutamate metabolism in a manner that influences their glutamate preference, consumption, and postingestive processing.
INTRODUCTION
l-Glutamic acid is a nonessential amino acid commonly found in proteins and in free form in some foods. It is used as a food additive, most commonly in the form of monosodium l-glutamate (MSG). l-Glutamic acid, especially its salts (for brevity, in this article, these compounds are referred to as “glutamate”), is perceived by humans as having the umami taste quality (1, 2) and probably evokes an equivalent taste sensation in nonhuman animals (3–5). Because umami taste likely evolved to detect the presence of nutrients and to induce their consumption, variation in umami taste responsiveness is likely to affect ingestive behavior. When glutamate is consumed, it can have postingestive effects that may be mediated by several different mechanisms, such as its nutritive value (which includes energy generated from its catabolism), production of metabolites, release of hormones, stimulation of vagus activity, or changes in gastrointestinal secretion (6–19). Therefore, glutamate consumption may depend on both chemosensory perception of its taste and on its postingestive effects.
Humans differ in perception of glutamate taste (20), and recent studies suggest that this variation may have a genetic basis (21–23). There is also evidence that mice have genetic differences in taste responsiveness to stimuli including glutamate (24). However, it is not known whether this variation in taste responses to glutamate influences glutamate preference or intake. Because glutamate has many beneficial effects for health (13, 14, 25, 26), it is important to understand genetic and physiologic mechanisms that regulate its consumption.
This article summarizes our studies of variation in glutamate consumption and the physiologic and genetic mechanisms underlying it. These studies involve inbred strains of mice. Animals within each inbred strain are genetically identical. Differences between inbred strains are attributed to allelic variation of polymorphic genes. Our studies have focused on 2 inbred mouse strains that differ in glutamate consumption, C57BL/6ByJ (B6) and 129P3/J (129).
GLUTAMATE CONSUMPTION
Strain differences in MSG consumption
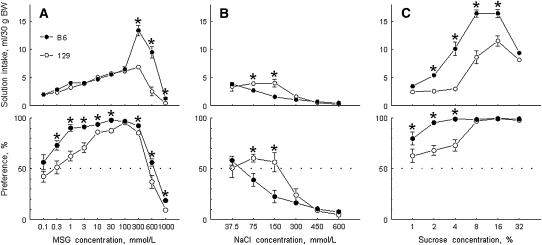
In 2-bottle preference tests with water and MSG solutions, mice from the B6 strain had higher MSG intakes and preferences than did mice from the 129 strain (Figure 1A). For preference scores, the strain differences were largest at lower MSG concentrations, which were near preference thresholds. These thresholds were lower in the B6 mice (0.3 mmol/L) than they were in the 129 mice (1 mmol/L). However, the strain differences in MSG solution intakes were largest at higher concentrations (300–600 mmol/L). Consumption of 300 mmol MSG/L by the B6 mice was remarkably high: some of the B6 mice consumed MSG solution during 24 h in amounts >50% of their body weight.
FIGURE 1.
Mean (±SEM) daily (A) monosodium l-glutamate (MSG), (B) sodium chloride (NaCl), and (C) sucrose intakes (upper panels) and preference scores (lower panels) in B6 and 129 mice in 48-h, 2-bottle preference tests. B6, C57BL/6ByJ mouse strain; 129, 129P3/J mouse strain; BW, body weight. *Significant difference between the B6 and 129 strains (P < 0.05, planned comparisons). Modified with permission from references 46 and 49.
These differences between the B6 and 129 strains allowed us to use these mice for physiologic and genetic analyses of mechanisms underlying glutamate consumption. Results of these analyses are described below.
Specificity of strain differences in MSG consumption
MSG contains sodium, which contributes a salty component to MSG taste and has its own postingestive effects when consumed. The salty (sodium chloride–like) taste quality component of MSG was shown in conditioned taste aversion experiments with mice (3, 4), rats (27), and hamsters (28) and in psychophysical experiments with humans (1, 2, 29, 30). Sodium consumed with MSG may affect sodium metabolism and osmotic equilibrium of the body. Therefore, strain differences in MSG consumption potentially could be influenced by differential responses to the Na+ anion of MSG. However, this is unlikely in this case because the B6 mice have lower sodium chloride intakes and preferences than do the 129 mice (Figure 1B; see references 31–34); in other words, the directions of the strain differences in MSG and sodium chloride consumption are opposite. Thus, the higher MSG consumption by B6 mice than by 129 mice is most likely due to specific effects of glutamate rather than to the effects of Na+ present in MSG.
There is evidence of some commonality between tastes of glutamate and sweeteners, which is supported by several types of experiments. First, conditioned taste aversions generalize between sucrose and a mixture of MSG with amiloride (35–38). Second, some sweet taste blockers can suppress taste responses to umami compounds (35, 39, 40). Third, some sweetener-responsive neural units in the gustatory nerves also respond to umami stimuli (5, 41, 42). Finally, both sweet and umami taste receptors include the T1R3 protein (43–45). We therefore examined whether strain differences in MSG consumption are associated with sweet taste responsiveness. We showed that compared with the 129 mice, the B6 mice have higher consumption of several different sweeteners (46–48), including sucrose (Figure 1C), which is consistent with the elevated consumption of MSG and sweeteners by B6 mice being due to a common underlying mechanism. However, our genetic and neurophysiologic experiments (described below in Genetics of MSG Consumption) show that this is not the case and that the similar direction of the strain differences in MSG and in sweetener consumption between the B6 mice and the 129 mice is coincidental.
Genetics of MSG consumption
To identify some of the genetic influences on MSG consumption, we out-crossed B6 mice and 129 mice to produce the first filial generation of hybrid mice, which were intercrossed to produce the second filial generation of hybrid mice (F2). The F2 mice (n = 455) were tested in 4-d, 2-bottle preference tests, with 1 and 300 mmol MSG/L and with 3 sweeteners: sucrose, saccharin, and d-phenylalanine (49). We showed that elevated MSG intake and preference in B6 mice is inherited as a recessive trait in the F2 generation.
