Abstract
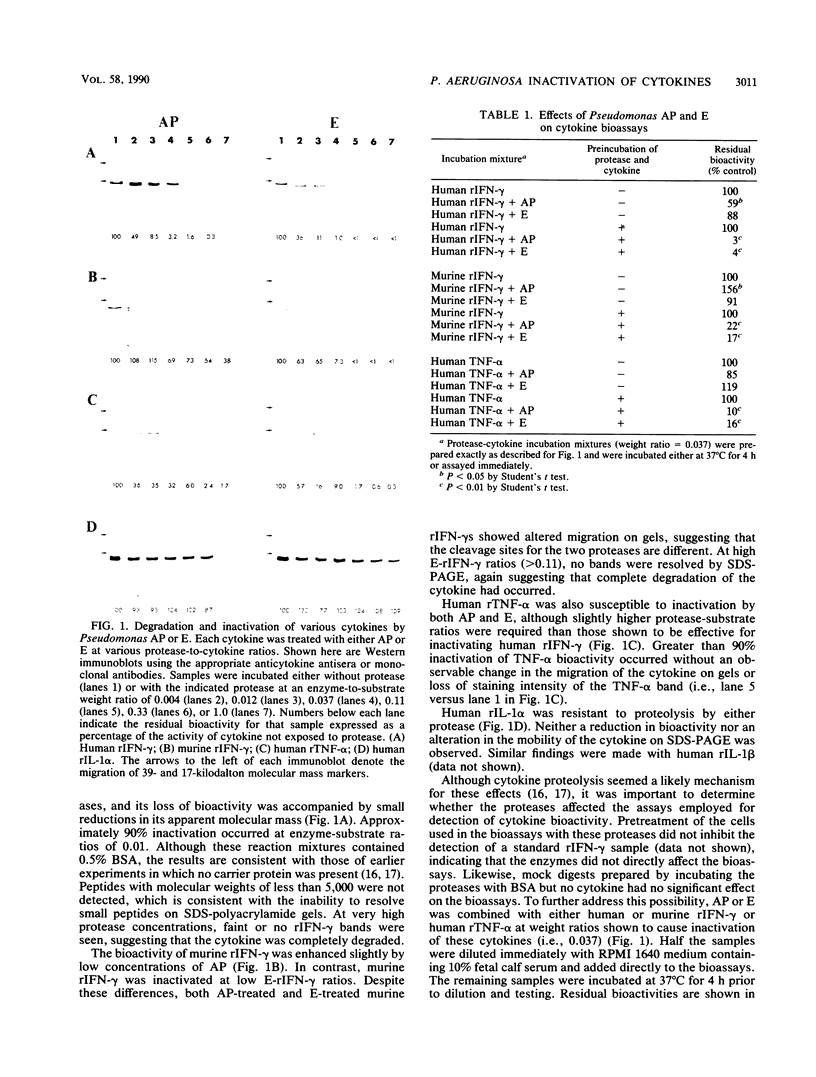
Pseudomonas aeruginosa alkaline protease and elastase are thought to contribute to bacterial invasiveness, tissue damage, and immune suppression in animals and patients infected with the bacterium. This study examined the ability of the two proteases to inactivate a number of cytokines that mediate immune and inflammatory responses. Human recombinant gamma interferon (rIFN-gamma) and human recombinant tumor necrosis factor alpha were inactivated by both proteases. Murine rIFN-gamma was relatively resistant to alkaline protease but was inactivated by elastase, and human recombinant interleukin-1 alpha and recombinant interleukin-1 beta were resistant to the effects of both proteases. Western immunoblots suggested that cytokine inactivation by these proteases, where it occurred, required only limited proteolysis of the polypeptides. The ability of different P. aeruginosa strains to inactivate IFN-gamma appeared to require the production of both proteases for optimum activity. These results indicate that in vitro cytokine inactivation by Pseudomonas proteases is selective, requires only limited proteolysis, and in certain instances reflects the cooperative effects of both proteases.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arakawa T., Hsu Y. R., Parker C. G., Lai P. H. Role of polycationic C-terminal portion in the structure and activity of recombinant human interferon-gamma. J Biol Chem. 1986 Jun 25;261(18):8534–8539. [PubMed] [Google Scholar]
- Beutler B. A., Cerami A. Recombinant interleukin 1 suppresses lipoprotein lipase activity in 3T3-L1 cells. J Immunol. 1985 Dec;135(6):3969–3971. [PubMed] [Google Scholar]
- Beutler B., Cerami A. The biology of cachectin/TNF--a primary mediator of the host response. Annu Rev Immunol. 1989;7:625–655. doi: 10.1146/annurev.iy.07.040189.003205. [DOI] [PubMed] [Google Scholar]
- Broxmeyer H. E., Williams D. E., Lu L., Cooper S., Anderson S. L., Beyer G. S., Hoffman R., Rubin B. Y. The suppressive influences of human tumor necrosis factors on bone marrow hematopoietic progenitor cells from normal donors and patients with leukemia: synergism of tumor necrosis factor and interferon-gamma. J Immunol. 1986 Jun 15;136(12):4487–4495. [PubMed] [Google Scholar]
- Buchmeier N. A., Schreiber R. D. Requirement of endogenous interferon-gamma production for resolution of Listeria monocytogenes infection. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7404–7408. doi: 10.1073/pnas.82.21.7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denizot F., Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986 May 22;89(2):271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A., Cannon J. G., Wolff S. M., Bernheim H. A., Beutler B., Cerami A., Figari I. S., Palladino M. A., Jr, O'Connor J. V. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin 1. J Exp Med. 1986 Jun 1;163(6):1433–1450. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and its biologically related cytokines. Adv Immunol. 1989;44:153–205. doi: 10.1016/s0065-2776(08)60642-2. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1. Rev Infect Dis. 1984 Jan-Feb;6(1):51–95. doi: 10.1093/clinids/6.1.51. [DOI] [PubMed] [Google Scholar]
- Georgilis K., Schaefer C., Dinarello C. A., Klempner M. S. Human recombinant interleukin 1 beta has no effect on intracellular calcium or on functional responses of human neutrophils. J Immunol. 1987 May 15;138(10):3403–3407. [PubMed] [Google Scholar]
