Abstract
Glutamate concentrations in plasma are 50–100 μmol/L; in whole brain, they are 10,000–12,000 μmol/L but only 0.5–2 μmol/L in extracellular fluids (ECFs). The low ECF concentrations, which are essential for optimal brain function, are maintained by neurons, astrocytes, and the blood-brain barrier (BBB). Cerebral capillary endothelial cells form the BBB that surrounds the entire central nervous system. Tight junctions connect endothelial cells and separate the BBB into luminal and abluminal domains. Molecules entering or leaving the brain thus must pass 2 membranes, and each membrane has distinct properties. Facilitative carriers exist only in luminal membranes, and Na+-dependent glutamate cotransporters (excitatory amino acid transporters; EAATs) exist exclusively in abluminal membranes. The EAATs are secondary transporters that couple the Na+ gradient between the ECF and the endothelial cell to move glutamate against the existing electrochemical gradient. Thus, the EAATs in the abluminal membrane shift glutamate from the ECF to the endothelial cell where glutamate is free to diffuse into blood on facilitative carriers. This organization does not allow net glutamate entry to the brain; rather, it promotes the removal of glutamate and the maintenance of low glutamate concentrations in the ECF. This explains studies that show that the BBB is impermeable to glutamate, even at high concentrations, except in a few small areas that have fenestrated capillaries (circumventricular organs). Recently, the question of whether the BBB becomes permeable in diabetes has arisen. This issue was tested in rats with diet-induced obesity and insulin resistance or with streptozotocin-induced diabetes. Neither condition produced any detectable effect on BBB glutamate transport.
INTRODUCTION
The brain is insulated from the plasma by the blood-brain barrier (BBB), which surrounds the entire central nervous system including the spinal cord (Figure 1). The BBB is necessary to provide an optimal chemical environment for cerebral function. Several layers exist between blood and the brain: capillary endothelial cells, a basement membrane that completely covers the capillaries, and finally, astrocyte processes that enclose the basement membrane. Each of these layers could potentially restrict the movement of solutes (Figure 2).
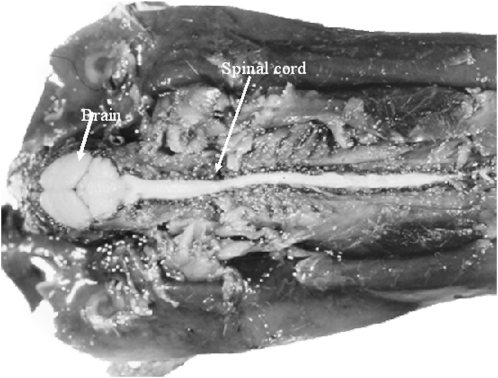
FIGURE 1.
The blood-brain barrier exists around the entire central nervous system. The photograph shows a mouse injected systemically with trypan blue (a dye that adheres to plasma proteins) and dissected from the dorsal side to reveal the central nervous system. Note that all structures throughout the body, except the central nervous system, take up the dye. Milton W Brightman and Thomas S Reese of the National Institute of Neurological Disorders and Stroke prepared the image, which was kindly provided by MW Brightman.
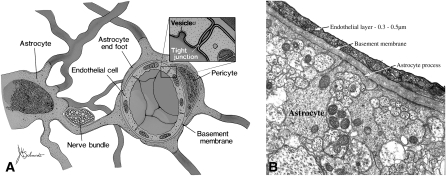
FIGURE 2.
Diagram of the blood-brain barrier. A: The blood-brain barrier exists at the level of the endothelial cells of cerebral capillaries. The endothelial cells are joined together by an extensive network of tight junctions. A basement membrane, within which pericytes reside, surrounds the endothelial cells as does a layer composed of astrocyte processes (so-called end feet). The pericytes are numerous and most likely function as phagocytes. The astrocyte layer serves as a metabolic barrier. For example, astrocytes incorporate NH4+ into glutamine and metabolize short-chain fatty acids. B: An electron micrograph of a cerebral capillary shows the basic elements (provided through the courtesy of Robert Page, Pennsylvania State University College of Medicine). Reproduced with permission from reference 1.
Endothelial cells were shown to be the site of the BBB in mammals (2–4) (Figure 3). The BBB has high electrical impedance (≈2000 Ω · cm2); therefore, even the passage of ions is restricted by the endothelial cell layer (5). Cerebral capillary endothelial cells differ from other mammalian capillary endothelial cells by having few cytoplasmic vesicles, more mitochondria (6), and a denser network of tight junctions between overlapping cells. The tight junctions restrict the movement of molecules between cells and also prevent membrane molecules from moving from one endothelial cell to another (7). Furthermore, the tight junctions divide the membranes of the endothelial cells into 2 discrete sides (8). Different populations of both lipids and intrinsic proteins (eg, transporters) exist in the luminal and abluminal membranes (9–11). Thus molecules must pass 2 sheaths of membrane to enter the brain. It is the combined characteristics of these 2 membrane domains that determine which particles traverse the barrier and how quickly.
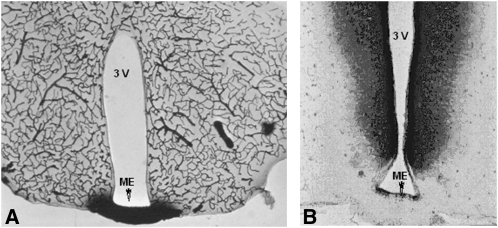
FIGURE 3.
A: Horseradish peroxidase injected into the general circulation. Note that the enzyme is restricted to capillaries except in the median eminence (ME), which has fenestrated capillaries. No horseradish peroxidase reaches any of the ventricles, including the third ventricle (3V). B: Horseradish peroxidase injected into the 3V. Note that the peroxidase readily penetrates the brain parenchyma, but not the area of the ME. It may be concluded that there is a barrier at the level of the epithelial lining of the ventricle that prevents leakage of metabolites into the ventricles. Milton W Brightman and Thomas S Reese of the National Institute of Neurological Disorders and Stroke prepared the image, which was kindly provided by MW Brightman.