We next analyzed correlations in preference scores of the solutions tested. Interestingly, preferences for 1 and 300 mmol MSG/L did not correlate in the F2 hybrid mice (Table 1), which suggested that ingestive responses to these solutions depend on different mechanisms and are determined by different genes. It is possible that weak and strong MSG solutions have different sensory properties. However, the difference in responses to these 2 solutions is also likely to depend on the postingestive effects of 300 mmol MSG/L (but not 1 mmol MSG/L), as discussed below in Postingestive Effects of Glutamate.
TABLE 1.
Correlations in preference scores for monosodium l-glutamate (MSG) and sweeteners in C57BL/6ByJ × 129P3/J F2 mice1
| Solutions | MSG (300 mmol/L) | Sucrose (120 mmol/L) | Saccharin (20 mmol/L) | d-Phenylalanine (30 mmol/L) |
| MSG (1 mmol/L) | +0.07 | +0.11 | +0.10 | +0.03 |
| MSG (300 mmol/L) | 0.00 | +0.01 | −0.04 | |
| Sucrose (120 mmol/L) | +0.722 | +0.502 | ||
| Saccharin (20 mmol/L) | +0.562 |
Reproduced with permission from reference 49. F2, second filial generation of hybrid mice.
Significant correlations; significance level was calculated with Bonferroni's correction for 10 correlation coefficients, which estimates critical P level as 0.05/10 = 0.005.
Preferences for sucrose, saccharin, and d-phenylalanine correlated strongly with each other in F2 mice (Table 1). However, no significant correlations were shown between MSG and sweetener preferences (Table 1), even though B6 mice have higher consumption of both MSG and sweeteners compared with 129 mice (Figure 1, A and C). Independence of the strain differences in taste responses to glutamate and sweeteners is also evident from studies of allelic variation of the Tas1r3 (taste receptor, type 1, member 3) gene (formerly the Sac, saccharin preference, locus). The Tas1r3 gene encodes the T1R3 protein (50–54). When T1R3 is coexpressed in a heterologous system with T1R2, it functions as a broad-spectrum sweet taste receptor (43–45). In contrast, a heterodimer of T1R1 and T1R3 proteins functions as an umami taste receptor in humans and is more broadly tuned in rodents to respond to l-amino acids (44, 45, 55). Thus, the T1R3 protein is involved in transduction of both sweet and umami tastes, and a disruption of the Tas1r3 gene diminishes taste responses to both sweet and umami taste stimuli (55, 56). Hence, natural allelic variation of the Tas1r3 gene could also affect umami taste responses. However, experiments with the F2 hybrids between the B6 and 129 inbred mouse strains (57) and with 129.B6-Tas1r3 congenic mice (58) have shown that although the Tas1r3 allelic variants influence taste responses to sweeteners, they do not affect behavioral or neural taste responses to umami stimuli, including MSG. Thus, although T1R3 is involved in the reception of umami taste, the B6/129-Tas1r3 sequence variants that affect its sensitivity to sweeteners do not affect taste responses to umami compounds, including MSG. These data demonstrate that the strain variation in MSG consumption depends on different genes from the strain variation in sweetener consumption.
The independent genetic determination of MSG and sweetener consumption must be a consequence of distinct physiologic mechanisms underlying strain differences in ingestive responses to these stimuli. This is consistent with available experimental data. Differences between B6 mice and 129 mice in sweetener consumption depend to a large degree on the T1R3-related peripheral taste mechanisms. Compared with 129 mice, B6 mice have higher peripheral neural responses to several sweeteners (59–61), and allelic variation of the Tas1r3 gene underlies the strain differences in both behavioral and neural taste responses to sweeteners (57, 58, 61). On the other hand, data presented below indicate that differences between B6 mice and 129 mice in consumption of concentrated MSG solutions depend on strain-specific postingestive effects of glutamate rather than on variation in umami taste responsiveness. Together, these results demonstrate that the elevated MSG and sweetener intakes by B6 mice and the decreased consumption of these compounds by 129 mice are the results of the incidental association of these traits during inbreeding of these 2 strains.
Possible mechanisms underlying strain differences in MSG consumption
Several possible mechanisms may be responsible for differences in MSG consumption between B6 mice and 129 mice. Moreover, strain variation in MSG preference at lower concentrations may depend on different mechanisms than does strain variation in intake of concentrated MSG. As discussed in the Introduction, when glutamate is consumed, it evokes taste sensations and may also produce postingestive effects. Therefore, strain variation in MSG consumption can be due to either differential umami taste responsiveness or differences in postingestive handling of glutamate or possibly to both. We have examined both of these possibilities in experiments that are described below in Taste Responses to Glutamate and Postingestive Effects of Glutamate.
TASTE RESPONSES TO GLUTAMATE
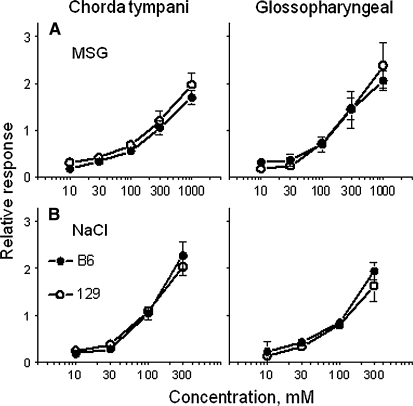
To examine whether differences between B6 mice and 129 mice in MSG consumption depend on peripheral taste responsiveness, we measured integrated responses of the whole chorda tympani and glossopharyngeal nerves to lingual application of MSG and several other taste stimuli in these mice (62). We showed that the B6 mice and the 129 mice have similar responses to MSG in both nerves (Figure 2A).
FIGURE 2.
Mean (±SEM) integrated whole-nerve responses [relative to 100 mmol ammonium chloride/L] to (A) monosodium l-glutamate (MSG) and (B) sodium chloride (NaCl) in the chorda tympani (left) and glossopharyngeal (right) nerves of B6 mice and 129 mice. B6, C57BL/6ByJ mouse strain; 129, 129P3/J mouse strain. There were no significant strain differences (ANOVA). Modified with permission from reference 62.
