Summary
Background
Factor VIII consists of a heavy chain (A1A2B domains) and light chain (A3C1C2 domains), while the contiguous A1A2 domains are separate subunits in the cofactor, factor VIIIa. Recently we reported that procofactor stability at elevated temperature and cofactor stability over an extended time course were increased following replacement of individual charged residues (Asp(D)519, Glu(E)665, or Glu(E)1984) with either Ala (A) or Val (V) (Wakabayashi et al., Blood, 112, 2761, 2008).
Objectives
In the current study we generated combination mutants at these three sites to examine any additive and/or synergistic effects of these mutations on the stability.
Methods
Studies assessing factor VIII stability involved monitoring decay rates of factor VIII at 55°C or in guanidinium, decay of factor VIIIa following A2 subunit dissociation, and thrombin generation at low (0.3 nM) factor VIII concentration.
Results and Conclusions
Similar tendencies were observed within each group of variants. Variants with mutations at D519 and either E665 or E1984 (Group A) generally showed significantly better stability as compared with single mutants. Most variants with mutations at E665 and at E1984 (Group B) did not show significant improvement. Triple mutants with mutations at D519, E665 and E1984 (Group C) showed improvement to a similar degree as the Group A double mutants. Overall, these results indicate that selected combinations of mutations to reduce charge and/or increase hydrophobicity at the A2/A1 and A2/A3 domain interfaces yield factor VIII reagents with improved stability parameters.
Keywords: factor VIII, factor Xa, guanidinium, thrombin generation, protein stability
Introduction
Factor VIII, a plasma protein that is decreased or defective in individuals with hemophilia A, is expressed as both single chain and heterodimer forms. The latter consists of a heavy chain (HC) comprised of A1(a1)A2(a2)B domains and a light chain (LC) comprised of (a3)A3C1C2 domains, with the lower case a representing short (~30–40 residue) segments rich in acidic residues (see Ref. [1] for review). Factor VIII is activated by proteolytic cleavages at the a1A2, a2B and a3A3 junctions catalyzed by thrombin or factor Xa. The resulting product, factor VIIIa, is a heterotrimer comprised of subunits designated A1, A2, and A3C1C2 that functions as a cofactor for the serine protease factor IXa in the membrane-dependent conversion of zymogen factor X to the serine protease, factor Xa (see Ref. [1] for review).
Reconstitution studies have shown that the factor VIII heterodimeric structure is supported by both electrostatic and hydrophobic interactions [2]. Metal ions also contribute to the inter-chain affinity and activity parameters [3]. Occupancy of a calcium site in the A1 domain is required to yield the active factor VIII conformation [4]. Recent intermediate resolution X-ray structures [5,6] showed occupancy of the two type 1 copper ion sites within the A1 and A3 domains. Earlier functional studies indicated that copper ions facilitate the association of HC and LC to form the heterodimer, increasing the inter-chain affinity by several-fold at physiologic pH [7,8].
The instability of factor VIIIa results from weak electrostatic interactions between the A2 subunit and the A1/A3C1C2 dimer [9,10] and leads to dampening of factor Xase activity [11,12]. Several factor VIII point mutations have been shown to facilitate the dissociation of A2 relative to wild type (WT) and these residues localize to either the A1–A2 domain interface [13,14] or the A2–A3 domain interface [15]. These factor VIII variants demonstrate a characteristic one-stage/two-stage assay discrepancy [16,17], with significant reductions in activity values determined by the latter assay as a result of increased rates of A2 subunit dissociation. Examination of hydrogen-bonding interactions at the A2 interface following mutation of selected charged/polar residues spatially separated by <2.8 Å showed loss of function, as judged by increased rates of factor VIII decay at 55°C and/or rates for factor VIIIa decay relative to WT, in approximately half of the 30 residues tested [18], suggesting that multiple residues at the A1A2 and A2A3 domain interfaces contribute to the stabilization of factor VIII.
Recently we identified four charged residues likely buried at the contiguous A2 interface and created charge removal/hydrophobic mutations [19] based on the assumption that mutation to increase buried hydrophobic area and/or reduce the buried hydrophilic area often results in enhanced protein stability [20]. Mutations at three of these sites, Asp519 and Glu665 in the A2 domain and Glu1984 in the A3 domain to Ala or Val yielded up to ~2 fold higher thermal stability in factor VIII and up to ~5 fold higher stability in factor VIIIa compared to WT [19]. In this report we created combined mutants at Asp519, Glu665, and/or Glu1984 and examined activity parameters assessing factor VIII thermal and chemical stability, A2 dissociation in factor VIIIa, and thrombin generation capacity to assess potential additive effects/synergy of the variants and the relationship to residue position. Results show that selective mutations possess enhanced gain-of-function dependent upon specific residue location.
Materials and Methods
Reagents
Recombinant factor VIII (Kogenate™) was a generous gift from Dr. Lisa Regan of Bayer Corporation (Berkeley, CA). Phospholipid vesicles containing 20% phosphatidylcholine (PC), 40% phosphatidylethanolamine (PE), and 40% phosphatidylserine (PS) were prepared using octylglucoside as described previously [21]. The reagents α-thrombin, factor VIIa, factor IXaβ, factor X, and factor Xa (Enzyme Research Laboratories, South Bend, IN), hirudin and phospholipids (DiaPharma, West Chester, OH), the chromogenic Xa substrate, Pefachrome Xa (Pefa-5523, CH3OCO-D-CHA-Gly-Arg-pNA·AcOH; Centerchem Inc. Norwalk CT), recombinant human tissue factor (rTF), Innovin (Dade Behring, Deerfield, IL), fluorogenic substrate, Z-Gly-Gly-Arg-AMC (Calbiochem, San Diego, CA), and thrombin calibrator (Diagnostica Stago, Parsippany, NJ) were purchased from the indicated vendors.
