Abstract
The upper gastrointestinal mucosa is exposed to endogenous and exogenous substances, including gastric acid, carbon dioxide, and foodstuffs. Physiologic processes such as secretion, digestion, absorption, and motility occur in the gastrointestinal tract in response to ingested substances, which implies the presence of mucosal sensors. We hypothesize that mucosal acid sensors and tastelike receptors are important components of the mucosal chemosensing system. We have shown that luminal acid/carbon dioxide is sensed via ecto- and cytosolic carbonic anhydrases and ion transporters in the epithelial cells and via acid sensors on the afferent nerves in the duodenum and esophagus. Furthermore, a luminal l-glutamate signal is mediated via mucosal l-glutamate receptors with activation of afferent nerves and cyclooxygenase in the duodenum, which suggests the presence of luminal l-glutamate sensing. These luminal chemosensors help to activate mucosal defense mechanisms to maintain the mucosal integrity and physiologic responses of the upper gastrointestinal tract. Because neural pathways are components of the luminal chemosensory system, investigation of these pathways may help to identify novel molecular targets in the treatment and prevention of mucosal injury and visceral sensation.
INTRODUCTION
The upper gastrointestinal mucosa is regularly exposed to endogenous and exogenous substances including gastric acid, CO2 (generated by the mixture of gastric acid and secreted HCO3−), and foodstuffs including nutrients. Physiologic processes such as secretion, digestion, absorption, and motility occur in response to ingested substances, which implys the presence of mucosal sensors. Because one of the endogenous chemical species in the upper gastrointestinal tract is gastric acid, how the upper gastrointestinal mucosa is protected from the secreted acid has been studied for many years (1, 2). Defense mechanisms are necessary in the mucosa to protect the epithelium because the cells cannot survive in such a low pH condition.
Mucosal defense mechanisms consist of premucosal, mucosal, and submucosal defense factors. We have studied these factors, including HCO3− and mucus secretion (premucosal), intracellular pH (pHi) regulation with ion transporters and enzyme activities (mucosal), and blood flow regulated via afferent nerves and mediator releases (submucosal). The esophageal, gastric, and duodenal mucosae individually possess unique mucosal defense mechanisms (2). Because disruption of these defenses causes mucosal injury, signals that enhance defense mechanisms may protect the mucosa from luminal substances to maintain epithelial integrity. Nevertheless, the mucosa needs to sense luminal acidity or substances to rapidly respond and enhance defense mechanisms.
Here, we will show how the upper gastrointestinal mucosa senses luminal acidity in the physiologic setting. Furthermore, we will discuss the presence of chemosensing receptors in the gastrointestinal tract that are very similar to those that populate the taste buds of the tongue, and in particular consider the receptor(s) for an umami substance, monosodium l-glutamate, which appears physiologically to be “sensed” by upper gastrointestinal mucosa. Understanding how the gastrointestinal mucosa “tastes” luminal chemicals may help to identify novel molecular targets in the treatment of mucosal injury and visceral sensation.
DUODENAL ACID SENSING AND H+/CO2 ABSORPTION
The duodenal mucosa, which is constantly exposed to luminal acid and high partial pressure of CO2 (PCO2) due to gastric acid and the secreted HCO3−, has multilayered, multistep defense mechanisms to counter mucosal injury due to constant exposure to luminal concentrated acid and high PCO2 (3). These mechanisms coordinately regulate premucosal, mucosal, and submucosal components, including the secretion of mucus and HCO3−, pHi and cellular buffering, and submucosal neuronal activation and blood flow responses. Because duodenal luminal pH rapidly changes between pH 2 and 7 as a result of the constant mixture of secreted HCO3− with jets of antrally propelled gastric acid, the duodenal mucosa must rapidly adjust its defense mechanisms according to luminal pH (4).
We examined the regulation of upper gastrointestinal defense factors in response to luminal acid by using a fluorescent microscopic system in vivo in rat stomach and duodenum. This system enables us to simultaneously measure epithelial pHi, which represents H+/HCO3− movement and cellular buffering; mucosal blood flow, which supplies oxygen and HCO3− and removes CO2 and H+; and mucus gel thickness, which corresponds to the mucus secretion rate (5–12). We have also investigated duodenal HCO3− secretion and H+/CO2 movement between the lumen and mucosa by using a duodenal loop perfusion system. We found that luminal acid is sensed by the capsaicin pathway, which consists of acid-induced intracellular acidification; H+ secretion across the epithelial-cell basolateral membrane via the Na+/H+ exchanger 1; activation of transient receptor potential vanilloid 1 (TRPV1) on capsaicin-sensitive afferent nerves by subepithelial H+; the release of vasoactive mediators such as calcitonin-gene-related peptide and nitric oxide; and an increase of mucosal blood flow and mucus secretion, followed by delayed cyclooxygenase-dependent mucus and HCO3− secretion (3, 13) (Figure 1). Such results show that the duodenal mucosa “tastes” luminal acidity by using epithelial ion transporters and neuronal acid sensors and that intracellular acidification triggers the enhancement of mucosal defense mechanisms.