Until recently, the BBB, at least with regard to metabolites, had been viewed as a passive system. The various facilitative transporters were considered to play a role in the regulation of brain metabolism through their ability to limit access (12). However, it was known that active transport of ions exists. Bicarbonate and other ions are actively secreted across the BBB (13, 14). Na+/K+-ATPase is present in the abluminal membrane, presumably to mediate blood-to-brain sodium flux. Mg++ and Ca++ ATPases exist in the BBB as well (15). Also, as mentioned, the BBB has a high capacity for energy production (6).
GLUTAMATE IN THE BRAIN AND THE CIRCULATION
Glutamate, a nonessential amino acid, is the most abundant free amino acid in the brain. In the central nervous system, glutamate functions as a neurotransmitter, as a link between the redox states of the pyridine nucleotides (NAD+ and NADP+), and as a fuel reserve. Glutamate metabolism is closely linked to the Krebs cycle. The reaction glutamate + NAD+ ↔ α-ketoglutarate + NADH + H+ is catalyzed by glutamate dehydrogenase. This mitochondrial reaction, which uses either NAD+ or NADP+ as cofactors, is believed to be at equilibrium and therefore serves to assure that the redox state of both pyridine nucleotides is similar (16). This is vital for those reactions that rely on NADPH (eg, isocitrate dehydrogenase) rather than NADH because it is NADH that is oxidized by the cytochrome system to produce energy.
Glutamate also serves as an important potential fuel reserve. The oxidation of glutamate to oxaloacetate yields 12 ATP per molecule of glutamate. Therefore, when the brain has insufficient glucose concentrations or glycolytic flux is reduced, the brain mobilizes glutamate as a fuel (17). In this regard, the energy available from glutamate is similar to glucose as a fuel reserve.
Compartmentalization of glutamate
In the brain, glutamate exists as a free amino acid divided between 2 separate metabolic compartments located in astrocytes and neurons. These compartments were first recognized in the brain on the basis of radioisotope precursor-product relations between glutamine and glutamate. To be incorporated into these amino acids from an oxidizable substrate, a label must first be converted into acetyl-coenzyme A (or succinyl-coenzyme A in the case of propionate) and then further oxidized in the Krebs cycle to α-ketoglutarate before exchanging with glutamate. Therefore, the specific radioactivity of glutamate is expected to exceed that of glutamine, as is the case with many substrates such as glucose, ketone bodies, lactate, and glycerol (18). However, several other oxidizable substrates, including some amino acids as well as short- to medium-chain-length fatty acids, label glutamine to a greater specific activity than glutamate (18–22). These results can be explained by the existence of ≥2 separate pools of Krebs cycle intermediates, one containing a large pool of glutamate with relatively little conversion to glutamine and the other containing a small glutamate pool that is rapidly metabolized to glutamine. On the basis of considerable evidence, these major pools can be assigned to the neurons and glial cells, respectively (19, 23–26). In support of this concept, compartmentation is almost absent at birth and develops in parallel with glial cells (26), and glutamine synthetase is found only in astrocytes (25).
Neuronal glutamate is contained in ≥2 pools, 1 composed of neuronal perikarya and dendrites and the 1 of nerve terminals (vesicles) (26). Nerve impulses trigger the release of glutamate from the presynaptic cell, which in turn binds to the glutamate receptors on the opposing postsynaptic cell. Neurotransmission is terminated by astrocytes and neurons that take up glutamate. Very little glutamate is believed to diffuse away from the synapse.
Excitotoxicity hypothesis of neuronal death
Early studies that used very high doses of glutamate, which were administered systemically, revealed brain damage in areas of the brain that were not protected by the BBB (27, 28). These studies led to the concept that neuronal death could be produced by overstimulation of excitatory amino acid receptors (29–31). Subsequently, this hypothesis became a popular explanation of the pathogenesis of neuronal death in a variety of acute conditions. However, in such cases, the source of glutamate arises from within the brain. For example, during an ischemic episode, release of glutamate (32, 33) from brain cells may result in an excessive concentration of glutamate in the extracellular fluid (ECF) (34, 35). The extreme excitation of neurons by glutamate in turn may result in the opening of receptor-coupled ionophores, of which calcium channels are of particular importance. A large influx of calcium associated with impaired intracellular calcium sequestration mechanisms, which activate a host of catabolic enzymes, may ultimately result in neuronal death (36). However, under normal conditions, plasma glutamate concentrations are stable and do not change appreciably unless raised by artificial means.
Glutamate in circulation
Plasma glutamate concentrations are in the range of 50–100 μmol/L in humans and other species (37). Even when relatively large quantities of monosodium glutamate have been added to the food of mice, monkeys, or humans, only very small changes in the plasma concentration of glutamate occur (38–41). This is because the intestinal mucosa preferentially metabolizes luminal glutamate and glutamine and uses their carbon skeletons as a source of energy (42, 43). Glutamine is also the primary amino acid by which nitrogen is carried from peripheral tissues to the splanchnic organs. Glutamine is deaminated by phosphate-dependent glutaminases in the intestinal mucosa to glutamate, which is then metabolized completely to produce energy. This process raises the ammonia in the hepatic portal vein to the degree that is optimal for the synthesis of urea (44).
FACILITATIVE AND ACTIVE TRANSPORT SYSTEMS FOR GLUTAMATE IN THE BBB
Early studies of the BBB that used whole-brain perfusions or animals in vivo identified facilitative transporters in the BBB membrane that are saturable and stereoselective (45–47). Because the substrate was presented to the capillary lumen, it was deduced that these transporters are present at least in the luminal membrane. On the other hand, it has been shown in several studies that glutamate does not enter the brain in material quantities, except in the circumventricular organs (48–50). Until recently, this has been a conundrum. Why should there be a transport system for an amino acid that is synthesized within the brain in large quantities? It required a different approach to the study of the BBB and new techniques to provide an explanation.
Studying each side of the BBB separately
The earlier models for studying the BBB in vivo gave incomplete information because metabolites have to pass both the luminal and the abluminal membranes to gain access to brain cells. In our laboratory, we studied both sides of the BBB by making use of isolated luminal and abluminal membranes obtained from fresh bovine brain.
The respective plasma membrane domain, when separated by the procedure developed by Betz et al (9, 10), demonstrated differences between the 2 sides of the BBB (polarity). We realized that both the luminal and the abluminal membranes form sealed spherical vesicles that are, for the most part, right side out and suitable for the study of transport in vitro (51, 52). The isolated membrane vesicles maintain their transport properties, and therefore they were used to characterize the contribution of each membrane domain to BBB activity. We found facilitative carriers for glutamate to exist exclusively in the luminal membranes and energy-dependent Na+-cotransporters in the abluminal membrane.