Construction, expression and purification of WT and variant factor VIII
Combination of Ala and Val mutants at charged residues (Asp519, Glu665, and Glu1984) and WT factor VIII forms were individually constructed as a B-domainless factor VIII, lacking residues Gln744-Ser1637 in the B-domain [22]. Recombinant WT and variant factor VIII forms were stably expressed in BHK cells and purified as described previously [4]. Protein yields for the variants ranged from >10 to ~100 μg from two 750 cm2 culture flasks, with purity from ~85% to >95% as judged by SDS-PAGE. The primary contaminant in the factor VIII preparations was albumin. Factor VIII concentration was measured using an Enzyme-Linked Immunoadsorbant Assay (ELISA) and factor VIII activity was determined by one-stage clotting and two-stage chromogenic factor Xa generation assays described below.
Assays
A sandwich ELISA was performed as previously described [23] using purified commercial recombinant factor VIII (Kogenate, Bayer Corporation) as a standard. Factor VIII capture used the anti-C2 antibody (ESH-8, American Diagnostica Inc., Stamford, CT), and R8B12 antibody (Green Mountain Antibody, Burlington, VT) was employed for factor VIII detection following biotinylation. One-stage clotting assays were performed using substrate plasma chemically depleted of factor VIII as previously described [19]. The rate of conversion of factor X to factor Xa was monitored in a purified system according to methods previously described [19].
Thrombin generation assay
The amount of thrombin generated in plasma was measured by Calibrated Automated Thrombography [24] using methods previously described [19]. Briefly, factor VIII deficient plasma (<1% residual activity, platelet-poor) from severe hemophilia A patients lacking factor VIII inhibitor (George King Bio-Medical, Overland Park, KS) was mixed at 37°C with a final concentration of 0.3 nM factor VIII, 0.5 pM rTF, 4 μM PSPCPE vesicles, 433 μM fluorogenic substrate, 13.3 mM CaCl2, and 105 nM thrombin calibrator. The development of a fluorescent signal was monitored at 8 second intervals using a Microplate Spectrofluorometer (Spectramax Gemini, Molecular Devices, Sunnyvale, CA) with a 355 nm (excitation)/460 nm (emission) filter set. Fluorescent signals were corrected by the reference signal from the thrombin calibrator samples [24] and actual thrombin generation in nM was calculated as previously described [19].
Factor VIII activity inhibition after guanidinium chloride exposure
WT and mutant factor VIII (50 nM) in buffer containing 20 mM N-[2-hydroxyethyl]piperazine-N′-[2-ethanesulfonic acid] (HEPES), pH 7.2, 0.1 M NaCl, 0.01% Tween 20, 0.01% BSA, and 5 mM CaCl2 plus 0–1.2 M guanidinium chloride were incubated for 2 hrs at 23°C. Aliquots were diluted (1/50) in the above buffer containing 10 μM PSPCPE vesicles and activated by 5 nM thrombin for 1 min. Reactions were immediately quenched with hirudin (10 U/ml) and activity was determined by factor Xa generation assay following addition of factor IXa (40 nM) and factor X (300 nM). Residual guanidinium chloride (< 24 mM) did not inhibit the activation of factor VIII or its cofactor activity.
Data analysis
Factor VIII/VIIIa activity values as a function of time were fitted to a single exponential decay curve by non-linear least squares regression using the equation,
where A is residual factor VIIIa activity (nM/min/nM factor VIII), A0 is the initial activity, k is the apparent rate constant, and t is the time (minutes) of reaction of either factor VIII at 55°C (for factor VIII decay experiments) or after thrombin activation was quenched (for factor VIIIa decay measurements).
Factor VIII activity inhibition data by guanidinium exposure were fitted to a concentration-response curve by non-linear least squares regression using the equation,
where IC50 is the 50% activity inhibitory concentration (M), X is the log-scale inhibitor (guanidinium chloride) concentration, and H is the Hill’s slope. Log(IC50) values were used for statistical analysis.
Nonlinear least-squares regression analysis was performed by Kaleidagraph (Synergy, Reading, PA). Comparison of average values was performed by the Student’s t-test.
Results
Generation of factor VIII point mutants in combination with Asp519Ala/Val, Glu665Ala/Val, and Glu1984Ala/Val
Previously we demonstrated increased factor VIII and VIIIa stability [19] resulting from Ala or Val mutation of 3 charged residues, Asp519, Glu665, and Glu1984, that appeared to be buried at the interface of the A2 and A1 domains (Asp519) or the A2 and A3 domains (Glu665 and Glu1984). Out of 12 possible combinations, 11 double mutants were successfully produced and designated as Group A variants with mutations at D519 and either E665 or E1984, and Group B variants with mutations at E665 and E1984. In addition, we combined Asp519Ala or -Val with Glu665Val/Glu1984Ala or Glu665Val/Glu1984Val to generate triple mutants (Group C variants).