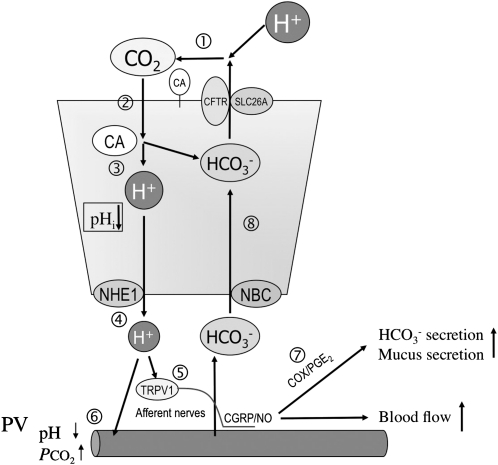
FIGURE 1.
Model of acid/CO2-sensing mechanisms in the duodenum. The scheme summarizes how H+/CO2 traverses the mucosa and is sensed by the duodenum. (1) Luminal H+ from the stomach is neutralized with the secreted HCO3− in the duodenum, generating CO2, which is facilitated by extracellular carbonic anhydrase (CA). (2) CO2 diffuses into the cytoplasm through the apical membrane of the epithelial cells. (3) CO2 is converted into H+ and HCO3− by cytosolic CA. (4) H+ acidifies cells and is extruded into the subepithelium via Na+/H+ exchanger 1 (NHE1). (5) H+ stimulates acid sensors such as transient receptor potential vanilloid 1 (TRPV1), followed by the release of calcitonin gene–related peptide (CGRP) and nitric oxide (NO), which increases blood flow. (6) H+ is carried by blood flow, which acidifies portal vein (PV) blood. (7) Concurrently, cyclooxygenase (COX) produces prostaglandin E2 (PGE2), which signals HCO3− and mucus secretion, further protecting the mucosa. (8) Cytoplasmic HCO3− generated from CO2 and HCO3− delivered by increased blood flow, and then loaded via Na+:HCO3− cotransporter (NBC1), is secreted through an apical solute carrier family 26Ax (SLC26A) anion exchanger or cystic fibrosis transmembrane conductance regulator (CFTR) to the lumen. Note that the net movement of CO2 is lumen→mucosa (absorption), whereas net HCO3− movement is mucosa→lumen (secretion). These simultaneous movements result in net H+ absorption, which is consistent with the function of the Jacobs-Stewart cycle. pHi, intracellular pH.
The high concentration of PCO2, which is generated in the proximal duodenum, gradually declines in the jejunum (14), which suggests rapid CO2 absorption by the duodenal mucosa. Because the duodenal mucosa has the highest carbonic anhydrase (CA) activity (15), which rapidly equilibrates H+ + HCO3−↔CO2 + H2O, we hypothesize that the duodenal mucosa absorbs luminal CO2 (pH 6.4, PCO2 = 260 mm Hg) effectively by cytosolic and membrane-bound CA activities. By using duodenal loop perfusion with flow-through pH and CO2 electrodes, we found that luminal CO2 is CA-dependently absorbed by the duodenal epithelium with stimulated HCO3− secretion, which is accompanied by portal venous acidification (16). Furthermore, CO2-induced intracellular acidification of epithelial cells is also CA dependent and accompanied by a TRPV1-dependent hyperemic response (17). These results suggest that luminal H+ is actively absorbed into the epithelium as CO2, which is converted into H+ and HCO3− and facilitated by membrane-bound and cytosolic CAs. Intracellular H+ is extruded via Na+/H+ exchanger 1 and sensed by the capsaicin pathway, which suggests that luminal H+ and CO2 provide equivalent acid loads that signal protective effector mechanisms (Figure 1). This mechanism resembles the Jacob-Stewart cycle, in which red blood cells absorb H+ in the peripheral tissues by absorbing CO2 and secreting HCO3−, facilitated by intra- and extracellular CA activities. The duodenum absorbs luminal H+ secreted by the stomach to maintain the acid-base balance between the stomach and duodenum (Figure 2). The acid-base balance between the stomach and duodenum is clinically important because loss of gastric content by vomiting in patients with pyloric obstruction induces acute metabolic alkalosis and hypochloremia (18, 19).
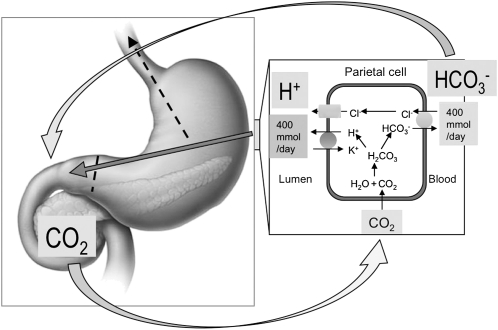
FIGURE 2.
Gastroduodenal acid-base balance: CO2 from the systemic circulation enters gastric parietal cells, where it is hydrated to carbonic acid by carbonic anhydrase. Carbonic acid is dissociated into H+ that is secreted by the apical membrane H+, K+-ATPase, and HCO3−, which is secreted into the circulation via a basolateral anion exchanger, most likely anion exchanger 2. Secreted acid enters the gastric lumen, where peristalsis carries it to the duodenal lumen. HCO3− alkalinizes the circulation, which creates the “alkaline tide.” This HCO3− is secreted by duodenal epithelial cells, the pancreatic ducts, and the bile ducts into the duodenal lumen, where it combines with H+, generating CO2. Luminal CO2 is absorbed by the mucosa, enters the portal vein, and is taken up by the parietal cells. When the gastric pylorus is obstructed, the gastric acid is lost by vomiting (dashed lines), inducing the acid-base unbalance and causing acute metabolic alkalosis and hypochloremia. Adapted with permission from reference 4.