Facilitative transport of glutamate in the luminal membrane
Lee et al (53) measured facilitative glutamate transport separately in luminal and abluminal membranes and found that facilitative glutamate transport exists only in the luminal border in a position to allow the release of glutamate from endothelial cells to the plasma. Luminal carriers of amino acids have no dependence on Na+ gradients (46, 54–58) and are therefore energy independent. Three broad classes of facilitative carriers exist: large neutral amino acids, cationic amino acids, and acidic amino acids, and each transports several amino acids (59). As mentioned, the presence of a transporter for acidic amino acids with a high affinity and a low capacity (12, 60, 61) was an enigma for many years because both glutamate and aspartate are nonessential amino acids that are synthesized and accumulated in high concentrations in the brain.
Active transport systems expel glutamate from the ECF
Ordinarily, ECF glutamate is kept very low (≈ 0.5–2 μmol/L) (61). In fact, the concentration of glutamate and aspartate in cerebrospinal fluid is lower than that of any other amino acid group (Figure 4). The large gradient between brain cells and ECF is maintained by a family of Na+-dependent glutamate transporters known as excitatory amino acid transporters (EAATs). These transporters couple the steep Na+ gradient that normally exists between the ECF and brain cells. Currently, 5 members of the EAAT family have been identified (62, 63). They reside in the plasma membranes of astrocytes (35, 62, 64, 65), neurons (62, 66–70), and the BBB (71). The Na+-dependent transporters work at the limit of their ability to maintain the glutamate gradient between the brain cells and the ECF and, of course, the steep Na+ gradient as well (extracellular >> intracellular) that is maintained by Na+/K+-ATPase. If the oxygen supply is insufficient to maintain ATP concentrations, membrane Na+/K+-ATPase ceases to function. Under these circumstances, the Na+ gradient is dissipated and glutamate is released from both astrocytes and neurons by reversal of the EAAT family of transporters. If ECF glutamate rises, nerve cells may be damaged.
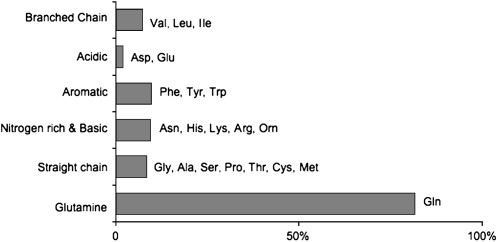
FIGURE 4.
Extracellular fluid (ECF) concentrations of amino acid groups compared with plasma. Note that the excitatory amino acids glutamate and aspartate are almost undetectable. Glutamine, which is believed to be nontoxic, has similar concentrations in the plasma and the ECF. Reproduced with permission from reference 1.
Of the 5 known Na+-dependent glutamate transporters (EAATs 1–5) (72), ≥3 exist in the abluminal membrane of the BBB (71, 73). They are voltage dependent and collectively have an apparent Km of 14 μmol/L at a transmembrane potential of −61 mV (53, 71). Western blot analysis confirmed that the glutamate transporters are present exclusively in the abluminal membranes; no EAATs were detectable in luminal membranes (71). Collectively, the EAAT family is the most powerful of the Na+-dependent amino acid transporters found in the abluminal membrane to date (1).
Current concept of glutamate transport across the BBB
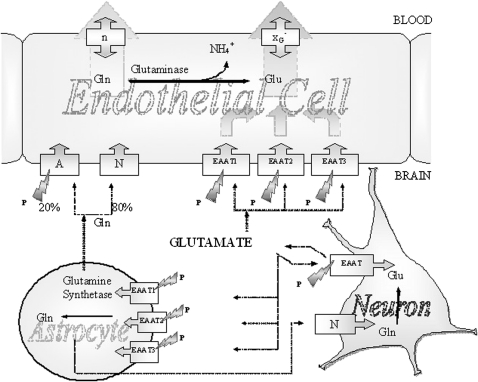
The current concept is that, when glutamate concentrations increase above optimal in the ECF, the abluminal membrane of the BBB pumps glutamate into the endothelial cells. The facilitative transport system in the luminal membrane allows glutamate egress to the circulation (Figure 5).
FIGURE 5.
Glutamate and glutamine transport between neurons, astrocytes, and endothelial cells of the blood-brain barrier. Glutamate is the most abundant excitatory neurotransmitter in the mammalian nervous system. At chemical synapses, glutamate is stored in vesicles. Nerve impulses trigger the release of glutamate from the presynaptic cell. Na+-dependent glutamate transporters (excitatory amino acid transporters; EAATs) are found in neuronal and glial membranes. These transporters play the important role of regulating concentrations of glutamate in the extracellular space, which maintains low concentrations. After glutamate is released as the result of an action potential, glutamate transporters quickly remove it from the extracellular space and thereby terminate the synaptic transmission. Without the activity of glutamate transporters, glutamate would build up and kill cells in a process called excitotoxicity, in which excessive amounts of glutamate act as a toxin to neurons. The activity of glutamate transporters also allows glutamate to be recycled. In cases of brain injury or oxygen insufficiency, the EAATs can work in reverse, and excess glutamate can accumulate outside cells, which rapidly halts neurotransmission. At least 3 EAATs are present in the abluminal membrane of the blood-brain barrier. These EAATs move glutamate into the endothelial cells from which egress is possible through the facilitative transporters in the luminal membrane. There are transporters capable of pumping glutamine from the extracellular fluid into endothelial cells, and glutaminase within endothelial cells may also hydrolyze glutamine to glutamate and NH4+. No carrier is necessary for NH4+, which may diffuse as NH3. A, Na+-dependent system A; N, Na+-dependent system N; XG–, facilitative glutamate transporter; n, facilitative glutamine transporter. The lightning symbols indicate Na+ dependence, and P indicates stimulation by pyroglutamate. Reproduced with permission from reference 1.