Factor VIII is expressed as a mixture of single chain and heterodimer forms [19]. Western blotting using an anti-A2 domain antibody to quantitate the stoichiometry of single chain and heterodimer forms yielded a value near unity for WT (results not shown). This value was somewhat lower for the factor VIII variants (typically ~0.5) indicating greater relative heterodimer content.
Purified proteins were assessed for specific activity using both one-stage and two-stage assays (results not shown). All Group A mutants retained >80% activity compared to WT. Interestingly, the activity by one-stage assay for the Asp519Ala/Glu665Ala, Asp519Val/Glu665Val, and Asp519Val/Glu1984Val variants were significantly increased (~140% to ~180% of WT activity). In Group B, the Glu665Ala/Glu1984Ala and Glu665Ala/Glu1984Val variants showed significant reductions in activity as measured by both one-stage and two-stage assays (~40% to ~80% of WT activity) compared to the value for the better single mutant in the combination, and suggested a detrimental effect of the combined mutations. Two of the Group C mutants retained similar levels as WT for both specific activity values. However, the activity of Asp519Ala/Glu665Ala/Glu1984Val by two-stage assay was reduced to ~40% of WT although the activity by one-stage was increased (160% of WT). The reason for this discrepancy is not clear.
Thermostability of factor VIII variants
The purified factor VIII mutant proteins were assessed for stability at 55°C as judged by rates of activity loss. Factor VIII (4 nM) was incubated and at the indicated times an aliquot was removed, cooled to room temperature, reacted with thrombin and residual cofactor activity was measured by factor Xa generation. Previously we showed that Ala and Val replacements for Asp519, Glu665, and Glu1984 all showed improved thermal stability [19]. Rates of factor VIII decay for the combination variants as well as the single point mutants are shown in Table 1. The decay rates for two Group A mutants (Asp519Ala/Glu665Ala and Asp519Ala/Glu665Val) were significantly improved compared with the value for the better single mutation in the combination, while rates of two Group B mutants (Glu665Ala/Glu1984Ala and Glu665Ala/Glu1984Val) and one Group C mutant (Asp519Val/Glu665Val/Glu1984Val) were somewhat increased. Although varying ratios of single chain and heterodimer forms likely impact these decay rate results, the stoichiometry of single chain to heterodimer for the combination variants (this study) and single point mutations [19] was consistently lower than that for WT. Since the single chain form of factor VIII shows greater thermal stability than the heterodimer [19], these results indicated the measured stability values underestimate that of the variants relative to WT.
Table 1.
Decay rates of factor VIII, factor VIIIa spontaneous decay rates, and IC50 values for the inhibition of factor VIII by guanidinium chloride.
| Factor VIII Decay Rate | Factor VIIIa Decay Rate | IC50 (M) | |
|---|---|---|---|
| WT | 0.0470 ± 0.0012 (1.00) | 0.1400 ± 0.0054 (1.00) | 0.814 ± 0.004 (1.00) |
| D519AE665A | 0.0254 ± 0.0017 (0.54)* | 0.0352 ± 0.0023 (0.25)† | 0.950 ± 0.005 (1.17)† |
| D519AE665V | 0.0212 ± 0.0007 (0.45)† | 0.0222 ± 0.0017 (0.16)† | 1.047 ± 0.003 (1.29)† |
| D519AE1984A | 0.0250 ± 0.0014 (0.53) | 0.0266 ± 0.0011 (0.19)† | 0.921 ± 0.009 (1.13)* |
| D519A/E1984V | 0.0247 ± 0.0019 (0.53) | 0.0318 ± 0.0021 (0.23)† | 0.946 ± 0.011 (1.16)† |
| D519VE665V | 0.0238 ± 0.0005 (0.51) | 0.0197 ± 0.0010 (0.14)† | 0.924 ± 0.005 (1.13)† |
| D519VE1984A | 0.0256 ± 0.0006 (0.54) | 0.0168 ± 0.0012 (0.12)† | 0.949 ± 0.007 (1.17)† |
| D519VE1984V | 0.0258 ± 0.0026 (0.54) | 0.0261 ± 0.0013 (0.19)† | 0.918 ± 0.003 (1.13)† |
| E665AE1984A | 0.0323 ± 0.0003 (0.69)† | 0.1302 ± 0.0046 (0.93)† | 0.879 ± 0.007 (1.08) |
| E665AE1984V | 0.0347 ± 0.0017 (0.74)† | 0.1266 ± 0.0043 (0.91)† | 0.831 ± 0.005 (1.02)† |
| E665VE1984A | 0.0231 ± 0.0010 (0.49) | 0.0360 ± 0.0009 (0.26) | 0.839 ± 0.007 (1.03)† |
| E665VE1984V | 0.0220 ± 0.0006 (0.46) | 0.0671 ± 0.0010 (0.48)† | 0.894 ± 0.009 (1.10)† |
| D519AE665VE1984A | 0.0246 ± 0.0004 (0.52) | 0.0234 ± 0.0018 (0.17)† | 1.002 ± 0.005 (1.23)† |
| D519VE665VE1984A | 0.0253 ± 0.0018 (0.54) | 0.0141 ± 0.0008 (0.10)† | 1.004 ± 0.006 (1.23)† |
| D519VE665VE1984V | 0.0306 ± 0.0024 (0.65)* | 0.0226 ± 0.0009 (0.16)† | 0.987 ± 0.011 (1.21)* |