ESOPHAGEAL CO2 CHEMOSENSING
In contrast to the “leaky” duodenal epithelium, the esophageal mucosa has high electrical resistance due to a multilayered epithelial structure. Because little H+ permeates the intact esophageal mucosa (20), heartburn sensation during acid reflux from the stomach in gastroesophageal reflux disease patients is believed to occur in the presence of macroscopic mucosal injury, which facilitates H+ permeation into the submucosa. However, in nonerosive reflux disease patients, luminal acid is sensed as heartburn in the absence of gross mucosal injury. Thus, another mechanism of permeation of H+ or H+ equivalents through the esophageal mucosa via dilated intercellular space has been hypothesized (21).
Although luminal acid (pH 1) has no effect on interstitial pH (pHint) of the esophagus (20), the esophagus responds consistently to acid perfusion by augmenting its intrinsic defense mechanisms, such as by increasing the thickness of the preepithelial gel layer, increasing HCO3− secretion (in humans, pigs, and frogs, but not in rodents or rabbits) and by increasing mucosal blood flow (20, 22, 23). Of these, the augmentation of mucosal blood flow is the most consistent and can be measured relatively easily in experimental animals and humans alike (20, 23). Acid-induced hyperemia is likely related to the activation of submucosal acid sensors on afferent nerves, such as TRPV1.
To explain the apparent inconsistency between low H+ permeation and the rapid response to luminal H+, we propose that CO2, the permeant gas, rather than H+, may penetrate the esophageal epithelium. Submucosal CO2 would then stimulate submucosal afferent nerves, enabling the subject to sense luminal acid. Sensitization or potentiation of the acid/CO2 sensing afferents may be related to the abnormal sensitivity of patients with nonerosive reflux disease to luminal acid. Because TRPV1 activation characteristically produces burning pain (24), we hypothesize that gastroesophageal reflux disease-related symptoms are also transduced by TRPV1 or other acid sensors and that the acid-related hyperemic response is a surrogate for this sensation. Furthermore, we hypothesize that CAs are involved in CO2-induced chemosensing in the esophageal mucosa, which is similar to the acid sensing in the duodenum described above.
By using an in vivo microscopic technique to measure pHint and blood flow in rat esophagus (20), we found that luminal CO2 challenge, similar to luminal acid challenge, induces hyperemia without a change in pHint in rat esophagus (25). The CO2 response is dependent on the activation of TRPV1, acid-sensing ion channels, capsaicin-sensitive afferent nerves, and cytosolic and membrane-bound CAs. Inhibition of CO2-induced hyperemia by any means is associated with interstitial acidification and a progressive decrease of esophageal blood flow. CAs and acid sensors are localized in the esophageal epithelium and in the submucosa. Furthermore, CA inhibition during luminal CO2 exposure induces portal venous acidification. These results suggest that luminal CO2 rather than H+ diffuses into the stratum epithelium, interacts with epithelial-membrane-bound and cytosolic CAs, TRPV1, and acid-sensing ion channels, and conducts the signals to capsaicin-sensitive afferent nerves via activation of acid sensors, producing hyperemia. Furthermore, the hyperemic response to luminal CO2 maintains a constant pHint. Irreversible interstitial acidification in the esophagus induced by the disruption of CO2-induced hyperemia may be related to the noxious stimuli, including the pain and heartburn sensation.
These results suggest that esophageal CO2 chemosensing may explain how luminal H+ equivalents are rapidly sensed in the esophagus. Alteration of one or more of these molecular targets in CO2 chemosensing may help to explain why patients with nonerosive reflux disease, without evidence of gross or microscopic injury, nevertheless experience heartburn and dyspepsia.
GASTRIC H+/CO2 SENSING
Luminal pH changes and proteins affect gastric acid secretion (26, 27). Increased luminal pH and proteins stimulate G cells in the antrum to secrete gastrin, which increases acid secretion from parietal cells in the fundic stomach, whereas decreased luminal pH activates D cells to secrete somatostatin, which decreases acid secretion. Although antral luminal pH changes stimulate these endocrine cells via activation of capsaicin-sensitive afferent nerves and calcitonin-gene-related peptide release (28), how luminal acidity is sensed by the endocrine cells or afferents is still unclear. Because the stomach does not absorb CO2 or H+ (18), alternative mechanisms of acid sensing that are different from those in the duodenum or esophagus may exist in the stomach. Nevertheless, acid-sensing studies in the duodenum and esophagus suggest that apical expression of acid sensors or acid-related receptors may be present in the gastric epithelium, including the endocrine cells (17, 29–31).
