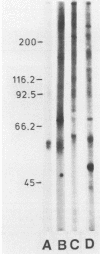
Abstract
We have compared the reactivities of antibodies developed by individuals frequently exposed to Plasmodium falciparum infections with the epitopes contained within the repeats of the circumsporozoite (CS) protein and their reactivities with the epitopes of a native molecule(s) accessible on the sporozoite surface. Results of direct-binding assays and competition assays between artificial and native molecules or between human antibodies and anti-CS monoclonal antibodies suggest that humans respond preferentially to epitopes not contained within the repeats of the CS protein and probably not contained in the whole CS protein. Human monoclonal antibodies reactive with P. falciparum sporozoite surface antigens were produced by Epstein-Barr virus transformation of human lymphocytes. Their pattern of reactivity with sporozoites from a number of different isolates indicates the existence of several distinct epitopes on the parasite surface. Differences between isolates and between sporozoites within a given sample were observed. No single human monoclonal antibody capable of detecting an epitope expressed in all the parasites studied was found.
Full text
PDF



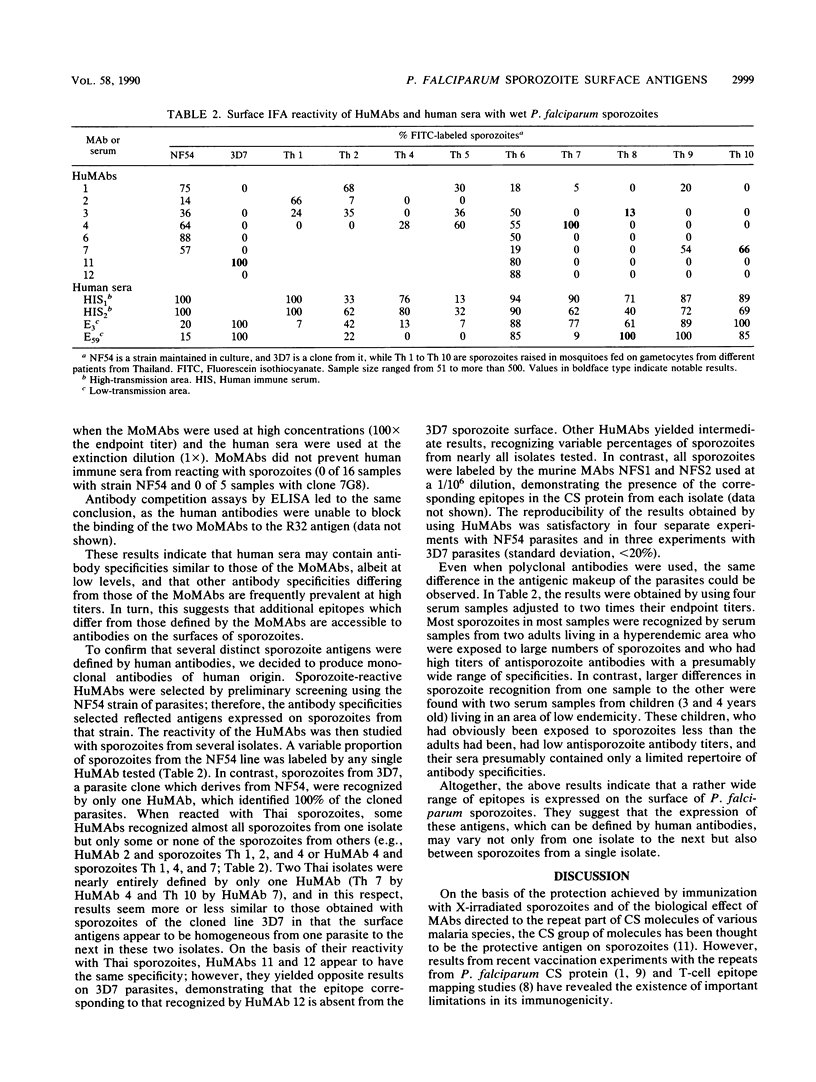


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballou W. R., Hoffman S. L., Sherwood J. A., Hollingdale M. R., Neva F. A., Hockmeyer W. T., Gordon D. M., Schneider I., Wirtz R. A., Young J. F. Safety and efficacy of a recombinant DNA Plasmodium falciparum sporozoite vaccine. Lancet. 1987 Jun 6;1(8545):1277–1281. doi: 10.1016/s0140-6736(87)90540-x. [DOI] [PubMed] [Google Scholar]
- Ballou W. R., Rothbard J., Wirtz R. A., Gordon D. M., Williams J. S., Gore R. W., Schneider I., Hollingdale M. R., Beaudoin R. L., Maloy W. L. Immunogenicity of synthetic peptides from circumsporozoite protein of Plasmodium falciparum. Science. 1985 May 24;228(4702):996–999. doi: 10.1126/science.2988126. [DOI] [PubMed] [Google Scholar]
- Charoenvit Y., Leef M. F., Yuan L. F., Sedegah M., Beaudoin R. L. Characterization of Plasmodium yoelii monoclonal antibodies directed against stage-specific sporozoite antigens. Infect Immun. 1987 Mar;55(3):604–608. doi: 10.1128/iai.55.3.604-608.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Giudice G., Verdini A. S., Pinori M., Pessi A., Verhave J. P., Tougne C., Ivanoff B., Lambert P. H., Engers H. D. Detection of human antibodies against Plasmodium falciparum sporozoites using synthetic peptides. J Clin Microbiol. 1987 Jan;25(1):91–96. doi: 10.1128/jcm.25.1.91-96.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desgranges C., Paire J., Pichoud C., Souche S., Frommel D., Trepo C. High affinity human monoclonal antibodies directed against hepatitis B surface antigen. J Virol Methods. 1987 Jul;16(4):281–292. doi: 10.1016/0166-0934(87)90013-9. [DOI] [PubMed] [Google Scholar]