| D519A | 0.0336 ± 0.0022 (0.71)‡ | 0.0898 ± 0.0035 (0.64)§ | 0.891 ± 0.007 (1.09)§ |
| D519V | 0.0262 ± 0.0012 (0.56)‡ | 0.0836 ± 0.0010 (0.59)§ | 0.848 ± 0.005 (1.04)§ |
| E665A | 0.0359 ± 0.0009 (0.76)‡ | 0.0834 ± 0.0017 (0.59)§ | 0.891 ± 0.007 (1.09)§ |
| E665V | 0.0309 ± 0.0007 (0.66)‡ | 0.0394 ± 0.0017 (0.28)§ | 0.963 ± 0.009 (1.18)§ |
| E1984A | 0.0240 ± 0.0011 (0.51)‡ | 0.0573 ± 0.0030 (0.40)§ | 0.892 ± 0.013 (1.10)§ |
| E1984V | 0.0211 ± 0.0016 (0.45)‡ | 0.0471 ± 0.0014 (0.33)§ | 0.867 ± 0.009 (1.07)§ |
Factor VIII decay at 55°C and factor VIIIa spontaneous decay were fitted to a single exponential decay curve. Guanidinium denaturation data as shown in Fig. 1 were fitted to a concentration response curve by non-linear least squares regression and IC50 values with standard deviations were obtained. Values in parentheses are relative to the WT value. The single letter code is used to designate amino acid residues, E (Glu), D (Asp), A (Ala) and V (Val). Factor VIII decay rate data for single mutants were taken from Ref. 19.
p < 0.05 or
p < 0.005 compared to the value for the best single mutant in the combination.
p < 0.05 or
p < 0.01 compared to the value for WT.
Factor VIIIa decay rates
Factor VIIIa decay rates previously reported for the stable single mutants were relatively slow, with >70% activity retention after >15 min incubation [19]. For direct comparisons, we performed the decay experiments using lower factor VIIIa concentration (1.5 nM) and in the absence of factor IXa. Under these conditions, the decay rates of the single mutants were somewhat increased compared to reported values [19], however, ratios to the WT value were not changed dramatically. Significant activity (>60% at 15 min; data not shown) was retained for Group A and C mutants which showed decay rates reduced as much as ~7–10-fold relative to WT and ~1.5–3-fold relative to the better single mutation in that combination (Table 1). On the other hand, two Group B mutants (Glu665Ala/Glu1984Ala and Glu665Ala/Glu1984Val) showed decay rates as high as WT, negating the stabilizing effect observed for the single mutation.
Factor VIII activity inhibition by guanidinium chloride
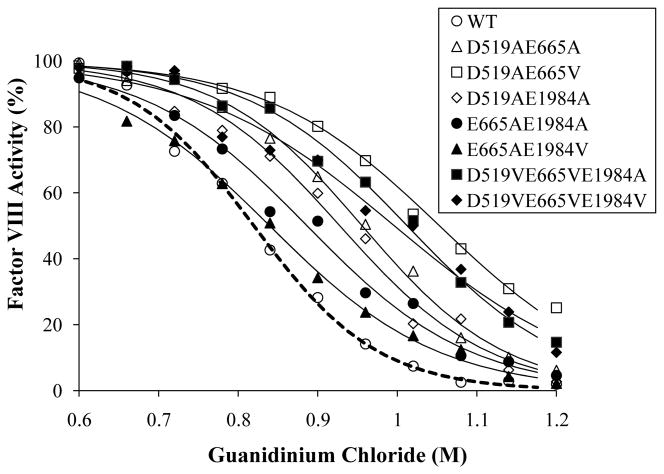
Factor VIII stability was examined following a 2h exposure to 0.6–1.2 M guanidinium (Fig. 1). Extended incubation beyond 2h did not appreciably alter the results (data not shown). The sigmoidal shape of the curve suggested a complicated denaturation process and these data were well fitted using a concentration-response curve. WT factor VIII activity reduction occurred at >0.6 M guanidinium, and at 1.2 M all of the activity was lost (Fig. 1). All of the mutants, including single mutants, showed increased resistance to denaturation (see Fig 1 for selected variants). IC50 values are shown in Table 1. The degree of improvement in IC50 compared to WT for the single mutants ranged from 4 - 18%, for Group A and C mutants this range was 13–29%, while that for Group B mutants was only 2 – 3%. When the IC50 values of the combined mutants were compared with the values of the best single mutant in the combination, significant improvement was observed in all Group A and C mutants except for Asp519Val/Glu665Val. On the other hand, 3 out of 4 Group B mutants showed significant reductions in IC50 values compared to the value of best single mutant in the combination.
Fig. 1.
Thrombin generation assay
Thrombin generation assays were performed at low rTF concentration (0.5 pM) using factor VIII deficient plasma. Titration of 0.25 to 1 nM WT or Asp519Val/Glu665Val variant factor VIII revealed increased peak values for the variant at all concentrations ranging from an ~2.5-fold increase at <0.5 nM to an ~1.5-fold increase at 1 nM (data not shown). This concentration-dependent increase in parameter value relative to WT at low variant concentration may reflect reduced rates of A2 dissociation for the variant protein. We speculate that at higher cofactor concentration the effects of A2 subunit dissociation become less apparent than at low factor VIIIa. Subsequent assays employed 0.3 nM factor VIII. At this factor VIII concentration, we observed significant increases in peak value and/or ETP for several of the single point mutations (see Table 2) not observed in our prior report using 1 nM factor VIII [19].