NUTRIENT SENSING IN THE GASTROINTESTINAL MUCOSA
The upper gastrointestinal mucosa senses not only endogenously generated H+/CO2, but also many exogenous substances such as the salts, fatty acids, glucose, and amino acids present in food. Whereas one mechanism for this detection is linked to nutrient absorption and processing by enterocytes (32), another relates to the occurrence of nutrient-specific receptors on enterocyte luminal cell membranes. This latter concept, and the approach to its study, has emerged from recent molecular studies that have identified the structure of specific receptors on the tongue for the basic tastes (sweet, sour, salty, bitter, umami) (33). And indeed, these receptors have been found in the gastrointestinal tract. In addition to “salty” sensed by epithelial Na+ channels and “sour” by H+-gated ion channels such as the acid-sensing ion channel, the “sweet” receptor heterodimer (T1R2/T1R3) is expressed in small intestinal mucosa (34–36). Bitter taste receptors of the type 2 taste receptor family are also expressed in the gastrointestinal tract (37). The expression of these taste receptors in the gastrointestinal mucosa suggests the need to sense the luminal contents, presumably to detect the presence of nutrients and unfavorable substances, to optimize digestion, absorption, secretion, and motility. Luminal chemosensing has been reported for glucose, bitter substances, and fatty acids in the gastrointestinal tract (36, 38, 39). Furthermore, because upper gastrointestinal acid/CO2 chemosensing is closely related to mucosal defense mechanisms, we suspect that mucosal defense factors may be modulated by luminal nutrients acting via taste receptors in the upper gastrointestinal mucosa.
l-GLUTAMATE SENSING AND MUCOSAL DEFENSES
The receptor on the tongue for l-glutamate, which is the primary nutrient conferring umami taste, is a heterodimer of T1R1 and T1R3 (40, 41) and/or a metabotropic l-glutamate receptor, mGluR1 and/or mGluR4 (there are several candidates; one or more may prove to be responsible for umami taste) (42, 43). These receptors belong to the G protein–coupled receptor superfamily, and they and their specific G protein, α-gustducin, are localized in the epithelial cells of the gastrointestinal tract (35, 36, 39, 44). This fact suggests that the mucosa directly “tastes” the luminal content, and in response, presumably releases mediators or otherwise transmits the luminal information to other signaling systems. Indeed, luminal l-glutamate stimulates gastric vagal afferents through the release of nitric oxide and 5-hyroxytryptamine (5-HT), which acts through 5-HT3 receptors (45). There thus seems to be a sensing pathway for l-glutamate in the upper gastrointestinal tract.
By using in vivo microscopic techniques, we have recently shown that luminal l-glutamate (0.1–10 mmol/L) dose-dependently increases pHi and mucus gel thickness, but not blood flow, in the gastroduodenal mucosa (46). In contrast, neither l-aspartate nor d-glutamate has these effects, which suggests that they are specific to l-glutamate. Furthermore, we observed that these actions of l-glutamate are mediated by capsaicin- and indomethacin-sensitive pathways in the duodenum, which suggests the activation of capsaicin-sensitive afferent nerves and cyclooxygenase activity, respectively. Reverse transcriptase–polymerase chain reaction detected the expression of possible l-glutamate receptor candidates including T1R1 and T1R3, mGluR1 and mGluR4, and a calcium-sensing receptor, but not T1R2, in the gastric and duodenal mucosa, which is consistent with the presence of l-glutamate receptors in the mucosa. Furthermore, because these receptors are G protein–coupled receptors, whose pathway involves phospholipase C activation, we studied the effect of a phospholipase C inhibitor, U73122, on the actions of l-glutamate in the duodenum. Pretreatment with U73122 (10 μmol/L) inhibited l-glutamate-induced cellular alkalinization and mucus secretion, which further suggests the involvement of l-glutamate receptors in the duodenal mucosa (47).
We note that the rise in intracellular pH caused by l-glutamate in the duodenum suggests that l-glutamate may stimulate HCO3− secretion because an increase in pHi precedes activated HCO3− secretion (48), although the secreted [HCO3−] and cellular [HCO3−] are not always correlated (48, 49). Luminal l-glutamate (10 mmol/L) had little effect on HCO3− secretion. Nevertheless, the addition of inosine 5′-monophosphate (IMP; 0.1 mmol/L) to l-glutamate synergistically increased HCO3− secretion, although IMP alone had a little effect. This result suggests that luminal l-glutamate activates epithelial l-glutamate receptors. l-glutamate with IMP reversed l-glutamate-induced cellular alkalinization, which is consistent with the reduction of cellular [HCO3−] by the stimulated HCO3− secretion. Furthermore, l-glutamate with IMP had no effect on the peak response in l-glutamate-induced mucus secretion but sustained the increased mucus gel thickness (47). Ongoing studies using selective agonists and antagonists of T1Rs and mGluR should identify which type of l-glutamate receptor is involved.
Because luminal l-glutamate enhances mucosal defense mechanisms, we hypothesize that luminal l-glutamate protects the mucosa from acid-induced injury. By using in vivo in situ propidium iodide staining (48), we have examined the effect of luminal l-glutamate (10 mmol/L) on supraphysiologic, pH 1.8 acid-induced epithelial injury in the duodenum. Perfusion with a pH 1.8 acid solution progressively increased the number of propidium-iodide-positive nuclei that corresponded to the injured cells, whereas preperfusion with l-glutamate inhibited the increased number of propidium-iodide-positive cells induced by pH 1.8 acid, which is consistent with enhanced mucosal defenses via luminal l-glutamate signaling (47). This result supports our hypothesis that luminal l-glutamate protects the mucosa from acid-induced injury in the gastrointestinal mucosa.