As discussed above in Specificity of Strain Differences in MSG Consumption, MSG contains sodium, which evokes a salty taste sensation and activates receptors distinct from the umami taste receptors (63). If there are strain differences in taste responses to Na+, they could also affect responses to MSG. We assessed the role of the Na+ component by measuring responses to a series of sodium chloride concentrations and showed that the B6 mice and the 129 mice had similar responses to sodium chloride in both nerves (Figure 2B). This is consistent with results of previous studies that used the B6 and 129 strains (33, 64–66) and implies that gustatory neural responses of B6 mice and 129 mice to MSG are not affected by differential responses to sodium present in this compound.
These data demonstrate that although B6 mice and 129 mice differ in MSG consumption in long-term 2-bottle tests (49), they have similar gustatory neural responses to MSG. In addition, there are no differences between these 2 strains in qualitative taste perception of MSG (3). Therefore, the strain differences in MSG consumption most likely depend on nongustatory mechanisms. Data presented below in Postingestive Effects of Glutamate suggest that strain-specific postingestive effects of glutamate can affect its intake.
POSTINGESTIVE EFFECTS OF GLUTAMATE
Strain-specific effects of exposure to MSG
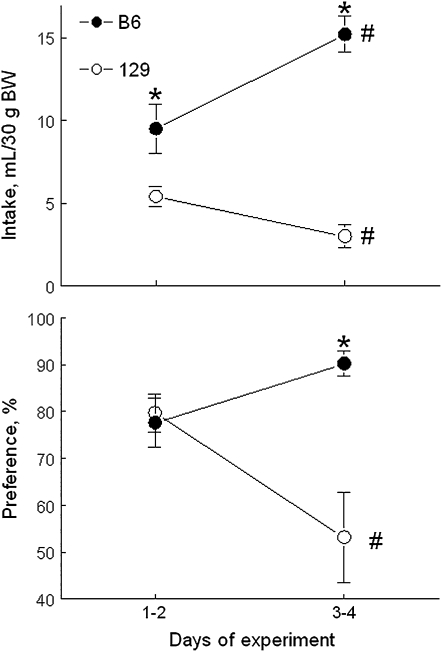
Mice given concentrated MSG solutions, in particular 300 mmol MSG/L, consume substantial amounts of glutamate (Figure 1A). It is likely that consumption of this amount of glutamate has postingestive effects. Such effects, if present, could alter initial levels of consumption by increasing (if the effects are rewarding) or decreasing (if the effects are aversive) intakes. To examine the possibility that MSG has postingestive effects that influence its consumption, we measured intake of 300 mmol MSG/L during the 4-d 2-bottle tests (49). We showed that MSG consumption changed over time in a strain-specific manner (Figure 3). Compared with the first 2 d of the test, during the last 2 d, MSG intakes and preferences increased in the B6 mice and decreased in the 129 mice (all changes were significant with the exception of nonsignificant increase in preference scores of the B6 mice). As a result, the strain differences in MSG consumption were larger at the end of the test than they were in the beginning of the test. These observations suggest that the postingestive effects of MSG are rewarding to B6 mice and aversive to 129 mice and that the strain differences in MSG consumption depend on its postingestive effects (at least for concentrated solutions).
FIGURE 3.
Mean (±SEM) daily 300 mmol monosodium l-glutamate (MSG)/L intakes (upper panel) and preference scores (lower panel) of B6 mice and 129 mice during 96-h, 2-bottle preference tests. B6, C57BL/6ByJ mouse strain; 129, 129P3/J mouse strain; BW, body weight. *Significant difference between B6 mice and 129 mice (P < 0.05, planned comparisons). #Significant difference between days 1–2 and days 3–4 of the test (P < 0.05, planned comparisons). Modified with permission from reference 49.
To investigate the nature of the strain-specific postingestive effects of glutamate, we conducted experiments, which are described below in Glutamate Metabolism. In these experiments, we examined whether B6 mice and 129 mice differ in postingestive metabolism of glutamate.
Glutamate metabolism
To examine whether strain-specific postingestive effects of MSG in B6 mice and 129 mice involve differences in glutamate metabolism, we administered MSG to mice from these strains intragastrically (by gavage) and measured perturbations in the main pathways of glutamate metabolism (67).
Metabolic pathways of ingested glutamate are complex. Glutamate is extensively metabolized in the small intestine into carbon dioxide, lactate, glutathione, glutamine, alanine, and several other amino acids (6, 10). If dietary glutamate is not completely metabolized in the intestine, then it (as well as some of its intestinal metabolites) is released into the hepatic portal circulation, and most of it is further metabolized in the liver. Because glutamate is a gluconeogenic amino acid, it (and its intestinal metabolites, alanine and lactate) can be converted to glucose in the liver. This glucose is either released into the general circulation (and stimulates insulin secretion) or stored in the liver in the form of glycogen. Alternatively, the carbon skeleton of glutamate can be oxidized through the tricarboxylic acid cycle to generate energy, and its nitrogen can be converted to urea, which is excreted in urine (68).
Our experiment was designed to characterize the main pathways of metabolism of ingested glutamate: its intestinal metabolism (by measuring plasma concentrations of glutamine, alanine, glutathione, and lactate), deamination (by measuring plasma and urine concentrations of urea and ammonia), gluconeogenesis (by measuring plasma glucose and insulin and liver glycogen), and thermogenesis (by measuring body temperature).
In this experiment, we intended to approximate conditions that mice experience during the 24-h 2-bottle tests. We calculated a dose of glutamate with voluntary intakes of 300 mmol MSG/L (the most consumed solution; Figure 1) in the 2-bottle tests. Mice were intragastrically administered (by gavage) 1000 mg MSG/kg body wt, which is a dose equivalent to the average amount of MSG voluntarily consumed in 1 h by a mouse from the 129 strain (49). Therefore, the strain differences in postingestive metabolism of glutamate shown in this experiment are likely to also influence postingestive processing of these amino acids consumed during the long-term 2-bottle tests.