Table 2.
Thrombin generation assay parameter values
| Latent Time (min) | Peak Time (min) | Peak Value (nM) | ETP (nM·min) | |
|---|---|---|---|---|
| WT | 9.25 ± 0.63 (1.00) | 20.1 ± 0.56 (1.00) | 41.7 ± 0.22 (1.00) | 870 ± 57 (1.00) |
| D519AE665A | 7.99 ± 0.81 (0.87) | 17.1 ± 2.37 (0.85) | 67.6 ± 4.33 (1.62) * | 1370 ± 92 (1.58)* |
| D519AE665V | 10.5 ± 0.72 (1.14) | 20.1 ± 0.18 (1.00) | 78.5 ± 5.81 (1.88) | 1356 ± 173 (1.56) |
| D519AE1984A | 8.44 ± 0.52 (0.91) | 16.7 ± 0.98 (0.83)* | 76.3 ± 6.77 (1.83) * | 1360 ± 53 (1.56)* |
| D519AE1984V | 8.33 ± 1.15 (0.90) | 16.8 ± 0.98 (0.84)* | 66.7 ± 4.59 (1.59) * | 1143 ± 272 (1.31) |
| D519VE665V | 9.12 ± 0.69 (0.99) | 17.3 ± 1.15 (0.86) | 92.8 ± 2.93 (2.22) * | 1496 ± 45 (1.71) |
| D519VE1984A | 9.46 ± 0.67 (1.02) | 18.2 ± 0.89 (0.91) | 79.0 ± 4.83 (1.89) * | 1206 ± 129 (1.39) |
| D519VE1984V | 7.46 ± 0.43 (0.81)* | 15.9 ± 0.63 (0.79)† | 74.8 ± 5.25 (1.79)* | 1327 ± 146 (1.53) |
| E665AE1984A | 9.62 ± 0.94 (1.04) | 20.5 ± 2.78 (1.02) | 52.3 ± 7.83 (1.25) | 1151 ± 169 (1.32) |
| E665VE1984A | 8.65 ± 0.56 (0.94) | 19.1 ± 1.86 (0.95) | 64.0 ± 7.06 (1.54) | 1272 ± 135 (1.46) |
| E665AE1984V | 9.04 ± 0.57 (0.98) | 20.4 ± 2.23 (1.02) | 57.7 ± 4.38 (1.38) | 1232 ± 51 (1.42) |
| E665VE1984V | 11.1 ± 0.54 (1.20) | 24.1 ± 0.26 (1.20)* | 46.6 ± 7.16 (1.12) | 1028 ± 151 (1.18) |
| D519AE665VE1984A | 8.48 ± 1.16 (0.92) | 17.1 ± 3.15 (0.85) | 68.8 ± 2.43 (1.65) | 1152 ± 58 (1.33) |
| D519VE665VE1984A | 8.72 ± 0.13 (0.94) | 17.6 ± 0.34 (0.88) | 68.9 ± 9.84 (1.65) | 1170 ± 88 (1.34) |
| D519VE665VE1984V | 9.52 ± 0.70 (1.03) | 19.0 ± 0.36 (0.94) | 75.9 ± 3.90 (1.82) | 1214 ± 45 (1.40) |
| D519A | 8.04 ± 0.31 (0.87) | 20.4 ± 1.58 (1.01) | 49.8 ± 5.51 (1.20) | 1138 ± 30 (1.31)‡ |
| D519V | 9.21 ± 0.46 (0.99) | 21.7 ± 1.98 (1.08) | 53.1 ± 6.54 (1.27) | 1121 ± 137 (1.29) |
| E665A | 9.04 ± 0.25 (0.98) | 21.2 ± 0.09 (1.06) | 39.7 ± 4.14 (0.95) | 1006 ± 87 (1.16) |
| E665V | 8.80 ± 0.07 (0.95) | 17.9 ± 1.25 (0.89) | 67.2 ± 7.19 (1.61)‡ | 1252 ± 101 (1.44)‡ |
| E1984A | 9.67 ± 0.20 (1.05) | 20.0 ± 0.65 (0.99) | 55.1 ± 6.22 (1.32) | 1068 ± 177 (1.23) |
| E1984V | 10.0 ± 0.66 (1.08) | 20.4 ± 0.47 (1.02) | 49.1 ± 4.41 (1.18) | 1028 ± 301 (1.18) |
Thrombin generation assays in the presence of 0.3 nM factor VIII proteins, 0.5 pM rTF, and 4 μM PSPCPE vesicles were performed and parameter values were calculated as described in Methods. Data represents the average values of triplicate samples. Values in parentheses are relative to the WT value. The single letter code is used to designate amino acid residues, E (Glu), D (Asp), A (Ala) and V (Val).
p < 0.05 or
p < 0.005 compared to the value for the best single mutant in the combination.
p < 0.05 compared to the value for WT.
