Abstract
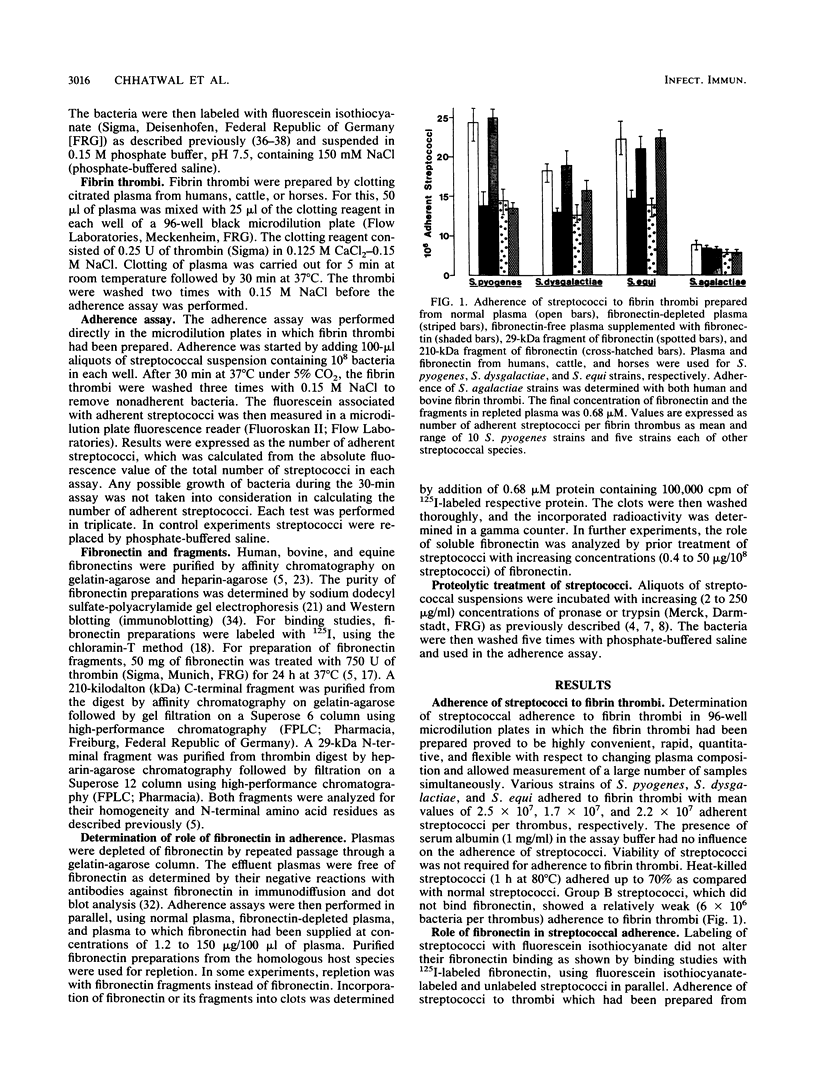
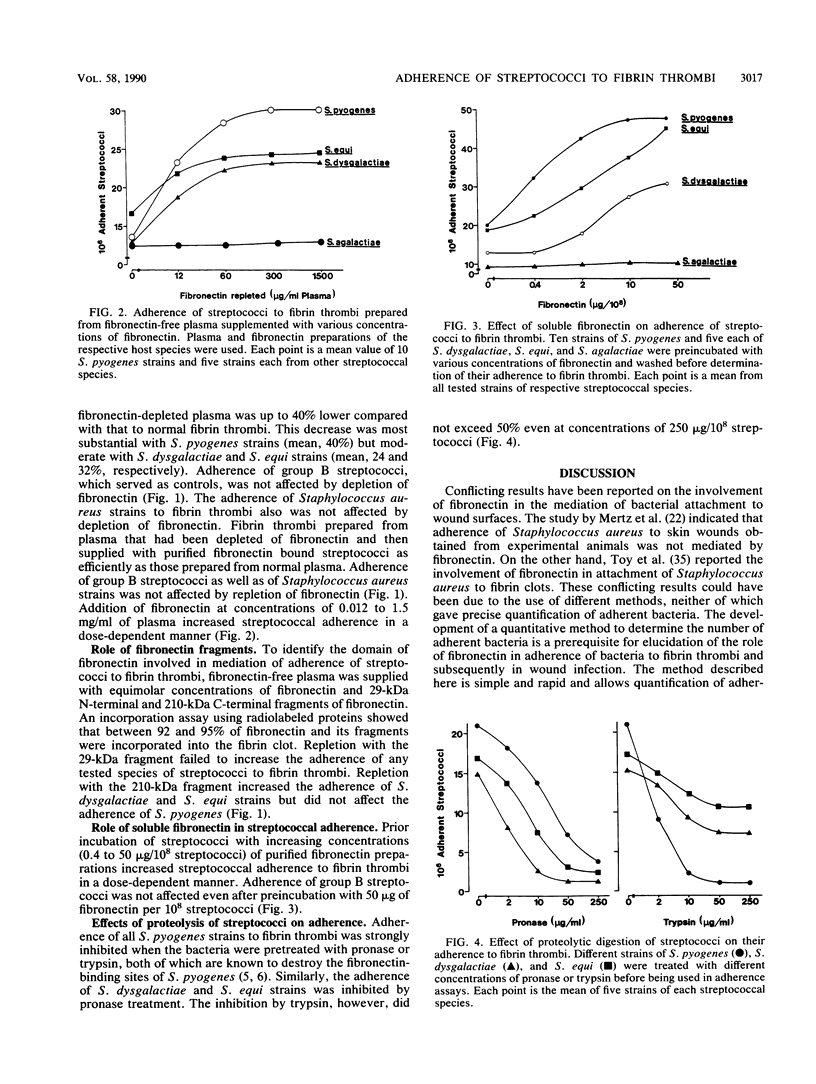
Adherence of group A, B, and C streptococci to fibrin thrombi was studied by using a novel fluorochrome microassay carried out in microdilution plates in which fibrin thrombi had been prepared by clotting citrated human, cattle, or horse plasma. Substantial adherence was observed with various strains of group A and C streptococci, whereas little was observed with group B streptococci. Adherence of all group A and C streptococcal strains decreased by up to 40% when fibronectin was depleted from the plasmas used for preparing fibrin thrombi, and fibronectin repletion increased adherence of streptococci in a dose-dependent manner. Addition of the 210-kilodalton C-terminal fragment of fibronectin to fibronectin-depleted plasma restored the adherence of group C but not group A streptococci, whereas addition of the 29-kilodalton N-terminal fragment was without any effect for all tested streptococcal strains. Prior incubation of group A and C streptococcal strains with fibronectin markedly increased their adherence, but treatment with proteases abolished their ability to adhere to fibrin thrombi. Adherence of group B streptococci was not affected by either fibronectin depletion or proteolytic digestion. These results indicate that both fibronectin incorporated into the fibrin matrix of thrombi and soluble fibronectin can mediate adherence of group A and C streptococci to fibrin thrombi and that binding sites for fibronectin located on the bacterial surface mediate this adherence.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P., Amrani D., Mosesson M. W., Bianco C. Receptors for cold-insoluble globulin (plasma fibronectin) on human monocytes. J Exp Med. 1981 Jan 1;153(1):42–60. doi: 10.1084/jem.153.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodén M. K., Flock J. I. Fibrinogen-binding protein/clumping factor from Staphylococcus aureus. Infect Immun. 1989 Aug;57(8):2358–2363. doi: 10.1128/iai.57.8.2358-2363.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhatwal G. S., Albohn G., Blobel H. Novel complex formed between a nonproteolytic cell wall protein of group A streptococci and alpha 2-macroglobulin. J Bacteriol. 1987 Aug;169(8):3691–3695. doi: 10.1128/jb.169.8.3691-3695.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhatwal G. S., Blobel H. Heterogeneity of fibronectin reactivity among streptococci as revealed by binding of fibronectin fragments. Comp Immunol Microbiol Infect Dis. 1987;10(2):99–108. doi: 10.1016/0147-9571(87)90003-8. [DOI] [PubMed] [Google Scholar]