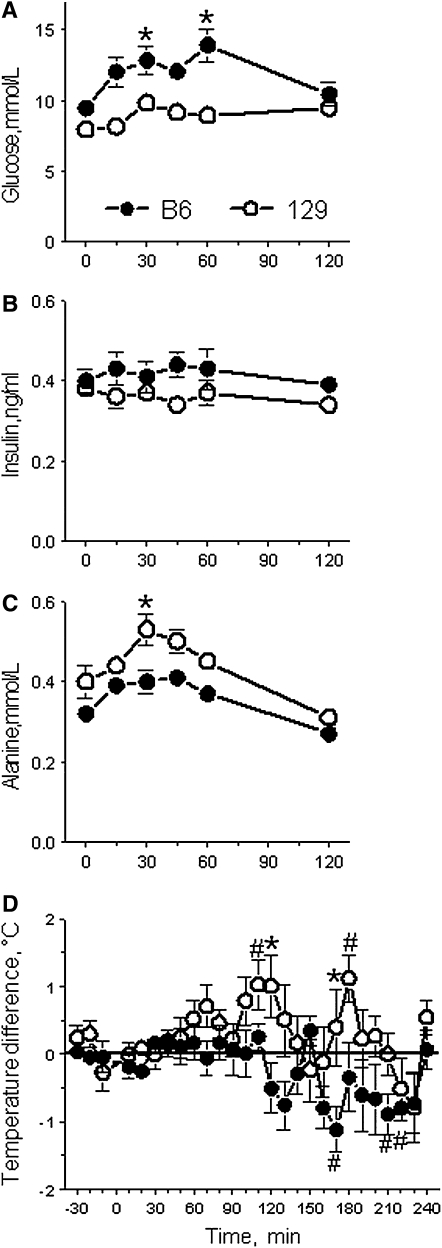
After administration of MSG, plasma glucose significantly increased above the baseline concentration in the B6 mice but not the 129 mice (Figure 4A). Correspondingly, plasma glucose and insulin (Figure 4B) concentrations were higher in the B6 mice than they were in the 129 mice [P < 0.05, effect of strain; analysis of variance (ANOVA)]. The opposite pattern of changes was present for plasma alanine concentration: it was significantly increased above baseline in the 129 mice but not the B6 mice (Figure 4C; P < 0.05, effect of strain, ANOVA). Body temperature measured with subcutaneously implanted temperature transponders did not increase above the baseline level in the B6 mice, but in the 129 mice significant increases above baseline and significantly higher levels than in the B6 mice were recorded (Figure 4D; P < 0.0001, effect of strain, ANOVA). The B6 mice and the 129 mice did not differ significantly in postingestive changes in plasma glutamine, lactate, glutathione, urea, or ammonia concentrations; in liver contents of glycogen; or in total nitrogen (ammonia plus urea) excretion in urine (67).
FIGURE 4.
Mean (±SEM) plasma concentrations of glucose (A), insulin (B), and alanine (C) and changes in body temperature (D) of B6 and 129 mice after intragastric administration of monosodium l-glutamate (MSG). Strain differences were significant for plasma glucose, insulin, and alanine [F(1,11) > 10.6, P < 0.05, ANOVA] and temperature changes [F(1,53) = 30.4, P < 0.0001]. B6, C57BL/6ByJ mouse strain; 129, 129P3/J mouse strain. *Significant difference between the 129 and B6 strains (P ≤ 0.05, post hoc tests; although insulin concentrations were significantly different between B6 mice and 129 mice in ANOVA, the strain differences did not reach the level of significance in the post hoc tests for any of the time points). #Temperature level significantly different from zero (P ≤ 0.05, one-sample t tests). At time 0, mice were administered with a single bolus of 591 mmol MSG/L (10% wt:vol) at the volume of 1 mL/100 g body wt and the dose 1 g/kg body wt. Blood was collected 6 times before and during the 2-h period after MSG administration. In separate groups of mice, body temperature was measured at 10-min intervals for 0.5 h before and for 4 h after MSG administration. In panel D values represent deviations in body temperature from those recorded after gavage with sodium chloride. Modified with permission from reference 67.
The time course of change was different for different measures. Peak alanine concentration in the 129 mice occurred 30 min after MSG administration, peak glucose concentration in the B6 mice occurred 60 min after MSG administration, and body temperature increase in the 129 mice occurred only at >90 min after MSG administration. These temporal differences point to different organs responsible for differential processing of glutamate. The relatively early time of the peak plasma alanine concentration suggests that it is produced from glutamate in the intestine. The later peak for glucose suggests that it is produced from absorbed glutamate (and its metabolites) in the liver through the gluconeogenic pathway and then released into the general circulation rather than stored as glycogen. The delayed body temperature rise suggests that the thermogenesis mainly occurred in the liver and/or other tissues but not in the intestine.
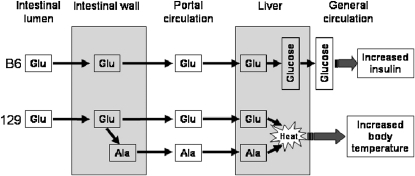
These observations indicate that the metabolic fate of oral glutamate differs in B6 mice and in 129 mice. B6 mice tend to use glutamate as a gluconeogenic precursor in the liver, whereas 129 mice convert glutamate to alanine in the intestine; then, alanine and possibly glutamate are further used for thermogenesis (Figure 5).
FIGURE 5.
Differences in glutamate metabolism between B6 mice and 129 mice. Arrows indicate predominant metabolic pathways in each strain. Glu, glutamate; Ala, alanine; B6, C57BL/6ByJ mouse strain; 129, 129P3/J mouse strain. Modified with permission from reference 67.
