Abstract
The Ferric Uptake Regulator (Fur) is a transcriptional regulator that is conserved across a broad number of bacterial species and has been shown to regulate expression of iron uptake and storage genes. Additionally, Fur has been shown to be an important colonization factor of the gastric pathogen Helicobacter pylori. In H. pylori, Fur-dependent regulation appears to be unique in that Fur is able to act as a transcriptional repressor when bound to iron as well as in its iron free (apo) form. To date, apo-regulation has not been identified in any other bacterium. To determine whether Fur from other species has the capacity for apo-regulation, we investigated the ability of Fur from Escherichia coli, Campylobacter jejuni, Desulfovibrio vulgaris Hildenborough, Pseudomonas aeruginosa, and Vibrio cholerae to complement both iron-bound and apo-Fur regulation within the context of an H. pylori fur mutant. We found that while some Fur species (E. coli, C. jejuni and V. cholerae) complemented iron-bound regulation, apo-regulation was unable to be complemented by any of the examined species. These data suggest that despite the conservation among bacterial Fur proteins, H. pylori Fur contains unique structure/function features that make it novel in comparison to Fur from other species.
Introduction
Helicobacter pylori persistently colonizes the gastric mucosa of the majority of the world’s human population (Blaser 1998). This fact seems remarkable when one considers that this site encounters large fluctuations in pH (McArthur and Feldman 1989), iron availability (Andrews et al. 2003), and other stresses (Seyler et al. 2001). Thus, in order to survive in this niche, H. pylori must be able to adapt to this dynamic, tumultuous environment. Indeed, a number of regulatory proteins in this organism have been shown to serve as essential components required for adaptation to stressful environments (Bury-Mone et al. 2004; Delany et al. 2005; Gancz et al. 2006). Included among these is the Ferric Uptake Regulator (Fur), which is involved in H. pylori colonization (Bury-Mone et al. 2004; Gancz et al. 2006) and is a necessary component for adaptation to low pH (Bijlsma et al. 2002) and iron limitation (Bijlsma et al. 2000).
In most organisms, iron is essential (Ratledge and Dover 2000) because it plays a role in respiration, electron transport, and is a required cofactor for many enzymes. Paradoxically, too much iron is as detrimental as insufficient amounts of iron since excess free iron leads to the Fenton reaction, which results in the formation of DNA-damaging and protein denaturing hydroxyl radicals (Gutteridge et al. 2001). Thus, there must be a delicate balance between acquiring a sufficient amount of iron but not so much as to overload the system. Indeed, this balance is achieved in many Gram positive and Gram negative bacterial species by intricate control over the transcription of iron uptake and storage genes by Fur.
Classically, Fur functions as a transcriptional repressor protein that binds to conserved promoter regulatory sequences known as Fur boxes (Hantke 2001). These Fur boxes often overlap the −10 and −35 promoter elements. Thus when iron is available, Fur binds its ferrous iron cofactor, dimerizes and binds to the Fur box. This complex prevents the binding of the RNA polymerase and gene expression is repressed. Conversely, as iron becomes limited, there is an insufficient amount of the ferrous cofactor to bind to Fur and thus, the protein is unable to dimerize and bind to the promoter elements. This allows RNA polymerase to bind and the gene is transcribed. H. pylori uses this type of classical regulation to control expression of several genes including the aliphatic amidase, amiE, which plays an important role in ammonia production through the hydrolysis of aliphatic amides (Ernst et al. 2005; Gancz et al. 2006; van Vliet et al. 2003). However, Fur regulation in H. pylori is more complex than this classic model since Fur has also been shown to repress expression of some additional promoters in an iron depleted (apo) form (Delany et al. 2001; Delany et al. 2001; Ernst et al. 2005). For this apo-regulation, in the absence of iron the apo-Fur protein can bind to the promoters of its target genes and block transcription. Thus, genes repressed by apo-Fur are transcribed in iron-replete conditions. Currently, the apo-Fur regulon is predicted to contain 16 gene targets (Ernst et al. 2005; Gancz et al. 2006). Of these targets, only sodB, a superoxide dismutase important for oxidative defense, and pfr, an iron storage molecule, have been definitively shown to be directly regulated by apo-Fur (Spiegelhalder et al. 1993; Delany et al. 2001; Ernst et al. 2005). Expression of both of these genes is repressed by apo-Fur when iron is limited, but this repression is lost in a fur mutant strain.