The organization of the BBB explains why various investigators have found glutamate entry into the brain is slow or almost undetectable (48–50, 61). Glutamate may enter the endothelial cells, but net movement of glutamate from endothelial cells to the brain is almost impossible. This is a consequence of the steep Na+ gradient that powers the EAAT family of glutamate transporters at the border between the ECF and the abluminal membrane of the endothelial cells. Because of this organization, the BBB is virtually impermeable to the net movement of glutamate from circulation into the brain.
GLUTAMATE, GLUTAMINE, AND AMMONIA REMOVAL
The description of the organization of the BBB also provides an explanation for a long-standing puzzle regarding brain NH4+ metabolism. Various measurements have shown that 20–50% of the NH4+ circulating through the blood vessels in the brain passes the BBB and is incorporated into the amide group of glutamine by astrocytes (23, 24). It is curious, however, that it has not been possible to consistently measure arteriovenous differences of NH4+ (24). If there were no mechanism for the removal of glutamine, it would accumulate in brain, thereby raising the osmolarity and causing swelling. The situation is now clearer. Glutamine and glutamate are pumped from the ECF into endothelial cells. Glutamine is at least partially metabolized to NH4+ and glutamate. The remaining glutamine as well as NH4+ and glutamate are free to diffuse across the luminal membrane into the blood (53, 71). Thus the rate of NH4+ uptake and release are balanced.
This new knowledge also explains how the entry of glutamine and glutamate into the central nervous system is restricted even though carrier activities for both amino acids have been described (49, 59, 74). Glutamine and glutamate can traverse the luminal membrane in facilitative systems. However, movement into the brain across the abluminal membrane is small because of the lack of facilitative carriers in the abluminal membrane. Furthermore, the 3 Na+-dependent carriers in the abluminal membrane that are driven by the steep Na+ gradient that exists between the brain ECF and the cell interior forcefully oppose glutamate entry and promote its removal from the brain.
The BBB seems to be arranged in such a manner as to not only restrict the entry of glutamine and glutamate into brain but also to actively export these amino acids and NH4+ to the circulation. Therefore, the BBB participates in the regulation of brain nitrogen metabolism and protects against the development of neurotoxicity by preventing the accumulation of glutamate as well as the accumulation of NH4+.
≫-Glutamyl cycle and the transport of amino acids across the BBB: the role of pyroglutamate in regulation
Meister et al (75, 76) noted that γ-glutamyl transpeptidase is present in tissues that were believed to actively transport amino acids. Such tissues include the brush border of the proximal convoluted tubules of the kidney (77), lactating mammary glands (78), the apical portion of the intestinal epithelium (79), the choroid plexus (80), and the BBB (81). However, the BBB differs from most tissues that actively transport amino acids. First, the BBB is composed of endothelial, not epithelial, cells. Second, there are no Na+-dependent amino acid transporters on the luminal surface of the BBB. Therefore, the BBB is not associated with energy-dependent amino acid uptake from plasma. Instead, the organization of the BBB provides a mechanism for active removal of glutamate, glutamine, and other amino acids. It has therefore been puzzling that brain capillaries have such high γ-glutamyl transpeptidase activity. γ-Glutamyl transpeptidase is the first enzyme in the γ-glutamyl cycle that produces pyroglutamate (pGlu) within the cell (82, 83) (also known as oxoproline—the cyclized amide of glutamate). pGlu can stimulate Na+-dependent glutamate transport at the BBB. It has been suggested that the generation of pGlu is part of a control mechanism that influences the concentration of glutamate in the brain ECF. This activity may be part of a short-term regulatory mechanism by which the removal of this potentially deleterious glutamate is accelerated. Furthermore, it suggests that EAAT transporters in the BBB may serve as a therapeutic target in circumstances where glutamate cytotoxicity occurs, eg, with ischemia or brain injury. Stimulation of EAAT transporters by pGlu or comparable molecules during reperfusion may accelerate the restoration of glutamate homeostasis and reduce the development of cerebral infarcts.
Diabetes does not affect glutamate transport at the BBB
A recent conference report on the use of glutamate in food suggested that, although addition of glutamate salts to foods may be considered harmless to the general population, this may not be the case in individuals in whom the BBB may be impaired, such as in patients with diabetes (84). We studied the possibility that the BBB permeability of glutamate is changed by diabetes in 2 rat models: 1) a model of diet-induced obesity that causes insulin resistance (85, 86), a mild form of diabetes, and 2) a model of a more severe form of diabetes, induced by streptozotocin, which destroys most of the pancreatic β cells and causes chronic hyperglycemia and ketonemia. Both forms showed no change in the BBB; that is, there was no evidence of increased permeability (RA Hawkins, A Mokashi, J Viña, and J Fernstrom, unpublished observations, 2008).
CONCLUSIONS
The current concept of the BBB is that cerebral endothelial cells are not simply passive barriers; rather, they participate actively in regulating the composition of the brain ECF. The abluminal and luminal membranes seem to be working in a complementary fashion with, for the most part, active transport occurring at the abluminal membrane and facilitative transport at the luminal membrane. The abluminal membrane is in direct contact with the ECF and has Na+-dependent transport systems and a Na+ gradient that can move metabolites out of the ECF against a concentration gradient. The luminal membrane primarily has facilitative transport systems that allow molecules to enter and exit the endothelial cells. (Other articles in this supplement to the Journal include references 87–115.)
Acknowledgments
I acknowledge Mary Regina DeJoseph and Ashwini Mokashi for their skilled technical assistance. I am grateful to the following individuals for generously sharing their ideas and advice: John Fernstrom, Juan Viña, Barry Levin, Darryl Peterson, Ian Simpson, and Andrew Ebert.
The author's travel expenses associated with participation in the symposium and an honorarium were paid by the conference sponsor, the International Glutamate Technical Committee, a nongovernmental organization funded by industrial producers and users of glutamate in food. There were no conflicts of interest in the material presented in this article.
