Abstract
Many of the receptors and downstream signaling elements involved in taste detection and transduction are also expressed in enteroendocrine cells where they underlie the chemosensory functions of the gut. In one well-known example of gastrointestinal chemosensation (the “incretin effect”), it is known that glucose that is given orally, but not systemically, induces secretion of glucagon-like peptide 1 and glucose-dependent insulinotropic peptide (the incretin hormones), which in turn regulate appetite, insulin secretion, and gut motility. Duodenal L cells express sweet taste receptors, the taste G protein gustducin, and several other taste transduction elements. Knockout mice that lack gustducin or the sweet taste receptor subunit T1r3 have deficiencies in secretion of glucagon-like peptide 1 and glucose-dependent insulinotropic peptide and in the regulation of plasma concentrations of insulin and glucose in response to orally ingested carbohydrate—ie, their incretin effect is dysfunctional. Isolated small intestine and intestinal villi from gustducin null mice displayed markedly defective glucagon-like peptide 1 secretion in response to glucose, indicating that this is a local circuit of sugar detection by intestinal cells followed by hormone secretion from these same cells. Modulating hormone secretion from gut “taste cells” may provide novel treatments for obesity, diabetes, and malabsorption syndromes.
MOLECULAR BASIS OF TASTE SIGNALING
The vertebrate sense of taste depends on specialized epithelial receptor cells contained in taste buds located in the surface papillae of the tongue. Taste is initiated by the interaction of tastants with receptors and ion channels in the apical microvilli of taste receptor cells. Some taste transduction pathways convert chemical information into a cellular second messenger code (eg, cyclic nucleotides and inositol trisphosphate) (reviewed in reference 1). These messengers are components of signaling cascades that typically lead to taste receptor cell depolarization and Ca2+ release. In other cases, the tastant itself may constitute all or part of the initial cellular signal (eg, Na+, K+, H+).
The sense of sweet taste is initiated by the binding of sugars or sweeteners to the sweet taste receptor: a heterodimer of 2 type 1 taste G protein–coupled receptors (T1R2 + T1R3) (2–6). The sweet taste receptor couples to heterotrimeric gustducin (α-gustducin, Gβ3, and Gγ13) (7, 8). Gustducin’s α-subunit (α-gustducin) activates taste cell phosphodiesterase to decrease cyclic nucleotide concentrations (1), whereas gustducin’s βγ-unit (Gβ3-Gγ13) activates phospholipase Cβ2 to generate inositol trisphosphate and diacylglycerol (9). Ca2+ influx and release from internal stores activates taste-cell-expressed transient receptor potential channel type M5 (TRPM5) (10), a Ca2+-activated cation channel (11–13), which leads to taste cell depolarization and signaling to other taste cells in the bud and to gustatory afferent nerves.
TASTE MOLECULES ARE EXPRESSED IN THE GUT
The taste G protein gustducin, originally identified in taste receptor cells (7), is expressed also in the cells of the stomach and small intestine (14, 15). In recent work from our group (16, 17) gustducin’s 3 subunits (α-gustducin, Gβ3, Gγ13), along with many other taste-signaling elements (eg, T1r1, T1r2, T1r3, TRPM5, PLCβ2, and others) were also found to be expressed in L-type enteroendocrine cells of the small intestine. Indeed, entire taste-signaling pathways are present in human duodenal L cells (16). The roles of gustducin, taste receptors, TRPM5, and other taste-signaling elements expressed in gut endocrine cells are now being made clear from physiologic studies in knockout mice. The expression of taste-signaling elements in gut endocrine cells and the characterization of their function as the gut’s luminal glucose sensor that initiates the incretin response to elicit the release of glucagon-like peptide 1 (GLP-1) from L cells are described here and elsewhere (16, 17).