- Chhatwal G. S., Lämmler C., Blobel H. Interactions of plasma proteins with group A, B, C and G streptococci. Zentralbl Bakteriol Mikrobiol Hyg A. 1985 Apr;259(2):219–227. doi: 10.1016/s0176-6724(85)80053-5. [DOI] [PubMed] [Google Scholar]
- Chhatwal G. S., Müller H. P., Blobel H. Characterization of binding of human alpha 2-macroglobulin to group G streptococci. Infect Immun. 1983 Sep;41(3):959–964. doi: 10.1128/iai.41.3.959-964.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhatwal G. S., Preissner K. T., Müller-Berghaus G., Blobel H. Specific binding of the human S protein (vitronectin) to streptococci, Staphylococcus aureus, and Escherichia coli. Infect Immun. 1987 Aug;55(8):1878–1883. doi: 10.1128/iai.55.8.1878-1883.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Dvorak H. F., Colvin R. B. Fibronectin in delayed-type hypersensitivity skin reactions: associations with vessel permeability and endothelial cell activation. J Immunol. 1981 Feb;126(2):787–793. [PubMed] [Google Scholar]
- Clark R. A., Lanigan J. M., DellaPelle P., Manseau E., Dvorak H. F., Colvin R. B. Fibronectin and fibrin provide a provisional matrix for epidermal cell migration during wound reepithelialization. J Invest Dermatol. 1982 Nov;79(5):264–269. doi: 10.1111/1523-1747.ep12500075. [DOI] [PubMed] [Google Scholar]
- Clark R. A., Quinn J. H., Winn H. J., Lanigan J. M., Dellepella P., Colvin R. B. Fibronectin is produced by blood vessels in response to injury. J Exp Med. 1982 Aug 1;156(2):646–651. doi: 10.1084/jem.156.2.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikawa L. S., Foster C. S., Harrist T. J., Lanigan J. M., Colvin R. B. Fibronectin in healing rabbit corneal wounds. Lab Invest. 1981 Aug;45(2):120–129. [PubMed] [Google Scholar]
- Gilchrest B. A., Nemore R. E., Maciag T. Growth of human keratinocytes on fibronectin -coated plates. Cell Biol Int Rep. 1980 Nov;4(11):1009–1016. doi: 10.1016/0309-1651(80)90173-3. [DOI] [PubMed] [Google Scholar]
- Grinnell F., Billingham R. E., Burgess L. Distribution of fibronectin during wound healing in vivo. J Invest Dermatol. 1981 Mar;76(3):181–189. doi: 10.1111/1523-1747.ep12525694. [DOI] [PubMed] [Google Scholar]
- Grinnell F., Feld M. K. Distribution of fibronectin on peripheral blood cells in freshly clotted blood. Thromb Res. 1981 Dec 1;24(5-6):397–404. doi: 10.1016/0049-3848(81)90074-8. [DOI] [PubMed] [Google Scholar]