REFERENCES
- 1.Hawkins RA, O'Kane RL, Simpson IA, Vina JR. Structure of the blood-brain barrier and its role in the transport of amino acids. J Nutr 2006;136:218S–26S [DOI] [PubMed] [Google Scholar]
- 2.Reese TS, Karnovsky MJ. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J Cell Biol 1967;34:207–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brightman MW, Reese TW. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol 1969;40:648–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brightman MW, Reese TS, Feder N. Assesment with the electron microscope of the permeability to peroxidase of cerebral endothelium and epithelium in mice and sharks. Crone C, Lassen NA, Capillary permeability: the transfer of molecules and ions between capillary blood and tissue. New York, NY: Academic Press, 1970:468–76 [Google Scholar]
- 5.Crone C, Olesen SP. Electrical resistance of brain microvascular endothelium. Brain Res 1982;241:49–55 [DOI] [PubMed] [Google Scholar]
- 6.Oldendorf WH, Brown WJ. Greater number of capillary endothelial cell mitochondria in brain than in muscle. Proc Soc Exp Biol Med 1975;149:736–8 [DOI] [PubMed] [Google Scholar]
- 7.van Meer G, Gumbiner B, Simons K. The tight junction does not allow lipid molecules to diffuse from one epithelial cell to the next. Nature 1986;322:639–41 [DOI] [PubMed] [Google Scholar]
- 8.van Meer G, Simons K. The function of tight junctions in maintaining differences in lipid composition between the apical and the basolateral cell surface domains of MDCK cells. EMBO J 1986;5:1455–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Betz AL, Goldstein GW. Polarity of the blood-brain barrier: neutral amino acid transport into isolated brain capillaries. Science 1978;202:225–7 [DOI] [PubMed] [Google Scholar]
- 10.Betz AL, Firth JA, Goldstein GW. Polarity of the blood-brain barrier: distribution of enzymes between the luminal and antiluminal membranes of brain capillary endothelial cells. Brain Res 1980;192:17–28 [DOI] [PubMed] [Google Scholar]
- 11.Tewes BJ, Galla H-J. Lipid polarity in brain capillary endothelial cells. Endothelium 2001;8:207–20 [DOI] [PubMed] [Google Scholar]
- 12.Pardridge WM. Brain metabolism: a perspective from the blood-brain barrier. Physiol Rev 1983;63:1481–535 [DOI] [PubMed] [Google Scholar]
- 13.Bradbury M. The concept of a blood-brain barrier. New York, NY: John Wiley & Sons, 1979 [Google Scholar]
- 14.Pappenheimer JR. Osmotic reflection coefficients in capillary membranes. Crone C, Lassen NA, Capillary permeability: the transfer of molecules and ions between capillary blood and tissue. Copenhagen, Denmark: Munksgaard, 1970:454–8 [Google Scholar]
- 15.Vorbrodt AW. Ultrastructural cytochemistry of blood-brain barrier endothelia. Prog Histochem Cytochem 1988;18:1–99 [DOI] [PubMed] [Google Scholar]
- 16.Krebs HA, Veech RL. Equilibrium relations between pyridine nucleotides and adenine nucleotides and their roles in the regulation of metabolic processes. Adv Enzyme Regul 1969;7:397–413 [DOI] [PubMed] [Google Scholar]
- 17.Miller AL, Hawkins RA, Veech RL. Decreased rate of glucose utilization by rat brain in vivo after exposure to atmospheres containing high concentrations of CO2. J Neurochem 1975;25:553–8 [DOI] [PubMed] [Google Scholar]
- 18.O'Neal RM, Koeppe RE. Precursors in vivo of glutamate, aspartate and their derivatives of rat brain. J Neurochem 1966;13:835–47 [DOI] [PubMed] [Google Scholar]
- 19.Balazs R, Patel AJ, Richter D. Metabolic compartmentation in the brain: their properties and relation to morphological structures. Balazs R, Cremer JE, Metabolic compartmentation in the brain. New York, NY: John Wiley & Sons, 1972:167–86 [Google Scholar]
- 20.Cremer JE, Heath DF, Patel AJ, Balazs R, Cavanagh JB. An experimental model of CNS change associated woth chronic liver disease: portocaval anastomosis in the rat. Berl S, Clarke DD, Schneider D, Metabolic compartmentation and neurotransmission: relation to brain structure and function. New York, NY: Plenum Press, 1975:461–78 [Google Scholar]
- 21.Cremer JE, Teal HM, Heath DF, Cavanagh JB. The influence of portocaval anastomosis on the metabolism of labelled octanoate, butyrate and leucine in rat brain. J Neurochem 1977;28:215–22 [DOI] [PubMed] [Google Scholar]
- 22.Van den Berg CJ, Ronda G, Reijnierse GLA, et al. A model of glutamate metabolism in brain: a biochemical analysis of a heterogeneous structure. Berl S, Clarke DD, Schneider D, Metabolic compartmentation and neurotransmission: relation to brain structure and function. New York, NY: Plenum Press, 1975:515–43 [Google Scholar]
- 23.Cooper AJ, McDonald JM, Gelbard AS, Gledhill RF, Duffy TE. The metabolic fate of 13N-labeled ammonia in rat brain. J Biol Chem 1979;254:4982–92 [PubMed] [Google Scholar]
- 24.Cooper AJ, Plum F. Biochemistry and physiology of brain ammonia. Physiol Rev 1987;67:440–519 [DOI] [PubMed] [Google Scholar]
- 25.Martinez-Hernandez A, Bell KP, Norenberg MD. Glutamine synthetase: glial localization in brain. Science 1977;195:1356–8 [DOI] [PubMed] [Google Scholar]
- 26.Balazs R, Machiyama Y, Patel AJ. Compartmentation and the metabolism of g-aminobutyrate. Balazs R, Cremer JE, Metabolic compartmentation in the brain. New York, NY: John Wiley & Sons, 1972:57–70 [Google Scholar]
- 27.Price MT, Olney JW, Lowry OH, Buchsbaum S. Uptake of exogenous glutamate and aspartate by circumventricular organs but not other regions of brain. J Neurochem 1981;36:1774–80 [DOI] [PubMed] [Google Scholar]