The role of luminal l-glutamate signaling in the gastroduodenum is still unclear. Because l-glutamate is the predominant amino acid in dietary proteins (50), l-glutamate may signal protein ingestion before protein digestion by the enzymes secreted by the stomach and pancreas (51). Only luminal l-glutamate among 20 amino acids activates vagal afferents in the rat stomach with the release of 5-HT and nitric oxide (45), a fact that supports such a hypothesis. In humans, the intestinal luminal concentration of l-glutamate after a protein meal is ≈2.6 mmol/L in the jejunum and ≈7.3 mmol/L in the ileum (51). This range of l-glutamate concentration in the bulk luminal solution may be high enough to produce the effects on mucosal defenses because perfusion of 1 mmol/L l-glutamate significantly increases pHi and mucus gel thickness in the duodenum (46). Applying food nutrients directly into the stomach, to eliminate the cephalic phase, differently stimulates acid secretion of the gastric phase. An intragastric peptone and amino acids mixture stimulates acid secretion and gastrin release (26, 27, 53), whereas luminal glucose, carbohydrate, or fat fails to affect acid secretion or gastrin release when eliminating its volume effect (26). Our findings that luminal l-glutamate enhances mucosal defenses and reduces acid-induced epithelial injury in the duodenum suggest that luminal l-glutamate signaling may precondition or prime the mucosa for subsequent acid exposure and protein digestion.
In conclusion, the upper gastrointestinal mucosa “tastes” luminal chemicals such as H+, CO2, and l-glutamate and in response enhances mucosal defense mechanisms through specific signaling cascades, including epithelial ion transporters, enzymes and receptors, and capsaicin-sensitive afferent nerves and the cyclooxygenase pathway. The result is the protection of the duodenal mucosa from acid injury. Understanding luminal chemosensory mechanisms may help to identify novel molecular targets for treating and preventing mucosal injury and abnormal visceral sensation. (Other articles in this supplement to the Journal include references 54–82.)
Acknowledgments
We thank Misa Mizumori, Maggie Ham, Chikako Watanabe, and Takanari Nakano for their research contributions.
The authors' responsibilities were as follows—YA: primary contributor to manuscript preparation and research data; and JDK: contributor to manuscript preparation and scientific support. YA's travel expenses associated with participation in the symposium were paid by the conference sponsor, the International Glutamate Technical Committee, a nongovernmental organization funded by industrial producers and users of glutamate in food. None of the authors had a conflict of interest related to this article.
REFERENCES
- 1.Allen A, Flemström G, Garner A, Kivilaakso E. Gastroduodenal mucosal protection. Physiol Rev 1993;73:823–57 [DOI] [PubMed] [Google Scholar]
- 2.Kaunitz JD, Akiba Y. Luminal acid elicits a protective duodenal mucosal response. Keio J Med 2002;51:29–35 [DOI] [PubMed] [Google Scholar]
- 3.Kaunitz JD, Akiba Y. Acid-sensing protective mechanisms of duodenum. J Physiol Pharmacol 2003;54:19–26 [PubMed] [Google Scholar]
- 4.Kaunitz JD, Akiba Y. Duodenal carbonic anhydrase: mucosal protection, luminal chemosensing, and gastric acid disposal. Keio J Med 2006;55:96–106 [DOI] [PubMed] [Google Scholar]
- 5.Kaunitz JD, Nishizaki Y, Kaneko K, Guth PH. Effect of orogastric nicotine on rat gastric mucosal gel thickness, surface cell viability, and intracellular pH. J Pharmacol Exp Ther 1993;265:948–54 [PubMed] [Google Scholar]
- 6.Nishizaki Y, Guth PH, Quintero E, Del Rivero M, Bover J, Kaunitz JD. Prostaglandin E2 enhances gastric defense mechanisms against acid injury in uremic rats. Gastroenterology 1994;107:1382–9 [DOI] [PubMed] [Google Scholar]
- 7.Tanaka S, Taché Y, Kaneko H, Guth PH, Kaunitz JD. Central vagal activation increases mucus gel thickness and surface cell intracellular pH in rat stomach. Gastroenterology 1997;112:409–17 [DOI] [PubMed] [Google Scholar]
- 8.Akiba Y, Kaunitz JD. Regulation of intracellular pH and blood flow in rat duodenal epithelium in vivo. Am J Physiol 1999;276:G293–302 [DOI] [PubMed] [Google Scholar]
- 9.Akiba Y, Guth PH, Engel E, Nastaskin I, Kaunitz JD. Acid-sensing pathways of rat duodenum. Am J Physiol 1999;277:G268–74 [DOI] [PubMed] [Google Scholar]