- Heck L. W., Morihara K., McRae W. B., Miller E. J. Specific cleavage of human type III and IV collagens by Pseudomonas aeruginosa elastase. Infect Immun. 1986 Jan;51(1):115–118. doi: 10.1128/iai.51.1.115-118.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoiby N., Flensborg E. W., Beck B., Friis B., Jacobsen S. V., Jacobsen L. Pseudomonas aeruginosa infection in cystic fibrosis. Diagnostic and prognostic significance of Pseudomonas aeruginosa precipitins determined by means of crossed immunoelectrophoresis. Scand J Respir Dis. 1977 Apr;58(2):65–79. [PubMed] [Google Scholar]
- Horvat R. T., Parmely M. J. Pseudomonas aeruginosa alkaline protease degrades human gamma interferon and inhibits its bioactivity. Infect Immun. 1988 Nov;56(11):2925–2932. doi: 10.1128/iai.56.11.2925-2932.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz Y., Strunk R. C. IL-1 and tumor necrosis factor. Similarities and differences in stimulation of expression of alternative pathway of complement and IFN-beta 2/IL-6 genes in human fibroblasts. J Immunol. 1989 Jun 1;142(11):3862–3867. [PubMed] [Google Scholar]
- Kawakami M., Cerami A. Studies of endotoxin-induced decrease in lipoprotein lipase activity. J Exp Med. 1981 Sep 1;154(3):631–639. doi: 10.1084/jem.154.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami M., Murase T., Ogawa H., Ishibashi S., Mori N., Takaku F., Shibata S. Human recombinant TNF suppresses lipoprotein lipase activity and stimulates lipolysis in 3T3-L1 cells. J Biochem. 1987 Feb;101(2):331–338. doi: 10.1093/oxfordjournals.jbchem.a121917. [DOI] [PubMed] [Google Scholar]
- Kaye J., Porcelli S., Tite J., Jones B., Janeway C. A., Jr Both a monoclonal antibody and antisera specific for determinants unique to individual cloned helper T cell lines can substitute for antigen and antigen-presenting cells in the activation of T cells. J Exp Med. 1983 Sep 1;158(3):836–856. doi: 10.1084/jem.158.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehrl J. H., Miller A., Fauci A. S. Effect of tumor necrosis factor alpha on mitogen-activated human B cells. J Exp Med. 1987 Sep 1;166(3):786–791. doi: 10.1084/jem.166.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt-Jones E. A., Beller D. I., Mizel S. B., Unanue E. R. Identification of a membrane-associated interleukin 1 in macrophages. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1204–1208. doi: 10.1073/pnas.82.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinikki P. O., Calderon J., Luquette M. H., Schreiber R. D. Reduced receptor binding by a human interferon-gamma fragment lacking 11 carboxyl-terminal amino acids. J Immunol. 1987 Nov 15;139(10):3360–3366. [PubMed] [Google Scholar]
- Mizel S. B. Physicochemical characterization of lymphocyte-activating factor (LAF). J Immunol. 1979 Jun;122(6):2167–2172. [PubMed] [Google Scholar]
- Moore M. A., Warren D. J. Synergy of interleukin 1 and granulocyte colony-stimulating factor: in vivo stimulation of stem-cell recovery and hematopoietic regeneration following 5-fluorouracil treatment of mice. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7134–7138. doi: 10.1073/pnas.84.20.7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morihara K., Tsuzuki H. Substrate specificity of elastolytic and nonelastolytic proteinases from Pseudomonas aeruginosa. Arch Biochem Biophys. 1966 Apr;114(1):158–165. doi: 10.1016/0003-9861(66)90317-1. [DOI] [PubMed] [Google Scholar]
- Mortensen R. F., Shapiro J., Lin B. F., Douches S., Neta R. Interaction of recombinant IL-1 and recombinant tumor necrosis factor in the induction of mouse acute phase proteins. J Immunol. 1988 Apr 1;140(7):2260–2266. [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Spitalny G. L., Nathan C. F. Activation of mouse peritoneal macrophages in vitro and in vivo by interferon-gamma. J Immunol. 1985 Mar;134(3):1619–1622. [PubMed] [Google Scholar]