Recent microarray analyses of Campylobacter jejuni (Holmes et al. 2005) and Desulfovibrio vulgaris Hildenborough (Bender et al. 2007) suggest that apo-Fur regulation may occur in these organisms; however, direct binding of apo-Fur to any identified target genes has not been shown in these organisms. Indeed, despite the fact that Fur has been extensively studied in many other organisms (Carpenter et al. 2009) there is currently no direct evidence that bacterial species other than H. pylori utilize apo-Fur regulation. This fact suggests that H. pylori Fur contains unique structure/function features in comparison to Fur from other bacterial species. Alternatively, it is possible that Fur from other bacterial species encodes the capacity for apo-regulation, but this form of regulation simply has not been identified in these organisms. To begin to examine these possibilities, herein we describe studies that investigate the ability of Fur from other bacterial species to complement both iron-bound and apo-Fur regulation within the context of an H. pylori fur mutant.
Materials and Methods
Bacterial strains and growth
The strains and plasmids used in this study are listed in Table 1. H. pylori strains were maintained as frozen stocks at −80° C in brain heart infusion medium supplemented with 20% glycerol and 10% fetal bovine serum (FBS). Bacteria were grown on horse blood agar plates containing 4% Columbia agar base (Neogen Corporation, USA), 5% defibrinated horse blood (HemoStat Labs, USA), 0.2% β-cyclodextrin, 10 μg/ml vancomycin (Sigma, USA), 5 μg/ml cefsulodin (Sigma, USA), 2.5 U/ml polymyxin B (Sigma, USA), 5 μg/ml trimethoprim (Sigma, USA), and 8 μg/ml of amphotericin B (Amresco, USA). As noted in Table 1, cultures and plates were supplemented with 8 μg/ml chloramphenicol (Cm) (EMD Chemicals Inc., USA), and/or 25 ug/ml kanamycin (Kan) (Gibco, USA). All H. pylori was grown in gas evacuation jars under microaerophilic conditions (5% O2, 10% CO2, 85% N2) generated by Anoxomat gas evacuation (Spiral Biotech, USA).
TABLE 1.
Plasmids and strains used in this study
| Plasmid or strain | Description | Reference |
|---|---|---|
| Plasmids | ||
| pDSM226 | pGEM T-easy:: H. pylori fur | (Carpenter et al. 2007) |
| pDSM340 | pTM117:: H. pylori fur | (Carpenter et al. 2007) |
| pDSM515 | pTM117:: Hp V. cholerae fur | This study |
| pDSM521 | pGEM T-easy:: Hp E. coli fur | This study |
| pDSM522 | pGEM T-easy:: Hp C. jejuni fur | This study |
| pDSM523 | pGEM T-easy:: Hp V. cholerae fur | This study |
| pDSM526 | pTM117:: Hp E. coli fur | This study |
| pDSM560 | pTM117:: Hp C. jejuni fur | This study |
| pDSM642 | pGEM T-easy:: Hp P. aeruginosa fur | This study |
| pDSM652 | pTM117:: Hp P. aeruginosa fur | This study |
| pDSM755 | pGEM T-easy:: Hp D. vulgaris Hildenborough fur | This study |
| pDSM758 | pTM117:: Hp D. vulgaris Hildenborough fur | This study |
| H. pylori strains | ||
| DSM300 | G27 Δfur:: cat, Cmr | (Carpenter et al. 2007) |
| DSM343 | G27 Δfur (pDSM340), Kanr Cmr | (Carpenter et al. 2007) |
| DSM554 | G27 Δfur (pDSM526), Kanr Cmr | This study |
| DSM557 | G27 Δfur (pDSM523), Kanr Cmr | This study |
| DSM583 | G27 Δfur (pDSM560), Kanr Cmr | This study |
| DSM712 | G27 Δfur (pDSM652), Kanr Cmr | This study |
| DSM761 | G27 Δfur (pDSM758), Kanr Cmr | This study |
Construction of heterologous Fur expression strains
Translational fusions in which the H. pylori fur promoter and 5′ nontranslated region, up to but not including the H. pylori Fur start codon, was directly fused to the start codon of the fur coding sequence of C. jejuni 11168 (Parkhill et al. 2000), D. vulgaris Hildenborough NCIMB 8303 (Heidelberg et al. 2004), E. coli O157::H7 EDL933 (Perna et al. 2001), P. aeruginosa PAO1 (Stover et al. 2000) or V. cholerae N16961 (Heidelberg et al. 2000) were constructed. In designing the translational fusions, the native H. pylori promoter and Ribosomal Binding Site (RBS) were used to bypass any potential problems with altered expression of a foreign fur promoter or RBS in the H. pylori system. For each construct, we utilized Splicing by Overlap Extension (SOE) PCR (Horton et al. 1993) to fuse the H. pylori promoter sequence to the heterologous Fur coding sequences. This was accomplished in a series of three PCR reactions using the primers listed in Table 2.