TASTE MOLECULES UNDERLIE THE INCRETIN EFFECT
GLP-1, secreted from enteroendocrine L cells of the gut, is an incretin hormone that augments the release of insulin from the pancreas. The incretin effect—the observation that orally ingested glucose is a much more effective stimulator of insulin secretion from the pancreas than is intravenously injected glucose (18)—is primarily mediated by GLP-1. It was recently determined that sugars in the gut lumen act on the taste-signaling proteins T1r3 and gustducin that are expressed in L cells to elicit the release of GLP-1 from enteroendocrine L cells (16). Analogously with their function in oral taste cells, the gut-expressed taste-signaling elements respond to sugars and artificial sweeteners in the gut’s lumen (16, 17). However, instead of transmitting their signals via gustatory afferents, these gut “taste cells” act via humoral mediators such as GLP-1 and glucose-dependent insulinotropic peptide (GIP).
TASTE MOLECULES ARE EXPRESSED IN INTESTINAL ENTEROENDOCRINE CELLS
Intestinal mucosal cells in humans and mice have been examined for the presence of α-gustducin, T1R taste receptors, and other known taste-signaling elements (16, 17). In human duodenal biopsy sections, α-gustducin was shown by indirect immunofluorescence to be present in 4 populations of intestinal mucosal cells: 1) GLP-1-expressing enteroendocrine L cells, 2) GIP-expressing enteroendocrine K cells, 3) GIP and GLP-1 co-expressing entereoendocrine K/L cells, and 4) mucosal cells (presumed to be brush cells) that expressed neither GLP-1 nor GIP (16). Most human duodenal L cells and a sizable minority of K cells expressed α-gustducin (16). Independent confirmation of the expression of α-gustducin in human duodenal enteroendocrine K and L cells came from laser capture followed by reverse transcriptase–polymerase chain reaction (16). This same technique was used to show that α-gustducin was absent from human duodenal enterocytes (16). In addition, multiple taste-signaling elements (T1R2, T1R3, Gβ3, Gγ13, PLCβ2, and TRPM5) were found to be co-expressed with α-gustducin and GLP-1 in human duodenal L cells (16).
TASTE MOLECULES IN ENTEROENDOCRINE L CELLS UNDERLIE THE INCRETIN EFFECT
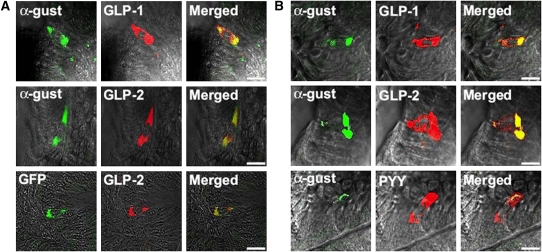
The function of taste-signaling elements in intestinal endocrine cells (mouse duodenal cells) has been examined by looking for the co-expression of taste-signaling elements. In mouse duodenum, GLP-1-expressing L cells frequently expressed α-gustducin and an α-gustducin marker (green fluorescent protein driven from the α-gustducin promoter) (Figure 1A). Mouse L cells in duodenum (Figure 1A), jejunum (Figure 1B), and ileum (16) frequently expressed α-gustducin along with GLP-2 and peptide YY (well known to be present in L cells).
FIGURE 1.
A: Indirect immunofluorescence staining of mouse duodenum showing co-expression of α-gustducin (α-gust) with glucagon-like peptide 1 (GLP-1), and GLP-2. Intrinsic fluorescence of green fluorescent protein (GFP) shows the expression of GFP and GLP-2 in the α-gustducin-expressing cells of α-gustducin–GFP transgenic mice. B: Indirect immunofluorescence of mouse jejunum showing the co-expression of α-gustin with GLP-1, GLP-2, and peptide YY (PYY). Bars, 15 μm. Data are modified with permission from reference 16.