- 10.Akiba Y, Guth PH, Engel E, Nastaskin I, Kaunitz JD. Dynamic regulation of mucus gel thickness in rat duodenum. Am J Physiol Gastrointest Liver Physiol 2000;279:G437–47 [DOI] [PubMed] [Google Scholar]
- 11.Akiba Y, Furukawa O, Guth PH, Engel E, Nastaskin I, Kaunitz JD. Acute adaptive cellular base uptake in rat duodenal epithelium. Am J Physiol Gastrointest Liver Physiol 2001;280:G1083–92 [DOI] [PubMed] [Google Scholar]
- 12.Akiba Y, Furukawa O, Guth PH, Engel E, Nastaskin I, Kaunitz JD. Sensory pathways and cyclooxygenase regulate mucus gel thickness in rat duodenum. Am J Physiol Gastrointest Liver Physiol 2001;280:G470–4 [DOI] [PubMed] [Google Scholar]
- 13.Akiba Y, Nakamura M, Nagata H, Kaunitz JD, Ishii H. Acid-sensing pathways in rat gastrointestinal mucosa. J Gastroenterol 2002;37:133–8 [DOI] [PubMed] [Google Scholar]
- 14.Rune SJ, Henriksen FW. Carbon dioxide tensions in the proximal part of the canine gastrointestinal tract. Gastroenterology 1969;56:758–62 [PubMed] [Google Scholar]
- 15.Sugai N, Okamura H, Tsunoda R. Histochemical localization of carbonic anhydrase in the rat duodenal epithelium. Fukushima J Med Sci 1994;40:103–17 [PubMed] [Google Scholar]
- 16.Mizumori M, Meyerowitz J, Takeuchi T, et al. Epithelial carbonic anhydrases facilitate PCO2 and pH regulation in rat duodenal mucosa. J Physiol 2006;573:827–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akiba Y, Ghayouri S, Takeuchi T, et al. Carbonic anhydrases and mucosal vanilloid receptors help mediate the hyperemic response to luminal CO2 in rat duodenum. Gastroenterology 2006;131:142–52 [DOI] [PubMed] [Google Scholar]
- 18.Gamble JL, Ross SG. The factors in the dehydration following pyloric obstruction. J Clin Invest 1925;1:403–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rune SJ. The metabolic alkalosis following aspiration of gastric acid secretion. Scand J Clin Lab Invest 1965;17:305–10 [DOI] [PubMed] [Google Scholar]
- 20.Tanaka S, Chu S, Hirokawa M, Montrose MH, Kaunitz JD. Direct measurement of acid permeation into rat oesophagus. Gut 2003;52:775–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Malenstein H, Farre R, Sifrim D. Esophageal dilated intercellular spaces (DIS) and nonerosive reflux disease. Am J Gastroenterol 2008;103:1021–8 [DOI] [PubMed] [Google Scholar]
- 22.Abdulnour-Nakhoul S, Nakhoul NL, Wheeler SA, Wang P, Swenson ER, Orlando RC. HCO3− secretion in the esophageal submucosal glands. Am J Physiol Gastrointest Liver Physiol 2005;288:G736–44 [DOI] [PubMed] [Google Scholar]
- 23.Bass BL, Trad KS, Harmon JW, Hakki FZ. Capsaicin-sensitive nerves mediate esophageal mucosal protection. Surgery 1991;110:419–25 [PubMed] [Google Scholar]
- 24.Tominaga M, Caterina MJ, Malmberg AB, et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 1998;21:531–43 [DOI] [PubMed] [Google Scholar]
- 25.Akiba Y, Mizumori M, Kuo M, et al. CO2 chemosensing in rat oesophagus. Gut 2008;57:1654–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richardson CT, Walsh JH, Hicks MI, Fordtran JS. Studies on the mechanisms of food-stimulated gastric acid secretion in normal human subjects. J Clin Invest 1976;58:623–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feldman EJ, Grossman MI. Liver extract and its free amino acids equally stimulate gastric acid secretion. Am J Physiol 1980;239:G493–6 [DOI] [PubMed] [Google Scholar]
- 28.Manela FD, Ren J, Gao J, McGuigan JE, Harty RF. Calcitonin gene-related peptide modulates acid-mediated regulation of somatostatin and gastrin release from rat antrum. Gastroenterology 1995;109:701–6 [DOI] [PubMed] [Google Scholar]
- 29.Quinn SJ, Bai M, Brown EM. pH sensing by the calcium receptor. J Biol Chem 2004;279:37241–9 [DOI] [PubMed] [Google Scholar]
- 30.Sugiura T, Dang K, Lamb K, Bielefeldt K, Gebhart GF. Acid-sensing properties in rat gastric sensory neurons from normal and ulcerated stomach. J Neurosci 2005;25:2617–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allen A, Flemström G. Gastroduodenal mucus bicarbonate barrier: protection against acid and pepsin. Am J Physiol Gastrointest Liver Physiol 2005;288:C1–19 [DOI] [PubMed] [Google Scholar]
- 32.Raybould HE, Glatzle J, Freeman SL, et al. Detection of macronutrients in the intestinal wall. Auton Neurosci 2006;125:28–33 [DOI] [PubMed] [Google Scholar]
- 33.Lindemann B. Receptors and transduction in taste. Nature 2001;413:219–25 [DOI] [PubMed] [Google Scholar]