TABLE 2.
Primers used in this study
| Primerb | Sequence (5′-3′)a | Reference |
|---|---|---|
| RPA primers | ||
| amiE-RPA-F | GGTTTGCCTGGGTTGGAT | (Gancz et al. 2006) |
| amiE-RPA-R | GATTTTGCGGTATTTTG | (Gancz et al. 2006) |
| pfr-RPA-F | GCGGCTGAAGAATACGAG | (Carpenter et al. 2007) |
| pfr-RPA-R | CTGATCAGCCAAATACAA | (Carpenter et al. 2007) |
| Hp fur RPA F | GAGCGCTTGAGGATGTCTATC | (Carpenter et al. 2007) |
| Hp fur RPA R | GTGATCATGGTGTTCTTTAGC | (Carpenter et al. 2007) |
| Cj fur RPA F | CCTGATTTAAATGTAGGAATTGC | This study |
| Cj fur RPA R | AAAGCTGCATCAAATGCCCTG | This study |
| Dv fur RPA F | CAACAGCCTCAAGGTGAC | This study |
| Dv fur RPA R | GTTCGATGTCGTCGTCGA | This study |
| Ec fur RPA F | GGAGCCGGACAACCATC | This study |
| Ec fur RPA R | CGCTTCGATGGAATCATC | This study |
| Pa fur RPA F | GACTCGGCCGAGCAAC | This study |
| Pa fur RPA R | ATTTCCTTCTGGCGCTTCTC | This study |
| Vc fur RPA F | CTCCCACGGCTTAAGATTTTAG | This study |
| Vc fur RPA R | GACGTTGTTCAATCACATCG | This study |
| SOE primers | ||
| FurCF1 (XbaI) | TCTAGAAAGGCTCACTCTACCCTATT | (Carpenter et al. 2007) |
| Cj furR (SalI) | GTCGACAAATGAGGATAAGGATTGATCCC | This study |
| Cj SOE F | CATTTTACGGATAAGGGAAATATCAGCATGCTGATAGAAAATGTGGAATATGATG | This study |
| Cj SOE R | CATCATATTCCACATTTTCTATCAGCATGCTGATATTTCCCTTATCCGTAAAATG | This study |
| Dv fur R | GGTACCTCGTTCACCCGCAC | This study |
| Dv SOE F | CATTTTACGGATAAGGGAAATATCAGCATGAAGGAACCCATCGCCGTATTTC | This study |
| Dv SOE R | GAAATACGGCGATGGGTTCCTTCATGCTGATATTTCCCTTATCCGTAAAATG | This study |
| Dv SOE F2 | GAAGCTCCTGTGCGACTCAGGTCTCGCCAAGGAAGTGC | This study |
| Dv SOE R2 | GCACTTCCTTGGCGAGACCTGAGTCGCACAGGAGCTTC | This study |
| Ec FurR (SalI) | GTCGACGATAAGGTCTGGCAGGAAATTCGC | This study |
| Ec SOE F | CATTTTACGGATAAGGGAAATATCAGCATGACTGATAACAATACCGCCCTAAAGAAAG | This study |
| Ec SOE R | CTTTCTTTAGGGCGGTATTGTTATCAGTCATGCTGATATTTCCCTTATCCGTAAAATG | This study |
| Pa furR (KpnI) | GGTACCTGGCCGCCCAGAACTGAAC | This study |
| Pa SOE F | CATTTTACGGATAAGGGAAATATCAGCATGGTTGAAAATAGCGAACTTCGAAAAGC | This study |
| Pa SOE R | GCTTTTCGAAGTTCGCTATTTTCAACCATGCTGATATTTCCCTTATCCGTAAAATG | This study |
| Vc FurR (SalI) | GTCGACAACCCACCATTCGGTGGG | This study |
| Vc SOE F | CATTTTACGGATAAGGGAAATATCAGCATGTCAGACAATAACCAAGCGCTAAAGG | This study |
| Vc SOE R | CCTTTAGCGCTTGGTTATTGTCTGACATGCTGATATTTCCCTTATCCGTAAAATG | This study |
Restriction endonuclease sites are underlined
Important restriction sites are included in parentheses
Briefly, template DNA from H. pylori G27 was isolated using the Invitrogen Easy DNA kit (USA), and used in combination with genomic DNA from C. jejuni 11168 (provided by D. Hendrixson), D. vulgaris Hildenborough NCIMB 8303 (provided by J. Wall), E. coli O157::H7 EDL933 (provided by A. O’Brien and L. Teele), P. aeruginosa PAO1 (provided by V. Lee), or V. cholerae N16961 (provided by A. Camilli). In the first and second PCR reactions, the H. pylori fur promoter was amplified such that the 3′ end of the fragment contained a complementary and overlapping region with the individual heterologous fur sequences and the heterologous fur coding sequences were amplified with a 5′ complementary overlapping extension for the H. pylori fur promoter sequence, respectively. In the final reaction, each of these products was mixed together, the complementary regions annealed and the fused product amplified using the extreme flanking primers (Table 2). Each of these H. pylori fur promoter – heterologous fur coding sequence products was initially subcloned into the pGEMT-Easy vector (Promega, USA) (Table 1) prior to digestion and ligation into the appropriately digested pTM117 