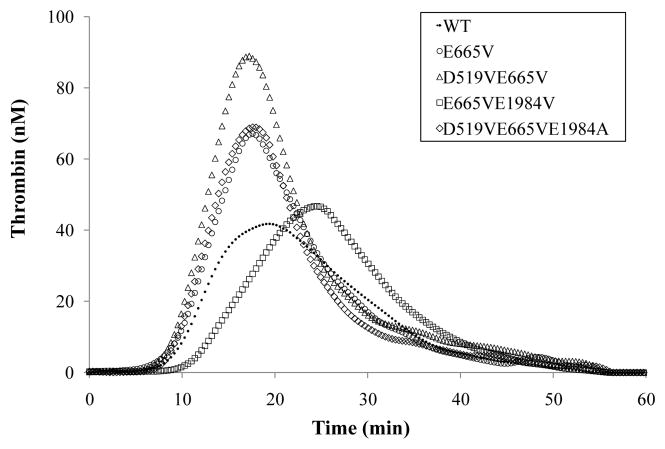
Examples of thrombin generation data are shown in Fig. 2 as represented for WT factor VIII, a single point mutant and one member each from Groups A, B and C. Thrombin generation initiated at ~8 – 9 min in all cases. Observed peak times were similar except for Glu665Val/Glu1984Val, that showed a significant delay by ~ 4 min. The largest difference was observed in values for peak height, that showed up to an ~2.2 fold increase for the Asp519Val/Glu665Val variant compared to the WT value. Thrombin generation parameters for all variants are shown in Table 2. All 7 group A mutants showed increases in peak value by 17–43% compared to the value of the better single mutant in the combination, with 6 out of 7 mutants showing statistical significance. Comparing ETP values, all mutants tested fell within the range of ± 20% difference compared to the value of the best single mutant in the combination, with two group A mutants (Asp519Ala/Glu665Ala and Asp519Ala/Glu1984Ala) showing significant increases. In addition, all mutants showed higher ETP values compared to WT, with increases ranging from 18% to 72%.
Fig. 2.
Discussion
Previously we reported that substitution of three charged residues with hydrophobic residues (Ala or Val) localized at sites predicted to be buried at the interface of the A2 domain with A1 (Asp519) or A3 (Glu665 and Glu1984) resulted in variable, but general increases in the stability of factor VIII when assessed following activity retention at elevated temperature and reduction in the rate of A2 subunit dissociation in the cofactor [19]. We proposed these results were due to elimination of detrimental inter-domain interactions at the interfaces. In the current study we now show that specific combinations of these mutations yield enhanced stability parameters suggesting additive as well as synergistic effects to the single point mutations. In the case of Group A variants where mutation at Asp519 was combined with mutation at Glu665 or Glu1984, we observed stability enhancement in all 4 parameters (factor VIII thermal and chemical stability, factor VIIIa stability, and thrombin generation capacity) with few exceptions. However, there was little enhancement of stability, but rather in many cases reductions in stability parameters with Group B variants that combined mutations at Glu665 and Glu1984. Based on the factor VIII and VIIIa stability results of Group B mutants, which showed somewhat better stability in Glu665Val containing mutants over Glu665Ala containing mutants, we generated triple mutants with the addition of mutation at Asp519. This additional mutation yielded higher stability parameters that approached those observed for Group A variants, particularly with respect to factor VIII chemical stability and factor VIIIa stability. Taken together, these results demonstrate that combinations of mutations at Asp519 with Glu665 or Glu1984 yield gain-of-function factor VIII reagents possessing improved protein stability as judged by several activity parameters.
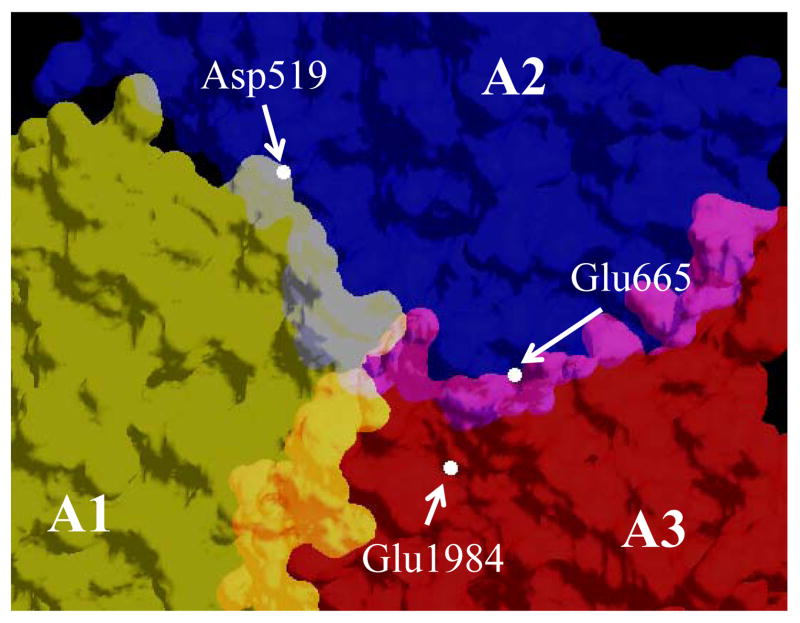
Based upon our results from a recent study examining H-bonding interactions in factor VIII, Asp519 appears to be located within a region where several residues (His281, Arg282, Ser524, and Arg531) that make important contribution to the A1 – A2 domain interaction are clustered [18]. Similarly, Glu665 and Glu1984 are located within a cluster of residues (Tyr664, Asn684, Tyr1786, Tyr1792, and Glu1829) that contributes to A2–A3 domain interactions [18]. Thus, the mutations we created occur within electrostatically high energy binding regions. The average Cα distances between the above residues calculated from the factor VIII homology model [25] and consistent with the recently reported factor VIII structures [5,6] were ~26, ~30, and ~10 Å for the Asp519 – Glu665, Asp519 – Glu1984, and Glu665 Glu1984 pairings, respectively (Fig. 3). The distances separating the mutated residues of the Group A mutants are far-removed (26 and 30 Å) compared to those of the Group B mutants (10 Å). While the reason(s) for the significant reductions in specific activity and some stability parameters for some of the Group B mutants is not clear, the introduction of two mutations at the A2-A3 interface in such close proximity possibly resulted in subtle changes in their relative coordination large enough to affect function.