- 34.Dyer J, Salmon KS, Zibrik L, Shirazi-Beechey SP. Expression of sweet taste receptors of the T1R family in the intestinal tract and enteroendocrine cells. Biochem Soc Trans 2005;33:302–5 [DOI] [PubMed] [Google Scholar]
- 35.Mace OJ, Affleck J, Patel N, Kellett GL. Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J Physiol 2007;582:379–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Margolskee RF, Dyer J, Kokrashvili Z, et al. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci USA 2007;104:15075–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu SV, Rozengurt N, Yang M, Young SH, Sinnett-Smith J, Rozengurt E. Expression of bitter taste receptors of the T2R family in the gastrointestinal tract and enteroendocrine STC-1 cells. Proc Natl Acad Sci USA 2002;99:2392–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raybould HE. Nutrient tasting and signaling mechanisms in the gut. I. Sensing of lipid by the intestinal mucosa. Am J Physiol 1999;277:G751–5 [DOI] [PubMed] [Google Scholar]
- 39.Rozengurt E. Taste receptors in the gastrointestinal tract. I. Bitter taste receptors and alpha-gustducin in the mammalian gut. Am J Physiol Gastrointest Liver Physiol 2006;291:G171–7 [DOI] [PubMed] [Google Scholar]
- 40.Nelson G, Chandrashekar J, Hoon MA, et al. An amino-acid taste receptor. Nature 2002;416:199–202 [DOI] [PubMed] [Google Scholar]
- 41.Zhao GQ, Zhang Y, Hoon MA, et al. The receptors for mammalian sweet and umami taste. Cell 2003;115:255–66 [DOI] [PubMed] [Google Scholar]
- 42.Chaudhari N, Landin AM, Roper SD. A metabotropic glutamate receptor variant functions as a taste receptor. Nat Neurosci 2000;3:113–9 [DOI] [PubMed] [Google Scholar]
- 43.Toyono T, Seta Y, Kataoka S, Kawano S, Shigemoto R, Toyoshima K. Expression of metabotropic glutamate receptor group I in rat gustatory papillae. Cell Tissue Res 2003;313:29–35 [DOI] [PubMed] [Google Scholar]
- 44.San Gabriel AM, Maekawa T, Uneyama H, Yoshie S, Torii K. mGluR1 in the fundic glands of rat stomach. FEBS Lett 2007;581:1119–23 [DOI] [PubMed] [Google Scholar]
- 45.Uneyama H, Niijima A, San GA, Torii K. Luminal amino acid sensing in the rat gastric mucosa. Am J Physiol Gastrointest Liver Physiol 2006;291:G1163–70 [DOI] [PubMed] [Google Scholar]
- 46.Akiba Y, Mizumori M, Kaunitz JD. Luminal L-glutamate enhances gastroduodenal mucosal defenses in rats. Gastroenterology 2008;134:A137 [Google Scholar]
- 47.Akiba Y, Watanabe C, Kaunitz JD. Luminal L-glutamate enhances duodenal mucosal defense mechanisms via multi glutamate receptors in rat. Gastroenterology 2009;136:W1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Akiba Y, Furukawa O, Guth PH, et al. Cellular bicarbonate protects rat duodenal mucosa from acid-induced injury. J Clin Invest 2001;108:1807–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hirokawa M, Takeuchi T, Chu S, et al. Cystic fibrosis gene mutation reduces epithelial cell acidification and injury in acid-perfused mouse duodenum. Gastroenterology 2004;127:1162–73 [DOI] [PubMed] [Google Scholar]
- 50.Giacometti T. Free and bound glutamate in natural products. Filer LJ, Garattini S, Kare MR, Wurtman RJ, Glutamic acid: advances in biochemistry and physiology. New York, NY: Raven Press, 1979:25–34 [Google Scholar]
- 51.Uneyama H, Gabriel AS, Kawai M, Tomoe M, Torii K. Physiological role of dietary free glutamate in the food digestion. Asia Pac J Clin Nutr 2008;17(suppl 1):372–5 [PubMed] [Google Scholar]
- 52.Adibi SA, Mercer DW. Protein digestion in human intestine as reflected in luminal, mucosal, and plasma amino acid concentrations after meals. J Clin Invest 1973;52:1586–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lam SK, Isenberg JI, Grossman MI, Lane WH, Walsh JH. Gastric acid secretion is abnormally sensitive to endogenous gastrin released after peptone test meals in duodenal ulcer patients. J Clin Invest 1980;65:555–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fernstrom JD. Introduction to the symposium. Am J Clin Nutr 2009;90(suppl):705S–6S [DOI] [PubMed] [Google Scholar]
- 55.Krebs JR. The gourmet ape: evolution and human food preferences. Am J Clin Nutr 2009;90(suppl):707S–11S [DOI] [PubMed] [Google Scholar]
- 56.Curtis RI. Umami and the foods of classical antiquity. Am J Clin Nutr 2009;90(suppl):712S–8S [DOI] [PubMed] [Google Scholar]
- 57.Kurihara K. Glutamate: from discovery as a food flavor to role as a basic taste (umami). Am J Clin Nutr 2009;90(suppl):719S–22S [DOI] [PubMed] [Google Scholar]