Can these strain differences in glutamate metabolism explain the differential consumption of glutamate by B6 mice and 129 mice? Our data suggest that rewarding postingestive effects of glutamate in B6 mice are associated with elevated blood glucose and insulin. Previous studies have shown that glucose can act as a rewarding unconditioned stimulus. Elevated blood glucose was shown to suppress intake in some (69–71) but not all (72) studies. At the same time, systemic intravenous glucose administration is not effective for conditioning flavor preferences (72, 73). On the contrary, intragastric and hepatic portal infusions of glucose and some other nutrients can condition flavor preferences (72, 74–76), which suggests that the rewarding signal of ingested glutamate in B6 mice may be initiated by glucose generated during gluconeogenesis in the liver before it enters the general circulation. In contrast, aversive postingestive effects of glutamate in the 129 mice are associated with thermogenesis. It was shown that changes in body temperature positively correlate with satiety (77–79). It is, therefore, likely that thermogenesis induced by glutamate consumption in the 129 mice suppresses ingestive behavior and thus limits glutamate intake in this strain.
These data are consistent with a hypothesis that the metabolic fate of glutamate plays an important regulatory role in control of its intake. We speculate that glucose produced during the gluconeogenesis of the ingested glutamate generates the rewarding stimulus in the liver before it enters the general circulation and that glutamate-induced postingestive thermogenesis generates the aversive stimulus. However, additional studies are needed to examine this hypothesis.
CONCLUSIONS
Our studies have identified genetically determined differences in glutamate consumption in mice and suggested that the metabolic fate of glutamate may play an important regulatory role in the control of its intake. In addition, these studies provide the first characterization of genetic variation in amino acid metabolism. Given the many similarities between mice and humans in metabolism and behavior, it is a small step to infer that genetic variation in glutamate metabolism may also exist in humans and may influence their glutamate preference, consumption and postingestive processing. (Other articles in this supplement to the Journal include references 14–17, 21–23, and 80–101.)
Acknowledgments
The authors' responsibilities were as follows—AAB: wrote the manuscript; and MI, HJ, YM, MGT, and GKB: edited the manuscript. The presenting author's (AAB) travel expenses associated with participation in the symposium were paid by the conference sponsor, the International Glutamate Technical Committee, a nongovernmental organization funded by industrial producers and users of glutamate in food. None of the authors had a personal or financial conflict of interest.
REFERENCES
- 1.Ninomiya T, Ikeda S, Yamaguchi S, Yoshikawa T. Tastes of various amino acids. Stat Quality Control 1966;17:69–73 (in Japanese) [Google Scholar]
- 2.Hayakawa Y, Kawai M. Taste properties of L-amino acid solutions at suprathreshold concentration. Jap J Taste Smell Res 2003;10:463–6 [Google Scholar]
- 3.Murata Y, Bachmanov AA, Beauchamp GK. Generalization of conditioned taste aversion (CTA) to monosodium glutamate (MSG) and inosine monophosphate (IMP) in 129P3/J and C57BL/6ByJ mice. Chem Senses 2005;30:A193 (abstr) [Google Scholar]
- 4.Ninomiya Y, Funakoshi M. Behavioural discrimination between glutamate and the four basic taste substances in mice. Comp Biochem Physiol A Comp Physiol 1989;92:365–70 [DOI] [PubMed] [Google Scholar]
- 5.Ninomiya Y, Funakoshi M. Peripheral neural basis for behavioural discrimination between glutamate and the four basic taste substances in mice. Comp Biochem Physiol A Comp Physiol 1989;92:371–6 [DOI] [PubMed] [Google Scholar]
- 6.Wu G. Intestinal mucosal amino acid catabolism. J Nutr 1998;128:1249–52 [DOI] [PubMed] [Google Scholar]
- 7.Brosnan JT. Glutamate, at the interface between amino acid and carbohydrate metabolism. J Nutr 2000;130:988S–90S [DOI] [PubMed] [Google Scholar]
- 8.Kondoh T, Mori M, Ono T, Torii K. Mechanisms of umami taste preference and aversion in rats. J Nutr 2000;130:966S–70S [DOI] [PubMed] [Google Scholar]
- 9.Niijima A. Effects of oral and intestinal stimulation with umami substance on gastric vagus activity. Physiol Behav 1991;49:1025–8 [DOI] [PubMed] [Google Scholar]
- 10.Reeds PJ, Burrin DG, Stoll B, Jahoor F. Intestinal glutamate metabolism. J Nutr 2000;130:978S–82S [DOI] [PubMed] [Google Scholar]
- 11.Young VR, Ajami AM. Glutamate: an amino acid of particular distinction. J Nutr 2000;130:892S–900S [DOI] [PubMed] [Google Scholar]
- 12.Uneyama H, Niijima A, San Gabriel A, Torii K. Luminal amino acid sensing in the rat gastric mucosa. Am J Physiol Gastrointest Liver Physiol 2006;291:G1163–70 [DOI] [PubMed] [Google Scholar]
- 13.Uneyama H, San Gabriel A, Kawai M, Tomoe M, Torii K. Physiological role of dietary free glutamate in the food digestion. Asia Pac J Clin Nutr 2008;17(suppl 1):372–5 [PubMed] [Google Scholar]
- 14.Kondoh T, Mallick HN, Torii K. Activation of the gut-brain axis by dietary glutamate and physiologic significance in energy homeostasis. Am J Clin Nutr 2009;90(suppl):832S–7S [DOI] [PubMed] [Google Scholar]