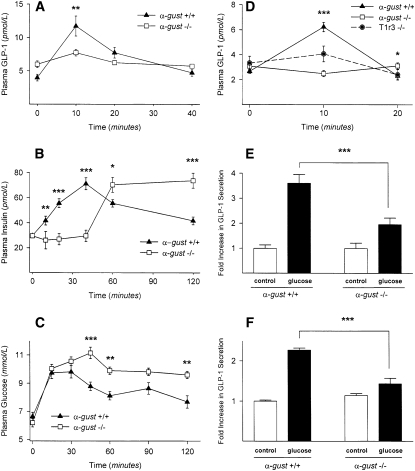
Knockout mice lacking either α-gustducin (α-gust−/−) or T1r3 (T1r3−/−) were examined for their enteroendocrine cell responses to glucose in the gut lumen. To determine the effects of directly stimulating the gut’s enteroendocrine cells and to eliminate the potential effects of the oral taste receptor cells, glucose was gavage administered by inserting feeding needles directly into the stomachs of α-gustducin knockout mice (α-gust−/−) and their wild-type (α-gust+/+) littermates (Figure 2). Gavage-administered glucose led to a significant rise (P < 0.01) in plasma concentrations of GLP-1 in wild-type mice with a peak ≈10 min after gavage (Figure 2A). In contrast, there was no significant rise in plasma concentrations of GLP-1 in the α-gust−/− mice after glucose gavage (Figure 2A). Gavage-administered glucose led to a significant rise (P < 0.001) in plasma concentrations of insulin in wild-type mice with a peak ≈45 min after gavage (Figure 2B). In contrast, there was a markedly delayed rise in plasma concentrations of insulin in α-gust−/− mice after glucose gavage (Figure 2B). In the α-gustducin-null mice, insulin reached a peak ≈60 min after gavage and remained elevated for 2 h (Figure 2B), which is a marked difference from wild-type mice whose insulin concentrations gradually returned to baseline between 60 and 120 min after gavage.
FIGURE 2.
Secretion of glucagon-like peptide 1 (GLP-1) (A) and insulin (B) in response to glucose gavage (5 g/kg body weight) in α-gustducin knockout (α-gust−/−) mice and wild-type (α-gust+/+) mice. (C) Postfasting plasma glucose concentrations after feeding standard rodent diet in α-gust−/− mice and α-gust+/+ mice. (D) Secretion of GLP-1 in response to 10% glucose injected into surgically isolated duodenum in α-gust−/− mice, T1r3 knockout (T1r3−/−) mice, and α-gust+/+ mice. The duodenum was ligated away from the stomach and the rest of the intestines, and circulatory contact was maintained. (E) Secretion of GLP-1 ex vivo from minced proximal duodenum from α-gust−/− and α-gust+/+ mice in response to the addition of 10% glucose to the culture medium. (F) Secretion of GLP-1 ex vivo from isolated duodenal villi from α-gust−/− and α-gust+/+ mice in response to the addition of 10% glucose to the culture medium. For in vivo experiments, n = 5–12 animals/genotype; in vitro experiments were carried out in triplicate and replicated at least twice. All values are means ± SEMs. *,**,*** Statistical significance was determined by ANOVA: *P < 0.05, **P < 0.01, ***P < 0.001. Data are modified with permission from Reference 16.
The α-gustducin-null mice also showed disrupted glucose homeostasis: plasma glucose concentrations were higher in α-gust−/− mice compared with wild type after overnight fasting followed by feeding (Figure 2C). Furthermore, plasma glucose concentrations in α-gust−/− mice remained high for >2 h after feeding (Figure 2C).
α-Gustducin is expressed in brush cells of the stomach (16) as well as in intestinal enteroendocrine cells (Figure 1). Thus, it is possible that glucose administered by gavage into the stomach might be acting directly on the brush cells and indirectly on the intestinal L cells via brush-cell-released factors. To rule out such indirect effects, glucose was injected directly into the duodena of wild-type, α-gust−/−, and T1r3−/− mice. In each animal, the duodenum was surgically isolated from the stomach and the distal portion of the small intestine, but remained in circulatory contact so that plasma concentrations of hormones could be monitored. After duodenal injection with glucose, plasma GLP-1 in wild-type mice peaked at 10 min after injection and then returned to baseline at 20 min (Figure 2D). In marked contrast, plasma concentrations of GLP-1 did not increase at all after duodenal injection with glucose in either α-gust−/− or T1r3−/− mice (Figure 2D).
To determine whether the glucose-stimulated release of GLP-1 was intrinsic to the enteroendocrine L cells of the duodenum, ex vivo experiments were carried out with dissected proximal duodena and isolated duodenal villi from wild-type and α-gustducin knockout mice. The addition of 10% glucose to the culture medium increased the release of GLP-1 from duodenal tissue from both wild-type and α-gust−/− mice (Figure 2E). However, the glucose stimulation of GLP-1 release was much greater in the wild-type mice. Similar results were observed when glucose stimulated a greater release of GLP-1 from isolated villi of wild-type mice than from those of α-gust−/− mice (Figure 2F).