- Grinnell F., Feld M., Minter D. Fibroblast adhesion to fibrinogen and fibrin substrata: requirement for cold-insoluble globulin (plasma fibronectin). Cell. 1980 Feb;19(2):517–525. doi: 10.1016/0092-8674(80)90526-7. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hörmann H., Richter H., Jelinić V. Fibrinmonomer binding to macrophages mediated by fibrin-binding fibronectin fragments. Thromb Res. 1985 Jul 15;39(2):183–194. doi: 10.1016/0049-3848(85)90106-9. [DOI] [PubMed] [Google Scholar]
- Krizek T. J., Robson M. C. Evolution of quantitative bacteriology in wound management. Am J Surg. 1975 Nov;130(5):579–584. doi: 10.1016/0002-9610(75)90516-4. [DOI] [PubMed] [Google Scholar]
- Kuusela P., Vartio T., Vuento M., Myhre E. B. Attachment of staphylococci and streptococci on fibronectin, fibronectin fragments, and fibrinogen bound to a solid phase. Infect Immun. 1985 Oct;50(1):77–81. doi: 10.1128/iai.50.1.77-81.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mertz P. M., Patti J. M., Marcin J. J., Marshall D. A. Model for studying bacterial adherence to skin wounds. J Clin Microbiol. 1987 Sep;25(9):1601–1604. doi: 10.1128/jcm.25.9.1601-1604.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miekka S. I., Ingham K. C., Menache D. Rapid methods for isolation of human plasma fibronectin. Thromb Res. 1982 Jul 1;27(1):1–14. doi: 10.1016/0049-3848(82)90272-9. [DOI] [PubMed] [Google Scholar]
- Mosher D. F. Fibronectin. Prog Hemost Thromb. 1980;5:111–151. [PubMed] [Google Scholar]
- Mosher D. F. Physiology of fibronectin. Annu Rev Med. 1984;35:561–575. doi: 10.1146/annurev.me.35.020184.003021. [DOI] [PubMed] [Google Scholar]
- Myhre E. B., Kuusela P. Binding of human fibronectin to group A, C, and G streptococci. Infect Immun. 1983 Apr;40(1):29–34. doi: 10.1128/iai.40.1.29-34.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E. Fibronectin and its receptors. Annu Rev Biochem. 1988;57:375–413. doi: 10.1146/annurev.bi.57.070188.002111. [DOI] [PubMed] [Google Scholar]
- Scheld W. M., Strunk R. W., Balian G., Calderone R. A. Microbial adhesion to fibronectin in vitro correlates with production of endocarditis in rabbits. Proc Soc Exp Biol Med. 1985 Dec;180(3):474–482. doi: 10.3181/00379727-180-42205. [DOI] [PubMed] [Google Scholar]