- 15.Tomé D, Schwarz J, Darcel N, Fromentin G. Protein, amino acids, vagus nerve signaling, and the brain. Am J Clin Nutr 2009;90(suppl):838S–43S [DOI] [PubMed] [Google Scholar]
- 16.Burrin DG, Stoll B. Metabolic fate and function of dietary glutamate in the gut. Am J Clin Nutr 2009;90(suppl):850S–6S [DOI] [PubMed] [Google Scholar]
- 17.Brosnan ME, Brosnan JT. Hepatic glutamate metabolism: a tale of 2 hepatocytes. Am J Clin Nutr 2009;90(suppl):857S–61S [DOI] [PubMed] [Google Scholar]
- 18.Shlygin GK, Vasilevskaia LS. [The mechanism of potentiating effect of glutamate on gastric secretion.] Dokl Akad Nauk SSSR 1990;312:1010–4 [PubMed] [Google Scholar]
- 19.Zolotarev V, Khropycheva R, Uneyama H, Torii K. Effect of free dietary glutamate on gastric secretion in dogs. Ann N Y Acad Sci (in press) [DOI] [PubMed] [Google Scholar]
- 20.Lugaz O, Pillias AM, Faurion A. A new specific ageusia: some humans cannot taste L-glutamate. Chem Senses 2002;27:105–15 [DOI] [PubMed] [Google Scholar]
- 21.Chen Q-Y, Alarcon S, Tharp A, et al. Perceptual variation in umami taste and polymorphisms in TAS1R taste receptor genes. Am J Clin Nutr 2009;90(suppl):770S–9S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shigemura N, Shirosaki S, Ohkuri T, et al. Variation in umami perception and in candidate genes for the umami receptor in mice and humans. Am J Clin Nutr 2009;90(suppl):764S–9S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raliou M, Wiencis A, Pillias A-M, et al. Nonsynonymous single nucleotide polymorphisms in human tas1r1, tas1r3, and mGluR1 and individual taste sensitivity to glutamate. Am J Clin Nutr 2009;90(suppl):789S–99S [DOI] [PubMed] [Google Scholar]
- 24.Ninomiya Y, Kurenuma S, Nomura T, Uebayashi H, Kawamura H. Taste synergism between monosodium glutamate and 5′-ribonucleotide in mice. Comp Biochem Physiol A Comp Physiol 1992;101:97–102 [DOI] [PubMed] [Google Scholar]
- 25.Toyama K, Tomoe M, Inoue Y, Sanbe A, Yamamoto S. A possible application of monosodium glutamate to nutritional care for elderly people. Biol Pharm Bull 2008;31:1852–4 [DOI] [PubMed] [Google Scholar]
- 26.Kochetkov AM, Shlygin GK, Loranskaia TI, Vasilevskaia LS, Kondrashev SI. [The use of monosodium glutamate in the combined therapy of patients with atrophic gastritis.] Vopr Pitan 1992;5–6:19–22 [PubMed] [Google Scholar]
- 27.Yamamoto T, Yuyama N, Kato T, Kawamura Y. Gustatory responses of cortical neurons in rats. III. Neural and behavioral measures compared. J Neurophysiol 1985;53:1370–86 [DOI] [PubMed] [Google Scholar]
- 28.Yamamoto T, Matsuo R, Kiyomitsu Y, Kitamura R. Taste effects of umami substances in hamsters as studied by electrophysiological and conditioned taste aversion techniques. Brain Res 1988;451:147–62 [DOI] [PubMed] [Google Scholar]
- 29.Yoshida M, Saito S. Multidimensional scaling of the taste of amino acids. Jpn Psychol Res 1969;11:149–66 [Google Scholar]
- 30.Bartoshuk LM, Cain WS, Cleveland CT, et al. Saltiness of monosodium glutamate and sodium intake. JAMA 1974;230:670 (letter). [DOI] [PubMed] [Google Scholar]
- 31.Bachmanov AA, Tordoff MG, Beauchamp GK. Voluntary sodium chloride consumption by mice: differences among five inbred strains. Behav Genet 1998;28:117–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beauchamp GK, Fisher AS. Strain differences in consumption of saline solutions by mice. Physiol Behav 1993;54:179–84 [DOI] [PubMed] [Google Scholar]
- 33.Gannon KS, Contreras RJ. Sodium intake linked to amiloride-sensitive gustatory transduction in C57BL/6J and 129/J mice. Physiol Behav 1995;57:231–9 [DOI] [PubMed] [Google Scholar]
- 34.Lush IE. The genetics of bitterness, sweetness, and saltiness in strains of mice. Wysocki CJ, Kare MR, Genetics of perception and communication. New York, NY: Marcel Dekker, 1991:227–41 [Google Scholar]
- 35.Yamamoto T, Matsuo R, Fujimoto Y, Fukanaga I, Miyasaka A, Imoto T. Electrophysiological and behavioral studies on the taste of umami substances in the rat. Physiol Behav 1991;49:919–25 [DOI] [PubMed] [Google Scholar]
- 36.Chaudhari N, Yang H, Lamp C, et al. The taste of monosodium glutamate: membrane receptors in taste buds. J Neurosci 1996;16:3817–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stapleton JR, Roper SD, Delay ER. The taste of monosodium glutamate (MSG), L-aspartic acid, and N-methyl-D-aspartate (NMDA) in rats: are NMDA receptors involved in MSG taste? Chem Senses 1999;24:449–57 [DOI] [PubMed] [Google Scholar]
- 38.Heyer BR, Taylor-Burds CC, Tran LH, Delay ER. Monosodium glutamate and sweet taste: generalization of conditioned taste aversion between glutamate and sweet stimuli in rats. Chem Senses 2003;28:631–41 [DOI] [PubMed] [Google Scholar]
- 39.Sako N, Yamamoto T. Analyses of taste nerve responses with special reference to possible receptor mechanisms of umami taste in the rat. Neurosci Lett 1999;261:109–12 [DOI] [PubMed] [Google Scholar]
- 40.Ninomiya Y, Nakashima K, Fukuda A, et al. Responses to umami substances in taste bud cells innervated by the chorda tympani and glossopharyngeal nerves. J Nutr 2000;130:950S–3S [DOI] [PubMed] [Google Scholar]
- 41.Formaker BK, Stapleton JR, Roper SD, Frank ME. Responses of the rat chorda tympani nerve to glutamate-sucrose mixtures. Chem Senses 2004;29:473–82 [DOI] [PubMed] [Google Scholar]
- 42.Sako N, Tokita K, Sugimura T, Yamamoto T. Synergistic responses of the chorda tympani to mixtures of umami and sweet substances in rats. Chem Senses 2003;28:261–6 [DOI] [PubMed] [Google Scholar]