CONCLUSIONS
In 2 recent studies (16, 17), gustducin-coupled sweet taste receptors were found to be present in enteroendocrine cells in the proximal intestines of mice and humans. In the human duodenum, α-gustducin was found in 3 types of enteroendocrine cells: K (GIP), L (GLP-1), and K/L (GIP and GLP-1) cells (16). In addition to expressing α-gustducin and the sweet receptor subunits T1R2 and T1R3, human duodenal L cells also expressed several other taste-signaling molecules, including Gβ3, Gγ13, PLCβ2, and TRPM5 (16).
In mice, α-gustducin was frequently found in L-type enteroendocrine cells but rarely in K cells. α-Gustducin knockout mice were disrupted in their glucose homeostasis and hormonal responses to glucose within the lumen of the small intestine. The primary deficit was the failure of enteroendocrine cells to release GLP-1 in response to glucose within the gut lumen (16). The absent GLP-1 response to glucose led to an abnormal insulin response and prolonged elevation of postprandial blood glucose. T1r3 knockout mice also failed to release GLP-1 from their duodenal cells after glucose injection into the intestine. Such data indicate that sweet receptors in intestinal L cells couple to heterotrimeric gustducin to detect extracellular glucose and then respond with secretion of GLP-1. In 2 L-cell lines, both α-gustducin and T1r3 were required for stimulating GLP-1 release in response to either sugars or noncaloric sweeteners (16, 17). The results indicate that the sensing of sugars and sweeteners by taste-signaling elements expressed in intestinal L cells in vivo leads to GLP-1 release from these same cells. (Other articles in this supplement to the Journal include references 19–47.)
Acknowledgments
The authors’ responsibilities were as follows—ZK, BM, and RFM: contributed to the design and analysis of the experiments and to writing the manuscript; RFM: wrote the final version of the manuscript. The travel expenses of the presenting author (RFM) associated with participation in the symposium and an honorarium were paid by the conference sponsor, the International Glutamate Technical Committee, a nongovernmental organization funded by industrial producers and users of glutamate in food. ZK and BM had no conflicts of interest. RFM has a personal financial interest in the form of stock ownership in the Redpoint Bio company, receives consulting fees from Redpoint Bio, and is an inventor on patents and patent applications that have been licensed to Redpoint Bio. Redpoint Bio is a biotechnology company that identifies and develops compounds to improve the taste of pharmaceutical, food, and beverage products.
REFERENCES
- 1.Kinnamon SC, Margolskee RF. Taste transduction Basbaum A, Bushnell M, Smith S, et al., The senses: a comprehensive reference, Vol 4. Amsterdam, Netherlands; Boston, MA: Elsevier, 2008:219–36 [Google Scholar]
- 2.Max M, Shanker YG, Huang L, et al. Tas1r3, encoding a new candidate taste receptor, is allelic to the sweet responsiveness locus Sac. Nat Genet 2001;28:58–63 [DOI] [PubMed] [Google Scholar]
- 3.Montmayeur JP, Liberles SD, Matsunami H, et al. A candidate taste receptor gene near a sweet taste locus. Nat Neurosci 2001;4:492–8 [DOI] [PubMed] [Google Scholar]
- 4.Nelson G, Hoon MA, Chandrashekar J, et al. Mammalian sweet taste receptors. Cell 2001;106:381–90 [DOI] [PubMed] [Google Scholar]
- 5.Li X, Staszewski L, Xu H, et al. Human receptors for sweet and umami taste. Proc Natl Acad Sci USA 2002;99:4692–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Damak S, Rong M, Yasumatsu K, et al. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science 2003;301:850–3 [DOI] [PubMed] [Google Scholar]