- Druilhe P., Pradier O., Marc J. P., Miltgen F., Mazier D., Parent G. Levels of antibodies to Plasmodium falciparum sporozoite surface antigens reflect malaria transmission rates and are persistent in the absence of reinfection. Infect Immun. 1986 Aug;53(2):393–397. doi: 10.1128/iai.53.2.393-397.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good M. F., Berzofsky J. A., Maloy W. L., Hayashi Y., Fujii N., Hockmeyer W. T., Miller L. H. Genetic control of the immune response in mice to a Plasmodium falciparum sporozoite vaccine. Widespread nonresponsiveness to single malaria T epitope in highly repetitive vaccine. J Exp Med. 1986 Aug 1;164(2):655–660. doi: 10.1084/jem.164.2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good M. F., Pombo D., Quakyi I. A., Riley E. M., Houghten R. A., Menon A., Alling D. W., Berzofsky J. A., Miller L. H. Human T-cell recognition of the circumsporozoite protein of Plasmodium falciparum: immunodominant T-cell domains map to the polymorphic regions of the molecule. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1199–1203. doi: 10.1073/pnas.85.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrington D. A., Clyde D. F., Losonsky G., Cortesia M., Murphy J. R., Davis J., Baqar S., Felix A. M., Heimer E. P., Gillessen D. Safety and immunogenicity in man of a synthetic peptide malaria vaccine against Plasmodium falciparum sporozoites. Nature. 1987 Jul 16;328(6127):257–259. doi: 10.1038/328257a0. [DOI] [PubMed] [Google Scholar]
- Mazier D., Mellouk S., Beaudoin R. L., Texier B., Druilhe P., Hockmeyer W., Trosper J., Paul C., Charoenvit Y., Young J. Effect of antibodies to recombinant and synthetic peptides on P. falciparum sporozoites in vitro. Science. 1986 Jan 10;231(4734):156–159. doi: 10.1126/science.3510455. [DOI] [PubMed] [Google Scholar]
- Nussenzweig V., Nussenzweig R. S. Development of a sporozoite malaria vaccine. Am J Trop Med Hyg. 1986 Jul;35(4):678–688. doi: 10.4269/ajtmh.1986.35.678. [DOI] [PubMed] [Google Scholar]
- Quakyi I. A., Otoo L. N., Pombo D., Sugars L. Y., Menon A., De Groot A. S., Johnson A., Alling D., Miller L. H., Good M. F. Differential non-responsiveness in humans of candidate Plasmodium falciparum vaccine antigens. Am J Trop Med Hyg. 1989 Aug;41(2):125–134. [PubMed] [Google Scholar]
- Weber J. L., Hockmeyer W. T. Structure of the circumsporozoite protein gene in 18 strains of Plasmodium falciparum. Mol Biochem Parasitol. 1985 Jun;15(3):305–316. doi: 10.1016/0166-6851(85)90092-1. [DOI] [PubMed] [Google Scholar]
- Wirtz R. A., Zavala F., Charoenvit Y., Campbell G. H., Burkot T. R., Schneider I., Esser K. M., Beaudoin R. L., Andre R. G. Comparative testing of monoclonal antibodies against Plasmodium falciparum sporozoites for ELISA development. Bull World Health Organ. 1987;65(1):39–45. [PMC free article] [PubMed] [Google Scholar]
- Wortman A., Rogers P., Charoenvit Y., McDermott A., Leef M., Sedegah M., Beaudoin R. L. Cloning of Plasmodium yoelii genes expressing three different sporozoite-specific antigens. Microb Pathog. 1989 Mar;6(3):227–231. doi: 10.1016/0882-4010(89)90072-7. [DOI] [PubMed] [Google Scholar]
- Young J. F., Hockmeyer W. T., Gross M., Ballou W. R., Wirtz R. A., Trosper J. H., Beaudoin R. L., Hollingdale M. R., Miller L. H., Diggs C. L. Expression of Plasmodium falciparum circumsporozoite proteins in Escherichia coli for potential use in a human malaria vaccine. Science. 1985 May 24;228(4702):958–962. doi: 10.1126/science.2988125. [DOI] [PubMed] [Google Scholar]
- Zavala F., Masuda A., Graves P. M., Nussenzweig V., Nussenzweig R. S. Ubiquity of the repetitive epitope of the CS protein in different isolates of human malaria parasites. J Immunol. 1985 Oct;135(4):2790–2793. [PubMed] [Google Scholar]