vector, which has previously been shown to be an efficient complementation vector for fur in H. pylori (Carpenter et al. 2007). In addition, DSM343, a strain carrying a pTM117 vector carrying the H. pylori fur promoter driving expression of H. pylori fur (pDSM340) was prepared for use as a positive control (Carpenter et al. 2007). Each of these vectors was next transformed into DSM300, which is a H. pylori Δfur mutant of strain G27 (Gancz et al. 2006). Transformants were selected on the appropriate antibiotics (Table 1). To verify that each of the individual heterologous fusions was correct and contained no mutations, each of the pTM117 vectors (pDSM515, pDSM526, pDSM560, pDSM652, pDSM758) was subsequently recovered from each of the H. pylori transformant strains and sequenced.
RNase Protection Assays
Each of the heterologous expression strains, as well as the wild type and Δfur H. pylori controls, were grown for 18 hours in liquid culture (Brucella broth (BB) supplemented with 10% FBS, 50 μg/ml vancomycin, and 25 μg/ml Kan to ensure maintenance of the plasmid). One half of each culture was removed for RNA extraction (t0) while the other half was depleted of iron by the addition of 200 μM of the iron chelator 2,2′ dipyridyl (dpp). After one hour of chelation (t60) these cells were then harvested for RNA extraction. RNA was extracted as previously described (Thompson et al. 2003). To examine expression of the fur transcript from the plasmid, riboprobe templates were constructed for C. jejuni, E. coli, D. vulgaris, H. pylori, P. aeruginosa, and V. cholerae fur using the primer pairs listed in Table 2. To measure iron-bound and apo-Fur regulation, riboprobe templates were also generated using the primer pairs listed in Table 2 for amiE and pfr, respectively. The resulting fur, amiE and pfr amplicons were ligated to pGEMT-easy (Promega, USA) and riboprobes were generated with the Maxiscript kit (Applied Biosystems, USA) and 50 μCi [32P] UTP (Perkin Elmer, USA). 1.5 μg of total RNA was then used to conduct RNase protection assays (RPAs) with the RPA III kit (Applied Biosystems, USA) as previously described (Carpenter et al. 2007). The gels were exposed to phosphor screens. The screens were scanned using a FLA-5100 scanner (Fujifilm, USA) and the intensity of protected bands was quantified with Multi-Gauge software (version 3.0, Fujifilm, USA).
Western blotting
To confirm expression of each Fur species, bacteriallysates were prepared from the heterologous strains grown as described above. Protein concentration was measured using the BCA protein assay (Thermo Scientific, USA) and equal concentrations of each sample were separated by sodium dodecyl sulfate-polyacrylamidegel electrophoresis (SDS-PAGE) using an 18% separating gel. The separated proteins were transferred to nitrocellulose membranes using a semidry transfer apparatus (Owl; Thermo Scientific, USA), and membranes were probed with anti-Fur antibodies. Given that antibodies specific for each of the individual Fur species were not available, we utilized polyclonal antibodies from available species and relied on the conservation of the protein to aid in the detection of Fur. Membranes were first probed with a 1:100 dilution of P. aeruginosa Martha 2472 polyclonal rabbit anti-Fur antibody (a kind gift from M. Vasil), followed by a 1:20,000 dilution of HRP-conjugated bovine anti-rabbit IgG secondary antibody (Santa Cruz Biotechnology). Proteins were detected using the SuperSignal West Pico Chemiluminescent Substrate kit (Thermo Scientific/Pierce, USA) and a LAS-3000 Intelligent Dark Box with LAS-3000 Lite capture software (Fujifilm, USA).