- Scheld W. M., Valone J. A., Sande M. A. Bacterial adherence in the pathogenesis of endocarditis. Interaction of bacterial dextran, platelets, and fibrin. J Clin Invest. 1978 May;61(5):1394–1404. doi: 10.1172/JCI109057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson W. A., Beachey E. H. Adherence of group A streptococci to fibronectin on oral epithelial cells. Infect Immun. 1983 Jan;39(1):275–279. doi: 10.1128/iai.39.1.275-279.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. E., Musich P. R., Johnson D. A. Sodium dodecyl sulfate enhancement of quantitative immunoenzyme dot-blot assays on nitrocellulose. Anal Biochem. 1989 Feb 15;177(1):212–219. doi: 10.1016/0003-2697(89)90043-2. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toy P. T., Lai L. W., Drake T. A., Sande M. A. Effect of fibronectin on adherence of Staphylococcus aureus to fibrin thrombi in vitro. Infect Immun. 1985 Apr;48(1):83–86. doi: 10.1128/iai.48.1.83-86.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin-Weigand P., Chhatwal G. S., Blobel H. A simple method for quantitative determination of bacterial adherence to human and animal epithelial cells. Microbiol Immunol. 1987;31(10):1017–1023. doi: 10.1111/j.1348-0421.1987.tb01334.x. [DOI] [PubMed] [Google Scholar]
- Valentin-Weigand P., Chhatwal G. S., Blobel H. Adherence of streptococcal isolates from cattle and horses to their respective host epithelial cells. Am J Vet Res. 1988 Sep;49(9):1485–1488. [PubMed] [Google Scholar]
- Valentin-Weigand P., Grulich-Henn J., Chhatwal G. S., Müller-Berghaus G., Blobel H., Preissner K. T. Mediation of adherence of streptococci to human endothelial cells by complement S protein (vitronectin). Infect Immun. 1988 Nov;56(11):2851–2855. doi: 10.1128/iai.56.11.2851-2855.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudaux P. E., Waldvogel F. A., Morgenthaler J. J., Nydegger U. E. Adsorption of fibronectin onto polymethylmethacrylate and promotion of Staphylococcus aureus adherence. Infect Immun. 1984 Sep;45(3):768–774. doi: 10.1128/iai.45.3.768-774.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercellotti G. M., Lussenhop D., Peterson P. K., Furcht L. T., McCarthy J. B., Jacob H. S., Moldow C. F. Bacterial adherence to fibronectin and endothelial cells: a possible mechanism for bacterial tissue tropism. J Lab Clin Med. 1984 Jan;103(1):34–43. [PubMed] [Google Scholar]
- Wideback K., Kronvall G. Surface receptors for serum albumin in group C and G streptococci show three different types of albumin specificity. Infect Immun. 1982 Dec;38(3):1154–1163. doi: 10.1128/iai.38.3.1154-1163.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]