Fig. 3.
In an attempt to identify the best variants based upon stability (factor VIII thermal and chemical stability and factor VIIIa decay) and thrombin generation parameter values, if we select the top three mutants from each of the categories examined, Asp519Ala/Glu665Val appears 3 times, while Asp519Val/Glu665Val, Asp519Val/Glu1984Ala, and Asp519Val/Glu665Val/Glu1984Ala appear twice. With respect to thrombin generation, using 0.3 nM factor VIII we now show similar enhancements in peak value (>150–220 % WT) and ETP (>130 – 170% WT) parameters as observed for a novel factor VIII variant, where the A2 domain is disulfide bridged to the A3 domain, following assays using similarly low levels of factor VIII [26]. Thus, we conclude that the above 4 mutants represent the best mutants in terms of gain-of-function produced from this panel.
There are still other areas of inter-subunit interfaces to explore for the purpose engineering superior factor VIII molecules with higher stability. For example, mutagenesis of many of the charged or polar residues comprising large areas of potential contacts between A2 and A1/A3 domains did not alter stability or functional parameters [18], suggesting little side chain involvement and/or non-H-bonding interactions. Therefore, creation of new, non-covalent interactions in these areas might induce further enhancements to protein stability. Furthermore, combining the high stability variants with other, non-related gain-of-function mutations could likely yield additional benefits as potential therapeutics. For example, preliminary results combining several of the high stability mutations in this report with Glu113Ala, a high specific activity variant resulting from mutation of a non-coordinating residue within a calcium binding site [23], yielded variants with the predicted enhancements in both parameters.
Acknowledgments
We thank Lisa M. Regan of Bayer Corporation for the gifts of recombinant human factor VIII and Pete Lollar and John Healey for the factor VIII cloning and expression vectors. This work was supported by NIH grants HL38199 and HL76213.
References
- 1.Fay PJ. Activation of factor VIII and mechanisms of cofactor action. Blood Rev. 2004;18:1–15. doi: 10.1016/s0268-960x(03)00025-0. [DOI] [PubMed] [Google Scholar]
- 2.Fay PJ. Reconstitution of human factor VIII from isolated subunits. Arch Biochem Biophys. 1988;262:525–31. doi: 10.1016/0003-9861(88)90404-3. [DOI] [PubMed] [Google Scholar]
- 3.Wakabayashi H, Koszelak ME, Mastri M, Fay PJ. Metal ion-independent association of factor VIII subunits and the roles of calcium and copper ions for cofactor activity and inter-subunit affinity. Biochemistry. 2001;40:10293–300. doi: 10.1021/bi010353q. [DOI] [PubMed] [Google Scholar]
- 4.Wakabayashi H, Freas J, Zhou Q, Fay PJ. Residues 110–126 in the A1 domain of factor VIII contain a Ca2+ binding site required for cofactor activity. J Biol Chem. 2004;279:12677–84. doi: 10.1074/jbc.M311042200. [DOI] [PubMed] [Google Scholar]
- 5.Shen BW, Spiegel PC, Chang CH, Huh JW, Lee JS, Kim J, Kim YH, Stoddard BL. The tertiary structure and domain organization of coagulation factor VIII. Blood. 2008;111:1240–7. doi: 10.1182/blood-2007-08-109918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ngo JC, Huang M, Roth DA, Furie BC, Furie B. Crystal structure of human factor VIII: implications for the formation of the factor IXa-factor VIIIa complex. Structure. 2008;16:597–606. doi: 10.1016/j.str.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Wakabayashi H, Zhou Q, Nogami K, Ansong C, Varfaj F, Miles S, Fay PJ. pH-dependent association of factor VIII chains: enhancement of affinity at physiological pH by Cu2+ Biochim Biophys Acta. 2006;1764:1094–101. doi: 10.1016/j.bbapap.2006.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ansong C, Fay PJ. Factor VIII A3 Domain Residues 1954–1961 Represent an A1 Domain- Interactive Site. Biochemistry. 2005;44:8850–7. doi: 10.1021/bi050145o. [DOI] [PubMed] [Google Scholar]
- 9.Fay PJ, Haidaris PJ, Smudzin TM. Human factor VIIIa subunit structure. Reconstruction of factor VIIIa from the isolated A1/A3-C1-C2 dimer and A2 subunit. J Biol Chem. 1991;266:8957–62. [PubMed] [Google Scholar]
- 10.Lollar P, Parker CG. pH-dependent denaturation of thrombin-activated porcine factor VIII. J Biol Chem. 1990;265:1688–92. [PubMed] [Google Scholar]