- 28.Olney JW, Sharpe LG. Brain lesions in an infant rhesus monkey treated with monosodium glutamate. Science 1969;166:386–8 [DOI] [PubMed] [Google Scholar]
- 29.Albin RL, Greenamyre JT. Alternative excitotoxic hypotheses. Neurology 1992;42:733–8 [DOI] [PubMed] [Google Scholar]
- 30.Kirino T. [Neuronal degeneration and glutamate] Rinsho Shinkeigaku 1989;29:1522–5 [PubMed] [Google Scholar]
- 31.Schwarcz R, Foster AC, French ED, Whetsell WO, Jr, Kohler C. Excitotoxic models for neurodegenerative disorders. Life Sci 1984;35:19–32 [DOI] [PubMed] [Google Scholar]
- 32.Castillo J, Davalos A, Naveiro J, Noya M. Neuroexcitatory amino acids and their relation to infarct size and neurological deficit in ischemic stroke. Stroke 1996;27:1060–5 [DOI] [PubMed] [Google Scholar]
- 33.Choi DW, Maulucci-Gedde M, Kriegstein AR. Glutamate neurotoxicity in cortical cell culture. J Neurosci 1987;7:357–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin RL, Lloyd HG, Cowan AI. The early events of oxygen and glucose deprivation: setting the scene for neuronal death? Trends Neurosci 1994;17:251–7 [DOI] [PubMed] [Google Scholar]
- 35.Rothstein JD, Dykes-Hoberg M, Pardo C, et al. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron 1996;16:675–86 [DOI] [PubMed] [Google Scholar]
- 36.Benveniste H, Drejer J, Schousboe A, Diemer NH. Elevations of the extracellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebral microdialysis. J Neurochem 1984;43:1369–74 [DOI] [PubMed] [Google Scholar]
- 37.Siegal GJ, Agranoff BW, Albers RW, Fisher SK, Uhler MD. Basic neurochemistry. 6th ed Philadelphia, PA: Lippincott-Raven, 1998 [Google Scholar]
- 38.Stegink LD, Filer LJ, Jr, Baker GL. Plasma and erythrocyte amino acid levels in normal adult subjects fed a high protein meal with and without added monosodium glutamate. J Nutr 1982;112:1953–60 [DOI] [PubMed] [Google Scholar]
- 39.Stegink LD, Filer LJ Jr, Baker GL. Plasma amino acid concentrations in normal adults fed meals with added monosodium L-glutamate and aspartame. J Nutr 1983;113:1851–60 [DOI] [PubMed] [Google Scholar]
- 40.Stegink LD, Filer LJ Jr, Baker GL. Plasma glutamate concentrations in adult subjects ingesting monosodium L-glutamate in consomme. Am J Clin Nutr 1985;42:220–5 [DOI] [PubMed] [Google Scholar]
- 41.Tsai PJ, Huang PC. Circadian variations in plasma and erythrocyte glutamate concentrations in adult men consuming a diet with and without added monosodium glutamate. J Nutr 2000;130:1002S–4S [DOI] [PubMed] [Google Scholar]
- 42.Hanson PJ, Parsons DS. Transport and metabolism of glutamine and glutamate in the small intestine. Kvamme E, Glutamine and glutamate in mammals. Boca Raton, FL: CRC Press Inc, 1988:235–53 [Google Scholar]
- 43.Windmueller HG. Metabolism of vascular and luminal glutamine by intestinal mucosa in vivo. Gayssubgerm D, Sies H, Glutamine metabolism in mammalian tissues. Berlin, Germany: Springer-Verlag, 1984:61–77 [Google Scholar]
- 44.Sies H, Haüssinger D. Hepatic glutamine and ammonia metabolism: nitrogen redox balance and the intracellular glutamine cycle. Haüssinger D, Sies H, Glutamine metabolism in mammalian tissues. New York, NY: Springer-Verlag, 1984:78–97 [Google Scholar]
- 45.Oldendorf WH. Measurement of brain uptake of radiolabeled substances using a tritiated water internal standard. Brain Res 1970;24:372–6 [DOI] [PubMed] [Google Scholar]
- 46.Oldendorf WH. Uptake of radiolabeled essential amino acids by brain following arterial injection. Proc Soc Exp Biol Med 1971;136:385–6 [DOI] [PubMed] [Google Scholar]
- 47.Oldendorf WH. Brain uptake of radiolabeled amino acids, amines, and hexoses after arterial injection. Am J Physiol 1971;221:1629–39 [DOI] [PubMed] [Google Scholar]
- 48.Drewes LR, Conway WP, Gilboe DD. Net amino acid transport between plasma and erythrocytes and perfused dog brain. Am J Physiol 1977;233:E320–5 [DOI] [PubMed] [Google Scholar]
- 49.Hawkins RA, DeJoseph MR, Hawkins PA. Regional brain glutamate transport in rats at normal and raised concentrations of circulating glutamate. Cell Tissue Res 1995;281:207–14 [DOI] [PubMed] [Google Scholar]
- 50.Vina JR, DeJoseph MR, Hawkins PA, Hawkins RA. Penetration of glutamate into brain of 7-day-old rats. Metab Brain Dis 1997;12:219–27 [PubMed] [Google Scholar]
- 51.Sánchez del Pino MM, Hawkins RA, Peterson DR. Biochemical discrimination between luminal and abluminal enzyme and transport activities of the blood-brain barrier. J Biol Chem 1995;270:14907–12 [DOI] [PubMed] [Google Scholar]
- 52.Sánchez del Pino M, Hawkins RA, Peterson DR. Neutral amino acid transport by the blood-brain barrier: membrane vesicle studies. J Biol Chem 1992;267:25951–7 [PubMed] [Google Scholar]
- 53.Lee WJ, Hawkins RA, Viña JR, Peterson DR. Glutamine transport by the blood-brain barrier: a possible mechanism for nitrogen removal. Am J Physiol 1998;274:C1101–7 [DOI] [PubMed] [Google Scholar]
- 54.Battistin L, Grynbaum A, Lajtha A. The uptake of various amino acids by the mouse brain in vivo. Brain Res 1971;29:85–99 [DOI] [PubMed] [Google Scholar]
- 55.Christensen HN. Developments in amino acid transport, illustrated for the blood-brain barrier. Biochem Pharmacol 1979;28:1989–92 [DOI] [PubMed] [Google Scholar]
- 56.Sershen H, Lajtha A. Capillary transport of amino acids in the developing brain. Exp Neurol 1976;53:465–74 [DOI] [PubMed] [Google Scholar]