- 7.McLaughlin SK, McKinnon PJ, Margolskee RF. Gustducin is a taste-cell-specific G protein closely related to the transducins. Nature 1992;357:563–9 [DOI] [PubMed] [Google Scholar]
- 8.Huang L, Shanker YG, Dubauskaite J, et al. Gγ13, a new G protein γ subunit expressed in gustducin-positive taste receptor cells, mediates IP3 responses to bitter denatonium. Nat Neurosci 1999;2:1055–62 [DOI] [PubMed] [Google Scholar]
- 9.Rossler P, Kroner C, Freitag J, et al. Identification of a phospholipase C beta subtype in rat taste cells. Eur J Cell Biol 1998;77:253–61 [DOI] [PubMed] [Google Scholar]
- 10.Perez CA, Huang L, Rong M, et al. A transient receptor potential channel expressed in taste receptor cells. Nat Neurosci 2002;5:1169–76 [DOI] [PubMed] [Google Scholar]
- 11.Hofmann T, Chubanov V, Gudermann T, et al. TRPM5 is a voltage-modulated and Ca(2+)-activated monovalent selective cation channel. Curr Biol 2003;13:1153–8 [DOI] [PubMed] [Google Scholar]
- 12.Liu D, Liman ER. Intracellular Ca2+ and the phospholipid PIP2 regulate the taste transduction ion channel TRPM5. Proc Natl Acad Sci USA 2003;100:15160–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prawitt D, Monteilh-Zoller MK, Brixel L, et al. TRPM5 is a transient Ca2+-activated cation channel responding to rapid changes in [Ca2+]i. Proc Natl Acad Sci USA 2003;100:15166–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Höfer D, Drenckhahn D. Identification of the taste cell G-protein, alpha-gustducin, in brush cells of the rat pancreatic duct system. Histochem Cell Biol 1998;110:303–9 [DOI] [PubMed] [Google Scholar]
- 15.Höfer D, Puschel B, Drenckhahn D. Taste receptor-like cells in the rat gut identified by expression of alpha-gustducin. Proc Natl Acad Sci USA 1996;93:6631–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jang HJ, Kokrashvili Z, Theodorakis MJ, et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci USA 2007;104:15069–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Margolskee RF, Dyer J, Kokrashvili Z, et al. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci USA 2007;104:15075–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McIntyre N, Holdsworth CD, Turner DS. New interpretation of oral glucose tolerance. Lancet 1964;4:20–1 [DOI] [PubMed] [Google Scholar]
- 19.Fernstrom JD. Introduction to the symposium. Am J Clin Nutr 2009;90(suppl):705S–6S [DOI] [PubMed] [Google Scholar]
- 20.Krebs JR. The gourmet ape: evolution and human food preferences. Am J Clin Nutr 2009;90(suppl):707S–11S [DOI] [PubMed] [Google Scholar]
- 21.Curtis RI. Umami and the foods of classical antiquity. Am J Clin Nutr 2009;90(suppl):712S–8S [DOI] [PubMed] [Google Scholar]
- 22.Kurihara K. Glutamate: from discovery as a food flavor to role as a basic taste (umami). Am J Clin Nutr 2009;90(suppl):719S–22S [DOI] [PubMed] [Google Scholar]
- 23.Beauchamp GK. Sensory and receptor responses to umami: an overview of pioneering work. Am J Clin Nutr 2009;90(suppl):723S–7S [DOI] [PubMed] [Google Scholar]
- 24.Sano C. History of glutamate production. Am J Clin Nutr 2009;90(suppl):728S–32S [DOI] [PubMed] [Google Scholar]
- 25.Li X. T1R receptors mediate mammalian sweet and umami taste. Am J Clin Nutr 2009;90(suppl):733S–7S [DOI] [PubMed] [Google Scholar]
- 26.Chaudhari N, Pereira E, Roper SD. Taste receptors for umami: the case for multiple receptors. Am J Clin Nutr 2009;90(suppl):738S–42S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.San Gabriel A, Maekawa T, Uneyama H, Torii K. Metabotropic glutamate receptor type 1 in taste tissue. Am J Clin Nutr 2009;90(suppl):743S–6S [DOI] [PubMed] [Google Scholar]