In order to detect the other heterologous Fur proteins, the membrane was then stripped by incubation at approximately 50°C in stripping solution (2% SDS, 62.5 mM Tris HCl pH 6.8, 10mM DTT) for 30 minutes, and reprobed with a 1:1,000 dilution of rabbit polyclonal anti-C. jejuni Fur antibody (a kind gift from A. Stintzi) followed by a 1:20,000 dilution of HRP-conjugated bovine anti-rabbit IgG secondary antibody (Santa Cruz Biotechnology, USA). After detection and scanning, the membrane was then stripped again and probed with a 1:100 dilution of rabbit polyclonal anti-H. pylori Fur antibody, which was prepared using the Rabbit Quick Draw protocol and produced by Pocono Rabbit Farm and Laboratory (Carpenter et al. 2010 Submitted). This was followed by a 1:20,000 dilution of HRP-conjugated bovine anti-rabbit IgG secondary antibody (Santa Cruz Biotechnology, USA) and detected as described above.
Results
Sequence conservation among Fur species
Comparison of the amino acid sequence of Fur from H. pylori to Fur encoded by several bacterial species in which Fur has been well studied (Pohl et al. 2003; Holmes et al. 2005; Bender et al. 2007; Sheikh and Taylor 2009) showed a moderate degree of conservation among the Fur proteins (Table 3, Fig. 1). Of note, of the species examined, the highest degrees of identity were found with C. jejuni, which is a close relative of H. pylori, and D. vulgaris, which is more distantly related to H. pylori. Together these two microbes remain the only other species that have been suggested to utilize apo-Fur regulation (Holmes et al. 2005; Bender et al. 2007). However, moderate levels of identity and similarity were also found in comparison to E. coli, P. aeruginosa, and V. cholerae Fur, none of which are currently suspected to utilize apo-Fur regulation. Based on this conservation, we wondered if any of these heterologous Fur species would be able to complement classic iron-bound and/or apo-Fur regulation when expressed within the context of a H. pylori fur mutant.
Table 3.
Percent identity and similarity of bacterial Fur amino acid sequences as compared to H. pylori Fura
| Identity to H. pylori | Similarity to H. pylori | |
|---|---|---|
| C. jejuni | 32.6% | 52.2% |
| D. vulgaris | 30.5% | 49.3% |
| E. coli | 29.1% | 52.7% |
| P. aeruginosa | 26.5% | 54.0% |
| V. cholerae | 25.6% | 52.0% |
Identity and similarity were calculated using MatGat 2.0 (Campanella et al. 2003).
Figure 1.
Alignments of Fur coding sequences. Amino acid sequence alignment of Fur from C. jejuni (CJ), E. coli (EC), D. vulgaris (DV), H. pylori (HP), P. aeruginosa (PA), and V. cholerae (VC). Identical residues are indicated by dark grey, conservative residues by medium grey, and similar residues by light grey. The alignment was constructed using AlignX software (Vector NTI, Invitrogen, USA).