- 43.Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell 2001;106:381–90 [DOI] [PubMed] [Google Scholar]
- 44.Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E. Human receptors for sweet and umami taste. Proc Natl Acad Sci USA 2002;99:4692–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nelson G, Chandrashekar J, Hoon MA, et al. An amino-acid taste receptor. Nature 2002;416:199–202 [DOI] [PubMed] [Google Scholar]
- 46.Bachmanov AA, Tordoff MG, Beauchamp GK. Ethanol consumption and taste preferences in C57BL/6ByJ and 129/J mice. Alcohol Clin Exp Res 1996;20:201–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bachmanov AA, Tordoff MG, Beauchamp GK. Sweetener preference of C57BL/6ByJ and 129P3/J mice. Chem Senses 2001;26:905–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bachmanov AA, Beauchamp GK. Amino acid and carbohydrate preferences in C57BL/6ByJ and 129P3/J mice. Physiol Behav 2008;93:37–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bachmanov AA, Tordoff MG, Beauchamp GK. Intake of umami-tasting solutions by mice: a genetic analysis. J Nutr 2000;130:935S–41S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bachmanov AA, Li X, Reed DR, et al. Positional cloning of the mouse saccharin preference (Sac) locus. Chem Senses 2001;26:925–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kitagawa M, Kusakabe Y, Miura H, Ninomiya Y, Hino A. Molecular genetic identification of a candidate receptor gene for sweet taste. Biochem Biophys Res Commun 2001;283:236–42 [DOI] [PubMed] [Google Scholar]
- 52.Max M, Shanker YG, Huang L, et al. Tas1r3, encoding a new candidate taste receptor, is allelic to the sweet responsiveness locus Sac. Nat Genet 2001;28:58–63 [DOI] [PubMed] [Google Scholar]
- 53.Montmayeur JP, Liberles SD, Matsunami H, Buck LB. A candidate taste receptor gene near a sweet taste locus. Nat Neurosci 2001;4:492–8 [DOI] [PubMed] [Google Scholar]
- 54.Sainz E, Korley JN, Battey JF, Sullivan SL. Identification of a novel member of the T1R family of putative taste receptors. J Neurochem 2001;77:896–903 [DOI] [PubMed] [Google Scholar]
- 55.Zhao GQ, Zhang Y, Hoon MA, et al. The receptors for mammalian sweet and umami taste. Cell 2003;115:255–66 [DOI] [PubMed] [Google Scholar]
- 56.Damak S, Rong M, Yasumatsu K, et al. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science 2003;301:850–3 [DOI] [PubMed] [Google Scholar]
- 57.Inoue M, Reed DR, Li X, Tordoff MG, Beauchamp GK, Bachmanov AA. Allelic variation of the Tas1r3 taste receptor gene selectively affects behavioral and neural taste responses to sweeteners in the F2 hybrids between C57BL/6ByJ and 129P3/J mice. J Neurosci 2004;24:2296–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Inoue M, Glendinning JI, Theodorides ML, et al. Allelic variation of the Tas1r3 taste receptor gene selectively affects taste responses to sweeteners: evidence from 129.B6-Tas1r3 congenic mice. Physiol Genomics 2007;32:82–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bachmanov AA, Reed DR, Ninomiya Y, et al. Sucrose consumption in mice: major influence of two genetic loci affecting peripheral sensory responses. Mamm Genome 1997;8:545–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Inoue M, McCaughey SA, Bachmanov AA, Beauchamp GK. Whole nerve chorda tympani responses to sweeteners in C57BL/6ByJ and 129P3/J mice. Chem Senses 2001;26:915–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li X, Inoue M, Reed DR, et al. High-resolution genetic mapping of the saccharin preference locus (Sac) and the putative sweet taste receptor (T1R1) gene (Gpr70) to mouse distal Chromosome 4. Mamm Genome 2001;12:13–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Inoue M, Beauchamp GK, Bachmanov AA. Gustatory neural responses to umami taste stimuli in C57BL/6ByJ and 129P3/J mice. Chem Senses 2004;29:789–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gilbertson TA, Boughter JD., Jr Taste transduction: appetizing times in gustation. Neuroreport 2003;14:905–11 [DOI] [PubMed] [Google Scholar]
- 64.Ninomiya Y, Fukami Y, Yamazaki K, Beauchamp GK. Amiloride inhibition of chorda tympani responses to NaCl and its temperature dependency in mice. Brain Res 1996;708:153–8 [DOI] [PubMed] [Google Scholar]
- 65.Shigemura N, Ohkuri T, Sadamitsu C, et al. Amiloride-sensitive NaCl taste responses are associated with genetic variation of ENaC alpha-subunit in mice. Am J Physiol Regul Integr Comp Physiol 2008;294:R66–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ohkuri T, Yasumatsu K, Shigemura N, Yoshida R, Ninomiya Y. Amiloride inhibition on NaCl responses of the chorda tympani nerve in two 129 substrains of mice, 129P3/J and 129X1/SvJ. Chem Senses 2006;31:565–72 [DOI] [PubMed] [Google Scholar]
- 67.Ji H, Bachmanov AA. Differences in postingestive metabolism of glutamate and glycine between C57BL/6ByJ and 129P3/J mice. Physiol Genomics 2007;31:475–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gearrett R, Grisham CM. Biochemistry. Fort Worth, TX: Saunders College Publishing, 1995 [Google Scholar]
- 69.Woods SC, Stein LJ, McKay LD, Porte D., Jr Suppression of food intake by intravenous nutrients and insulin in the baboon. Am J Physiol 1984;247:R393–401 [DOI] [PubMed] [Google Scholar]
- 70.Walls EK, Koopmans HS. Differential effects of intravenous glucose, amino acids, and lipid on daily food intake in rats. Am J Physiol 1992;262:R225–34 [DOI] [PubMed] [Google Scholar]