- 11.Lollar P, Parker ET. Structural basis for the decreased procoagulant activity of human factor VIII compared to the porcine homolog. J Biol Chem. 1991;266:12481–6. [PubMed] [Google Scholar]
- 12.Fay PJ, Beattie TL, Regan LM, O'Brien LM, Kaufman RJ. Model for the factor VIIIa-dependent decay of the intrinsic factor Xase. Role of subunit dissociation and factor IXa-catalyzed proteolysis. J Biol Chem. 1996;271:6027–32. doi: 10.1074/jbc.271.11.6027. [DOI] [PubMed] [Google Scholar]
- 13.Pipe SW, Eickhorst AN, McKinley SH, Saenko EL, Kaufman RJ. Mild hemophilia A caused by increased rate of factor VIII A2 subunit dissociation: evidence for nonproteolytic inactivation of factor VIIIa in vivo. Blood. 1999;93:176–83. [PubMed] [Google Scholar]
- 14.Pipe SW, Saenko EL, Eickhorst AN, Kemball-Cook G, Kaufman RJ. Hemophilia A mutations associated with 1-stage/2-stage activity discrepancy disrupt protein-protein interactions within the triplicated A domains of thrombin-activated factor VIIIa. Blood. 2001;97:685–91. doi: 10.1182/blood.v97.3.685. [DOI] [PubMed] [Google Scholar]
- 15.Hakeos WH, Miao H, Sirachainan N, Kemball-Cook G, Saenko EL, Kaufman RJ, Pipe SW. Hemophilia A mutations within the factor VIII A2–A3 subunit interface destabilize factor VIIIa and cause one-stage/two-stage activity discrepancy. Thromb Haemost. 2002;88:781–7. [PubMed] [Google Scholar]
- 16.Duncan EM, Duncan BM, Tunbridge LJ, Lloyd JV. Familial discrepancy between the one-stage and two-stage factor VIII methods in a subgroup of patients with haemophilia A. Br J Haematol. 1994;87:846–8. doi: 10.1111/j.1365-2141.1994.tb06749.x. [DOI] [PubMed] [Google Scholar]
- 17.Rudzki Z, Duncan EM, Casey GJ, Neumann M, Favaloro EJ, Lloyd JV. Mutations in a subgroup of patients with mild haemophilia A and a familial discrepancy between the one-stage and two-stage factor VIII:C methods. Br J Haematol. 1996;94:400–6. doi: 10.1046/j.1365-2141.1996.d01-1792.x. [DOI] [PubMed] [Google Scholar]
- 18.Wakabayashi H, Fay PJ. Identification of Residues Contributing to A2 Domain-dependent Structural Stability in Factor VIII and Factor VIIIa. J Biol Chem. 2008;283:11645–51. doi: 10.1074/jbc.M710252200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wakabayashi H, Varfaj F, Deangelis J, Fay PJ. Generation of enhanced stability factor VIII variants by replacement of charged residues at the A2 domain interface. Blood. 2008;112:2761–9. doi: 10.1182/blood-2008-02-142158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sammond DW, Eletr ZM, Purbeck C, Kimple RJ, Siderovski DP, Kuhlman B. Structure-based protocol for identifying mutations that enhance protein-protein binding affinities. J Mol Biol. 2007;371:1392–404. doi: 10.1016/j.jmb.2007.05.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mimms LT, Zampighi G, Nozaki Y, Tanford C, Reynolds JA. Phospholipid vesicle formation and transmembrane protein incorporation using octyl glucoside. Biochemistry. 1981;20:833–40. doi: 10.1021/bi00507a028. [DOI] [PubMed] [Google Scholar]
- 22.Doering C, Parker ET, Healey JF, Craddock HN, Barrow RT, Lollar P. Expression and characterization of recombinant murine factor VIII. Thromb Haemost. 2002;88:450–8. [PubMed] [Google Scholar]
- 23.Wakabayashi H, Su YC, Ahmad SS, Walsh PN, Fay PJ. A Glu113Ala Mutation within a Factor VIII Ca(2+)-Binding Site Enhances Cofactor Interactions in Factor Xase. Biochemistry. 2005;44:10298–10304. doi: 10.1021/bi050638t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hemker HC, Giesen P, Al Dieri R, Regnault V, de Smedt E, Wagenvoord R, Lecompte T, Beguin S. Calibrated automated thrombin generation measurement in clotting plasma. Pathophysiol Haemost Thromb. 2003;33:4–15. doi: 10.1159/000071636. [DOI] [PubMed] [Google Scholar]
- 25.Pemberton S, Lindley P, Zaitsev V, Card G, Tuddenham EG, Kemball-Cook G. A molecular model for the triplicated A domains of human factor VIII based on the crystal structure of human ceruloplasmin. Blood. 1997;89:2413–21. [PubMed] [Google Scholar]
- 26.Radtke KP, Griffin JH, Riceberg J, Gale AJ. Disulfide bond-stabilized factor VIII has prolonged factor VIIIa activity and improved potency in whole blood clotting assays. J Thromb Haemost. 2007;5:102–8. doi: 10.1111/j.1538-7836.2006.02283.x. [DOI] [PubMed] [Google Scholar]