- 57.Smith QR, Stoll J. Blood-brain barrier amino acid transport. In: Pardridge WM, ed Introduction to the blood-brain barrier: methodology, biology, and pathology. Cambridge, United Kingdom: Cambridge University Press, 1998:188–97 [Google Scholar]
- 58.Schain RJ, Watanabe KS. Distinct patterns of entry of two non-metabolizable amino acids into brain and other organs of infant guinea pigs. J Neurochem 1972;19:2279–88 [DOI] [PubMed] [Google Scholar]
- 59.Oldendorf WH, Szabo J. Amino acid assignment to one of three blood-brain barrier amino acid carriers. Am J Physiol 1976;230:94–8 [DOI] [PubMed] [Google Scholar]
- 60.Benrabh H, Lefauconnier JM. Glutamate is transported across the rat blood-brain barrier by a sodium-independent system. Neurosci Lett 1996;210:9–12 [DOI] [PubMed] [Google Scholar]
- 61.Smith QR. Transport of glutamate and other amino acids at the blood-brain barrier. J Nutr 2000;130:1016S–22S [DOI] [PubMed] [Google Scholar]
- 62.Meldrum BS. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr 2000;130:1007S–15S [DOI] [PubMed] [Google Scholar]
- 63.Eliasof S, Arriza J, Leighton B, Amara S, Kavanaugh M. Localization and function of five glutamate transporters cloned from the salamander retina. Vision Res 1998;38:1443–54 [DOI] [PubMed] [Google Scholar]
- 64.Rothstein JD, Martin L, Levey AI, et al. Localization of neuronal and glial glutamate transporters. Neuron 1994;13:713–25 [DOI] [PubMed] [Google Scholar]
- 65.Swanson RA, Liu J, Miller J, et al. Neuronal regulation of glutamate transporter subtype expression in astrocytes. J Neurosci 1997;17:932–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kanai Y, Hediger MA. Primary structure and functional characterization of a high-affinity glutamate transporter. Nature 1992;360:467–71 [DOI] [PubMed] [Google Scholar]
- 67.Kanai Y, Hediger MA. The glutamate/neutral amino acid transporter family SLC1: molecular, physiological and pharmacological aspects. Pflugers Arch 2004;447:469–79 [DOI] [PubMed] [Google Scholar]
- 68.Velaz-Faircloth M, McGraw TS, Alandro MS, Fremeau RT, Jr, Kilberg MS, Anderson KJ. Characterization and distribution of the neuronal glutamate transporter EAAC1 in rat brain. Am J Physiol 1996;270:C67–75 [DOI] [PubMed] [Google Scholar]
- 69.Attwell D. Brain uptake of glutamate: food for thought. J Nutr 2000;130:1023S–5S [DOI] [PubMed] [Google Scholar]
- 70.Lehre KP, Levy LM, Ottersen OP, Storm-Mathisen J, Danbolt NC. Differential expression of two glial glutamate transporters in the rat brain: quantitative and immunocytochemical observations. J Neurosci 1995;15:1835–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.O'Kane RL, Martinez-Lopez I, DeJoseph MR, Viña JR, Hawkins RA. Na(+)-dependent glutamate transporters (EAAT1, EAAT2, and EAAT3) of the blood-brain barrier: a mechanism for glutamate removal. J Biol Chem 1999;274:31891–5 [DOI] [PubMed] [Google Scholar]
- 72.Shigeri Y, Seal RP, Shimamoto K. Molecular pharmacology of glutamate transporters, EAATs and VGLUTs. Brain Res Brain Res Rev 2004;45:250–65 [DOI] [PubMed] [Google Scholar]
- 73.Hutchison HT, Eisenberg HM, Haber B. High-affinity transport of glutamate in rat brain microvessels. Exp Neurol 1985;87:260–9 [DOI] [PubMed] [Google Scholar]
- 74.Smith QR, Momma S, Aoyagi M, Rapoport SI. Kinetics of neutral amino acid transport across the blood-brain barrier. J Neurochem 1987;49:1651–8 [DOI] [PubMed] [Google Scholar]
- 75.Meister A. Transport and metabolism of glutathione and gamma-glutamyl amino acids. Biochem Soc Trans 1983;11:793–4 [DOI] [PubMed] [Google Scholar]
- 76.Meister A, Anderson ME. Glutathione. Annu Rev Biochem 1983;52:711–60 [DOI] [PubMed] [Google Scholar]
- 77.Curto KA, Sweeney WE, Avner ED, Piesco NP, Curthoys NP. Immunocytochemical localization of gamma-glutamyl transpeptidase during fetal development of mouse kidney. J Histochem Cytochem 1988;36:159–66 [DOI] [PubMed] [Google Scholar]
- 78.Viña JR, Palacin M, Puertes IR, Hernandez R, Viña J. Role of the g-glutamyl cycle in the regulation of amino acid translocation. Am J Physiol 1989;257:E916–22 [DOI] [PubMed] [Google Scholar]
- 79.Garvey TQ, Hyman PE, Isselbacher KJ. Gamma-glutamyl transpeptidase of rat intestine: localization and possible role in amino acid transport. Gastroenterology 1976;71:778–85 [PubMed] [Google Scholar]
- 80.Anderson ME, Underwood M, Bridges RJ, Meister A. Glutathione metabolism at the blood-cerebrospinal fluid barrier. FASEB J 1989;3:2527–31 [DOI] [PubMed] [Google Scholar]
- 81.Orlowski M, Sessa G, Green JP. Gamma-glutamyl transpeptidase in brain capillaries: possible site of a blood-brain barrier for amino acids. Science 1974;184:66–8 [DOI] [PubMed] [Google Scholar]
- 82.Orlowski M, Meister A. The g-glutamyl cycle: a possible transport system for amino acids. Proc Natl Acad Sci USA 1970;67:1248–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meister A. On the enzymology of amino acid transport. Science 1973;180:33–9 [DOI] [PubMed] [Google Scholar]
- 84.Beyreuther K, Biesalski HK, Fernstrom JD, et al. Consensus meeting: monosodium glutamate—an update. Eur J Clin Nutr 2007;61:304–13 [DOI] [PubMed] [Google Scholar]
- 85.Levin BE, Magnan C, Migrenne S, Chua SC, Jr, Dunn-Meynell AA. F-DIO obesity-prone rat is insulin resistant before obesity onset. Am J Physiol Regul Integr Comp Physiol 2005;289:R704–11 [DOI] [PubMed] [Google Scholar]