- 58.Beauchamp GK. Sensory and receptor responses to umami: an overview of pioneering work. Am J Clin Nutr 2009;90(suppl):723S–7S [DOI] [PubMed] [Google Scholar]
- 59.Sano C. History of glutamate production. Am J Clin Nutr 2009;90(suppl):728S–32S [DOI] [PubMed] [Google Scholar]
- 60.Li X. T1R receptors mediate mammalian sweet and umami taste. Am J Clin Nutr 2009;90(suppl):733S–7S [DOI] [PubMed] [Google Scholar]
- 61.Chaudhari N, Pereira E, Roper SD. Taste receptors for umami: the case for multiple receptors. Am J Clin Nutr 2009;90(suppl):738S–42S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.San Gabriel A, Maekawa T, Uneyama H, Torii K. Metabotropic glutamate receptor type 1 in taste tissue. Am J Clin Nutr 2009;90(suppl):743S–6S [DOI] [PubMed] [Google Scholar]
- 63.Yasumatsu K, Horio N, Murata Y, et al. Multiple receptors underlie glutamate taste responses in mice. Am J Clin Nutr 2009;90(suppl):747S–52S [DOI] [PubMed] [Google Scholar]
- 64.Kinnamon SC. Umami taste transduction mechanisms. Am J Clin Nutr 2009;90(suppl):753S–5S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bachmanov AA, Inoue M, Ji H, Murata Y, Tordoff MG, Beauchamp GK. Glutamate taste and appetite in laboratory mice: physiologic and genetic analyses. Am J Clin Nutr 2009;90(suppl):756S–63S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shigemura N, Shirosaki S, Ohkuri T, et al. Variation in umami perception and in candidate genes for the umami receptor in mice and humans. Am J Clin Nutr 2009;90(suppl):764S–9S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen Q-Y, Alarcon S, Tharp A, et al. Perceptual variation in umami taste and polymorphisms in TAS1R taste receptor genes. Am J Clin Nutr 2009;90(suppl):770S–9S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mennella JA, Forestell CA, Morgan LK, Beauchamp GK. Early milk feeding influences taste acceptance and liking during infancy. Am J Clin Nutr 2009;90(suppl):780S–8S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Raliou M, Wiencis A, Pillias A-M, et al. Nonsynonymous single nucleotide polymorphisms in human tas1r1, tas1r3, and mGluR1 and individual taste sensitivity to glutamate. Am J Clin Nutr 2009;90(suppl):789S–99S [DOI] [PubMed] [Google Scholar]
- 70.Donaldson LF, Bennett L, Baic S, Melichar JK. Taste and weight: is there a link? Am J Clin Nutr 2009;90(suppl):800S–3S [DOI] [PubMed] [Google Scholar]
- 71.Rolls ET. Functional neuroimaging of umami taste: what makes umami pleasant? Am J Clin Nutr 2009;90(suppl):804S–13S [DOI] [PubMed] [Google Scholar]
- 72.Blachier F, Boutry C, Bos C, Tomé D. Metabolism and functions of l-glutamate in the epithelial cells of the small and large intestines. Am J Clin Nutr 2009;90(suppl):814S–21S [DOI] [PubMed] [Google Scholar]
- 73.Kokrashvili Z, Mosinger B, Margolskee RF. Taste signaling elements expressed in gut enteroendocrine cells regulate nutrient-responsive secretion of gut hormones. Am J Clin Nutr 2009;90(suppl):822S–5S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kondoh T, Mallick HN, Torii K. Activation of the gut-brain axis by dietary glutamate and physiologic significance in energy homeostasis. Am J Clin Nutr 2009;90(suppl):832S–7S [DOI] [PubMed] [Google Scholar]
- 75.Tomé D, Schwarz J, Darcel N, Fromentin G. Protein, amino acids, vagus nerve signaling, and the brain. Am J Clin Nutr 2009;90(suppl):838S–43S [DOI] [PubMed] [Google Scholar]
- 76.Yamamoto S, Tomoe M, Toyama K, Kawai M, Uneyama H. Can dietary supplementation of monosodium glutamate improve the health of the elderly? Am J Clin Nutr 2009;90(suppl):844S–9S [DOI] [PubMed] [Google Scholar]
- 77.Burrin DG, Stoll B. Metabolic fate and function of dietary glutamate in the gut. Am J Clin Nutr 2009;90(suppl):850S–6S [DOI] [PubMed] [Google Scholar]
- 78.Brosnan ME, Brosnan JT. Hepatic glutamate metabolism: a tale of 2 hepatocytes. Am J Clin Nutr 2009;90(suppl):857S–61S [DOI] [PubMed] [Google Scholar]
- 79.Stanley CA. Regulation of glutamate metabolism and insulin secretion by glutamate dehydrogenase in hypoglycemic children. Am J Clin Nutr 2009;90(suppl):862S–6S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hawkins RA. The blood-brain barrier and glutamate. Am J Clin Nutr 2009;90(suppl):867S–74S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Magistretti PJ. Role of glutamate in neuron-glia metabolic coupling. Am J Clin Nutr 2009;90(suppl):875S–80S [DOI] [PubMed] [Google Scholar]
- 82.Fernstrom JD. Symposium summary. Am J Clin Nutr 2009;90(suppl):881S–5S [DOI] [PubMed] [Google Scholar]