- Nacy C. A., Fortier A. H., Meltzer M. S., Buchmeier N. A., Schreiber R. D. Macrophage activation to kill Leishmania major: activation of macrophages for intracellular destruction of amastigotes can be induced by both recombinant interferon-gamma and non-interferon lymphokines. J Immunol. 1985 Nov;135(5):3505–3511. [PubMed] [Google Scholar]
- Okusawa S., Gelfand J. A., Ikejima T., Connolly R. J., Dinarello C. A. Interleukin 1 induces a shock-like state in rabbits. Synergism with tumor necrosis factor and the effect of cyclooxygenase inhibition. J Clin Invest. 1988 Apr;81(4):1162–1172. doi: 10.1172/JCI113431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onozaki K., Matsushima K., Aggarwal B. B., Oppenheim J. J. Human interleukin 1 is a cytocidal factor for several tumor cell lines. J Immunol. 1985 Dec;135(6):3962–3968. [PubMed] [Google Scholar]
- Parmely M. J., Horvat R. T. Antigenic specificities of Pseudomonas aeruginosa alkaline protease and elastase defined by human T cell clones. J Immunol. 1986 Aug 1;137(3):988–994. [PubMed] [Google Scholar]
- Pedersen B. K., Kharazmi A., Theander T. G., Odum N., Andersen V., Bendtzen K. Selective modulation of the CD4 molecular complex by Pseudomonas aeruginosa alkaline protease and elastase. Scand J Immunol. 1987 Jul;26(1):91–94. doi: 10.1111/j.1365-3083.1987.tb02239.x. [DOI] [PubMed] [Google Scholar]
- Ranges G. E., Zlotnik A., Espevik T., Dinarello C. A., Cerami A., Palladino M. A., Jr Tumor necrosis factor alpha/cachectin is a growth factor for thymocytes. Synergistic interactions with other cytokines. J Exp Med. 1988 Apr 1;167(4):1472–1478. doi: 10.1084/jem.167.4.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinderknecht H., Geokas M. C., Silverman P., Haverback B. J. A new ultrasensitive method for the determination of proteolytic activity. Clin Chim Acta. 1968 Aug;21(2):197–203. doi: 10.1016/0009-8981(68)90127-7. [DOI] [PubMed] [Google Scholar]
- Schreiber R. D. Identification of gamma-interferon as a murine macrophage-activating factor for tumor cytotoxicity. Contemp Top Immunobiol. 1984;13:171–198. doi: 10.1007/978-1-4757-1445-6_9. [DOI] [PubMed] [Google Scholar]
- Theander T. G., Kharazmi A., Pedersen B. K., Christensen L. D., Tvede N., Poulsen L. K., Odum N., Svenson M., Bendtzen K. Inhibition of human lymphocyte proliferation and cleavage of interleukin-2 by Pseudomonas aeruginosa proteases. Infect Immun. 1988 Jul;56(7):1673–1677. doi: 10.1128/iai.56.7.1673-1677.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomassen M. J., Demko C. A., Doershuk C. F. Cystic fibrosis: a review of pulmonary infections and interventions. Pediatr Pulmonol. 1987 Sep-Oct;3(5):334–351. doi: 10.1002/ppul.1950030510. [DOI] [PubMed] [Google Scholar]
- Wirth J. J., Kierszenbaum F., Sonnenfeld G., Zlotnik A. Enhancing effects of gamma interferon on phagocytic cell association with and killing of Trypanosoma cruzi. Infect Immun. 1985 Jul;49(1):61–66. doi: 10.1128/iai.49.1.61-66.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dissel J. T., Stikkelbroeck J. J., Michel B. C., van den Barselaar M. T., Leijh P. C., van Furth R. Inability of recombinant interferon-gamma to activate the antibacterial activity of mouse peritoneal macrophages against Listeria monocytogenes and Salmonella typhimurium. J Immunol. 1987 Sep 1;139(5):1673–1678. [PubMed] [Google Scholar]