- 86.Levin BE, Dunn-Meynell AA, McMinn JE, Alperovich M, Cunningham-Bussel A, Chua SC., Jr A new obesity-prone, glucose-intolerant rat strain (F.DIO). Am J Physiol Regul Integr Comp Physiol 2003;285:R1184–91 [DOI] [PubMed] [Google Scholar]
- 87.Fernstrom JD. Introduction to the symposium. Am J Clin Nutr 2009;90(suppl):705S–6S [DOI] [PubMed] [Google Scholar]
- 88.Krebs JR. The gourmet ape: evolution and human food preferences. Am J Clin Nutr 2009;90(suppl):707S–11S [DOI] [PubMed] [Google Scholar]
- 89.Curtis RI. Umami and the foods of classical antiquity. Am J Clin Nutr 2009;90(suppl):712S–8S [DOI] [PubMed] [Google Scholar]
- 90.Kurihara K. Glutamate: from discovery as a food flavor to role as a basic taste (umami). Am J Clin Nutr 2009;90(suppl):719S–22S [DOI] [PubMed] [Google Scholar]
- 91.Beauchamp GK. Sensory and receptor responses to umami: an overview of pioneering work. Am J Clin Nutr 2009;90(suppl):723S–7S [DOI] [PubMed] [Google Scholar]
- 92.Sano C. History of glutamate production. Am J Clin Nutr 2009;90(suppl):728S–32S [DOI] [PubMed] [Google Scholar]
- 93.Li X. T1R receptors mediate mammalian sweet and umami taste. Am J Clin Nutr 2009;90(suppl):733S–7S [DOI] [PubMed] [Google Scholar]
- 94.Chaudhari N, Pereira E, Roper SD. Taste receptors for umami: the case for multiple receptors. Am J Clin Nutr 2009;90(suppl):738S–42S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.San Gabriel A, Maekawa T, Uneyama H, Torii K. Metabotropic glutamate receptor type 1 in taste tissue. Am J Clin Nutr 2009;90(suppl):743S–6S [DOI] [PubMed] [Google Scholar]
- 96.Yasumatsu K, Horio N, Murata Y, et al. Multiple receptors underlie glutamate taste responses in mice. Am J Clin Nutr 2009;90(suppl):747S–52S [DOI] [PubMed] [Google Scholar]
- 97.Kinnamon SC. Umami taste transduction mechanisms. Am J Clin Nutr 2009;90(suppl):753S–5S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bachmanov AA, Inoue M, Ji H, Murata Y, Tordoff MG, Beauchamp GK. Glutamate taste and appetite in laboratory mice: physiologic and genetic analyses. Am J Clin Nutr 2009;90(suppl):756S–63S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shigemura N, Shirosaki S, Ohkuri T, et al. Variation in umami perception and in candidate genes for the umami receptor in mice and humans. Am J Clin Nutr 2009;90(suppl):764S–9S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen Q-Y, Alarcon S, Tharp A, et al. Perceptual variation in umami taste and polymorphisms in TAS1R taste receptor genes. Am J Clin Nutr 2009;90(suppl):770S–9S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mennella JA, Beauchamp GK, Forestell CA, Morgan LK. Early milk feeding influences taste acceptance and liking during infancy. Am J Clin Nutr 2009;90(suppl):780S–8S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Raliou M, Wiencis A, Pillias A-M, et al. Nonsynonymous single nucleotide polymorphisms in human tas1r1, tas1r3, and mGluR1 and individual taste sensitivity to glutamate. Am J Clin Nutr 2009;90(suppl):789S–99S [DOI] [PubMed] [Google Scholar]
- 103.Donaldson LF, Bennett L, Baic S, Melichar JK. Taste and weight: is there a link? Am J Clin Nutr 2009;90(suppl):800S–3S [DOI] [PubMed] [Google Scholar]
- 104.Rolls ET. Functional neuroimaging of umami taste: what makes umami pleasant? Am J Clin Nutr 2009;90(suppl):804S–13S [DOI] [PubMed] [Google Scholar]
- 105.Blachier F, Tomé D. Metabolism and functions of l-glutamate in the epithelial cells of the small and large intestines. Am J Clin Nutr 2009;90(suppl):814S–21S [DOI] [PubMed] [Google Scholar]
- 106.Kokrashvili Z, Mosinger B, Margolskee RF. Taste signaling elements expressed in gut enteroendocrine cells regulate nutrient-responsive secretion of gut hormones. Am J Clin Nutr 2009;90(suppl):822S–5S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Akiba Y, Kaunitz JD. Luminal chemosensing and upper gastrointestinal mucosal defenses. Am J Clin Nutr 2009;90(suppl):826S–31S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kondoh T, Mallick HN, Torii K. Activation of the gut-brain axis by dietary glutamate and physiologic significance in energy homeostasis. Am J Clin Nutr 2009;90(suppl):832S–7S [DOI] [PubMed] [Google Scholar]
- 109.Tomé D, Schwarz J, Darcel N, Fromentin G. Protein, amino acids, vagus nerve signaling, and the brain. Am J Clin Nutr 2009;90(suppl):838S–43S [DOI] [PubMed] [Google Scholar]
- 110.Yamamoto S, Tomoe M, Toyama K, Kawai M, Uneyama H. Can dietary supplementation of monosodium glutamate improve the health of the elderly? Am J Clin Nutr 2009;90(suppl):844S–9S [DOI] [PubMed] [Google Scholar]
- 111.Burrin DG, Stoll B. Metabolic fate and function of dietary glutamate in the gut. Am J Clin Nutr 2009;90(suppl):850S–6S [DOI] [PubMed] [Google Scholar]
- 112.Brosnan ME, Brosnan JT. Hepatic glutamate metabolism: a tale of 2 hepatocytes. Am J Clin Nutr 2009;90(suppl):857S–61S [DOI] [PubMed] [Google Scholar]
- 113.Stanley CA. Regulation of glutamate metabolism and insulin secretion by glutamate dehydrogenase in hypoglycemic children. Am J Clin Nutr 2009;90(suppl):862S–6S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Magistretti PJ. Role of glutamate in neuron-glia metabolic coupling. Am J Clin Nutr 2009;90(suppl):875S–80S [DOI] [PubMed] [Google Scholar]
- 115.Fernstrom JD. Symposium summary. Am J Clin Nutr 2009;90(suppl):881S–5S [DOI] [PubMed] [Google Scholar]