- 28.Yasumatsu K, Horio N, Murata Y, et al. Multiple receptors underlie glutamate taste responses in mice. Am J Clin Nutr 2009;90(suppl):747S–52S [DOI] [PubMed] [Google Scholar]
- 29.Kinnamon SC. Umami taste transduction mechanisms. Am J Clin Nutr 2009;90(suppl):753S–5S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bachmanov AA, Inoue M, Ji H, Murata Y, Tordoff MG, Beauchamp GK. Glutamate taste and appetite in laboratory mice: physiologic and genetic analyses. Am J Clin Nutr 2009;90(suppl):756S–63S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shigemura N, Shirosaki S, Ohkuri T, et al. Variation in umami perception and in candidate genes for the umami receptor in mice and humans. Am J Clin Nutr 2009;90(suppl):764S–9S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Q-Y, Alarcon S, Tharp A, et al. Perceptual variation in umami taste and polymorphisms in TAS1R taste receptor genes. Am J Clin Nutr 2009;90(suppl):770S–9S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mennella JA, Forestell CA, Morgan LK, Beauchamp GK. Early milk feeding influences taste acceptance and liking during infancy. Am J Clin Nutr 2009;90(suppl):780S–8S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raliou M, Wiencis A, Pillias A-M, et al. Nonsynonymous single nucleotide polymorphisms in human tas1r1, tas1r3, and mGluR1 and individual taste sensitivity to glutamate. Am J Clin Nutr 2009;90(suppl):789S–99S [DOI] [PubMed] [Google Scholar]
- 35.Donaldson LF, Bennett L, Baic S, Melichar JK. Taste and weight: is there a link? Am J Clin Nutr 2009;90(suppl):800S–3S [DOI] [PubMed] [Google Scholar]
- 36.Rolls ET. Functional neuroimaging of umami taste: what makes umami pleasant? Am J Clin Nutr 2009;90(suppl):804S–13S [DOI] [PubMed] [Google Scholar]
- 37.Blachier F, Boutry C, Bos C, Tomé D. Metabolism and functions of l-glutamate in the epithelial cells of the small and large intestines. Am J Clin Nutr 2009;90(suppl):814S–21S [DOI] [PubMed] [Google Scholar]
- 38.Akiba Y, Kaunitz JD. Luminal chemosensing and upper gastrointestinal mucosal defenses. Am J Clin Nutr 2009;90(suppl):826S–31S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kondoh T, Mallick HN, Torii K. Activation of the gut-brain axis by dietary glutamate and physiologic significance in energy homeostasis. Am J Clin Nutr 2009;90(suppl):832S–7S [DOI] [PubMed] [Google Scholar]
- 40.Tomé D, Schwarz J, Darcel N, Fromentin G. Protein, amino acids, vagus nerve signaling, and the brain. Am J Clin Nutr 2009;90(suppl):838S–43S [DOI] [PubMed] [Google Scholar]
- 41.Yamamoto S, Tomoe M, Toyama K, Kawai M, Uneyama H. Can dietary supplementation of monosodium glutamate improve the health of the elderly? Am J Clin Nutr 2009;90(suppl):844S–9S [DOI] [PubMed] [Google Scholar]
- 42.Burrin DG, Stoll B. Metabolic fate and function of dietary glutamate in the gut. Am J Clin Nutr 2009;90(suppl):850S–6S [DOI] [PubMed] [Google Scholar]
- 43.Brosnan ME, Brosnan JT. Hepatic glutamate metabolism: a tale of 2 hepatocytes. Am J Clin Nutr 2009;90(suppl):857S–61S [DOI] [PubMed] [Google Scholar]
- 44.Stanley CA. Regulation of glutamate metabolism and insulin secretion by glutamate dehydrogenase in hypoglycemic children. Am J Clin Nutr 2009;90(suppl):862S–6S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hawkins RA. The blood-brain barrier and glutamate. Am J Clin Nutr 2009;90(suppl):867S–74S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Magistretti PJ. Role of glutamate in neuron-glia metabolic coupling. Am J Clin Nutr 2009;90(suppl):875S–80S [DOI] [PubMed] [Google Scholar]
- 47.Fernstrom JD. Symposium summary. Am J Clin Nutr 2009;90(suppl):881S–5S [DOI] [PubMed] [Google Scholar]