- 71.Burggraf KK, Willing AE, Koopmans HS. The effects of glucose or lipid infused intravenously or intragastrically on voluntary food intake in the rat. Physiol Behav 1997;61:787–93 [DOI] [PubMed] [Google Scholar]
- 72.Tordoff MG, Friedman MI. Hepatic portal glucose infusions decrease food intake and increase food preference. Am J Physiol 1986;251:R192–6 [DOI] [PubMed] [Google Scholar]
- 73.Gowans SE, Weingarten HP. Elevations of plasma glucose do not support taste-to-postingestive consequence learning. Am J Physiol 1991;261:1409–17 [DOI] [PubMed] [Google Scholar]
- 74.Sclafani A. Oral and postoral determinants of food reward. Physiol Behav 2004;81:773–9 [DOI] [PubMed] [Google Scholar]
- 75.Sclafani A, Glendinning JI. Flavor preferences conditioned in C57BL/6 mice by intragastric carbohydrate self-infusion. Physiol Behav 2003;79:783–8 [DOI] [PubMed] [Google Scholar]
- 76.Sclafani A, Glendinning JI. Sugar and fat conditioned flavor preferences in C57BL/6J and 129 mice: oral and postoral interactions. Am J Physiol Regul Integr Comp Physiol 2005;289:R712–20 [DOI] [PubMed] [Google Scholar]
- 77.Westerterp-Plantenga MS, van Marken Lichtenbelt WD, Cilissen C, Top S. Energy metabolism in women during short exposure to the thermoneutral zone. Physiol Behav 2002;75:227–35 [DOI] [PubMed] [Google Scholar]
- 78.Westerterp-Plantenga MS, van Marken Lichtenbelt WD, Strobbe H, Schrauwen P. Energy metabolism in humans at a lowered ambient temperature. Eur J Clin Nutr 2002;56:288–96 [DOI] [PubMed] [Google Scholar]
- 79.Westerterp-Plantenga MS, Rolland V, Wilson SA, Westerterp KR. Satiety related to 24 h diet-induced thermogenesis during high protein/carbohydrate vs high fat diets measured in a respiration chamber. Eur J Clin Nutr 1999;53:495–502 [DOI] [PubMed] [Google Scholar]
- 80.Fernstrom JD. Introduction to the symposium. Am J Clin Nutr 2009;90(suppl):705S–6S [DOI] [PubMed] [Google Scholar]
- 81.Krebs JR. The gourmet ape: evolution and human food preferences. Am J Clin Nutr 2009;90(suppl):707S–11S [DOI] [PubMed] [Google Scholar]
- 82.Curtis RI. Umami and the foods of classical antiquity. Am J Clin Nutr 2009;90(suppl):712S–8S [DOI] [PubMed] [Google Scholar]
- 83.Kurihara K. Glutamate: from discovery as a food flavor to role as a basic taste (umami). Am J Clin Nutr 2009;90(suppl):719S–22S [DOI] [PubMed] [Google Scholar]
- 84.Beauchamp GK. Sensory and receptor responses to umami: an overview of pioneering work. Am J Clin Nutr 2009;90(suppl):723S–7S [DOI] [PubMed] [Google Scholar]
- 85.Sano C. History of glutamate production. Am J Clin Nutr 2009;90(suppl):728S–32S [DOI] [PubMed] [Google Scholar]
- 86.Li X. T1R receptors mediate mammalian sweet and umami taste. Am J Clin Nutr 2009;90(suppl):733S–7S [DOI] [PubMed] [Google Scholar]
- 87.Chaudhari N, Pereira E, Roper SD. Taste receptors for umami: the case for multiple receptors. Am J Clin Nutr 2009;90(suppl):738S–42S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.San Gabriel A, Maekawa T, Uneyama H, Torii K. Metabotropic glutamate receptor type 1 in taste tissue. Am J Clin Nutr 2009;90(suppl):743S–6S [DOI] [PubMed] [Google Scholar]
- 89.Yasumatsu K, Horio N, Murata Y, et al. Multiple receptors underlie glutamate taste responses in mice. Am J Clin Nutr 2009;90(suppl):747S–52S [DOI] [PubMed] [Google Scholar]
- 90.Kinnamon SC. Umami taste transduction mechanisms. Am J Clin Nutr 2009;90(suppl):753S–5S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mennella JA, Beauchamp GK, Forestell CA, Morgan LK. Early milk feeding influences taste acceptance and liking during infancy. Am J Clin Nutr 2009;90(suppl):780S–8S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Donaldson LF, Bennett L, Baic S, Melichar JK. Taste and weight: is there a link? Am J Clin Nutr 2009;90(suppl):800S–3S [DOI] [PubMed] [Google Scholar]
- 93.Rolls ET. Functional neuroimaging of umami taste: what makes umami pleasant? Am J Clin Nutr 2009;90(suppl):804S–13S [DOI] [PubMed] [Google Scholar]
- 94.Blachier F, Tomé D. Metabolism and functions of l-glutamate in the epithelial cells of the small and large intestines. Am J Clin Nutr 2009;90(suppl):814S–21S [DOI] [PubMed] [Google Scholar]
- 95.Kokrashvili Z, Mosinger B, Margolskee RF. Taste signaling elements expressed in gut enteroendocrine cells regulate nutrient-responsive secretion of gut hormones. Am J Clin Nutr 2009;90(suppl):822S–5S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Akiba Y, Kaunitz JD. Luminal chemosensing and upper gastrointestinal mucosal defenses. Am J Clin Nutr 2009;90(suppl):826S–31S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yamamoto S, Tomoe M, Toyama K, Kawai M, Uneyama H. Can dietary supplementation of monosodium glutamate improve the health of the elderly? Am J Clin Nutr 2009;90(suppl):844S–9S [DOI] [PubMed] [Google Scholar]
- 98.Stanley CA. Regulation of glutamate metabolism and insulin secretion by glutamate dehydrogenase in hypoglycemic children. Am J Clin Nutr 2009;90(suppl):862S–6S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hawkins RA. The blood-brain barrier and glutamate. Am J Clin Nutr 2009;90(suppl):867S–74S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Magistretti PJ. Role of glutamate in neuron-glia metabolic coupling. Am J Clin Nutr 2009;90(suppl):875S–80S [DOI] [PubMed] [Google Scholar]
- 101.Fernstrom JD. Symposium summary. Am J Clin Nutr 2009;90(suppl):881S–5S [DOI] [PubMed] [Google Scholar]