Analysis of iron-bound Fur complementation
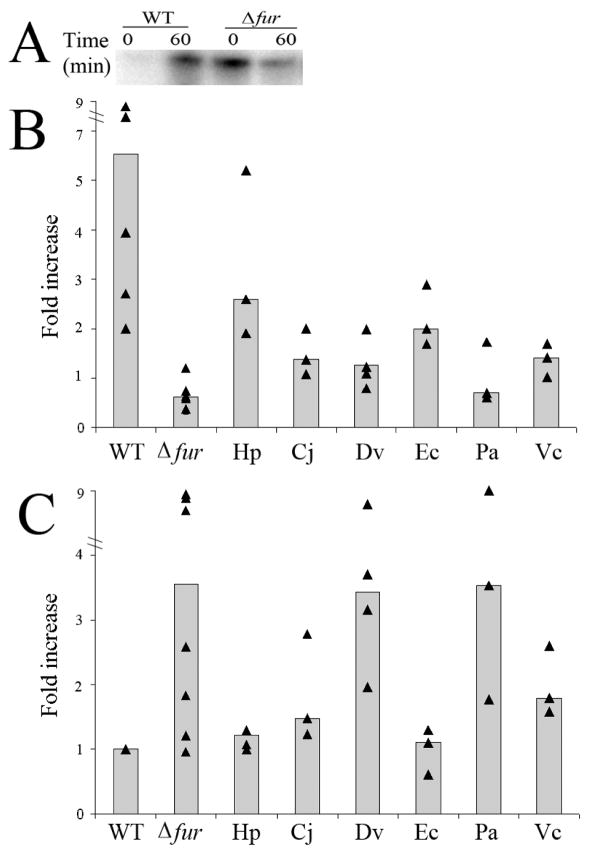
To determine whether the individual heterologous Fur constructs could complement iron-bound Fur regulation in the Δfur G27 strain, changes in the transcription of amiE were monitored in response to iron availability; amiE, encodes an aliphatic amidase and is known to be repressed by iron-bound Fur (van Vliet et al. 2003). As shown in Figure 2A, addition of the iron chelator, dpp, to the wild type strain resulted in a large increase in amiE expression (4.9 fold). However, this increase is lost in the Δfur strain (0.6 fold), which additionally shows increased basal level expression of amiE even in the presence of iron (Fig. 2A). These results are in accordance with amiE being repressed by the iron bound form of Fur; in the absence of iron, iron-free Fur is no longer able to bind to the Fur box and repress expression of amiE. For each of the heterologous strains, three to four biological repeats of the chelation and RPAs were repeated and the fold change relative to t0 calculated. In order to show the reproducibility of the RPA data, the data is represented in a graphical format in Figures 2B and 2C. In these graphs, the fold change for each strain and biological repeat is displayed as a point on the graph. Additionally, to allow for easy comparison between the strains, the median fold change is depicted as a bar. As expected (Carpenter et al. 2007), increased amiE expression in response to iron chelation was partially restored (2.6 fold) in the strain expressing G27 Fur in the context of the complementation vector pTM117 (Fig. 2B). Analysis of amiE expression in the strains carrying the heterologous Fur constructs showed the following changes: C. jejuni (1.4 fold), D. vulgaris (1.2 fold), E. coli (2.0 fold), P. aeruginosa (0.7 fold) and V. cholerae (1.4 fold).
Figure 2.
Determination of the ability of the heterologous constructs to complement iron-bound Fur regulation of amiE. Wild type H. pylori (WT), Δfur H. pylori (Δfur), and Δfur H. pylori carrying the heterologous Fur constructs from C. jejuni (Cj), D. vulgaris (Dv), E. coli (Ec), H. pylori (Hp), P. aeruginosa (Pa), and V. cholerae (Vc) were grown to exponential phase in iron replete liquid media. On the subsequent day, one half was used for RNA isolation (t0). The other half was exposed to iron deplete conditions for one hour by the addition of 200 μM dpp prior to isolation of the RNA (t60). Each triangle represents a biologically independent set of RNA. Median fold change is represented as a bar for each strain. (A) RPA using an amiE riboprobe showed classical Fur dependent changes in amiE expression. (B) Graphic depiction of the fold increase in expression of amiE calculated by comparing the relative amount of protected riboprobe in the iron deplete (t60) condition to the iron replete condition (t0). (C) Basal level of repression of amiE at t0 in each of the heterologous strains as compared to WT.
Given that the Δfur strain showed an increased basal level expression of amiE (3.6 fold) even in the presence of iron (Fig. 2A), we also assessed whether there was a difference in the relative level of expression of amiE between strains at the t0 time point since this also would be an indication of complementation. For this analysis, the level of amiE for each of the heterologous constructs at t0 was calculated relative to the level expressed in the wild type at t0. As expected (Carpenter et al. 2007), basal level expression of amiE in the strain expressing G27 Fur in the context of pTM117 was similar to wild type (1.2 fold), thus indicating that Fur carried on this vector is able to complement a chromosomal fur mutation (Fig. 2C). Analysis of amiE basal level expression in the strains carrying the heterologous Fur constructs showed the following changes: C. jejuni (1.5 fold), D. vulgaris (3.0 fold), E. coli (1.1 fold), P. aeruginosa (3.5 fold), and V. cholerae (1.8 fold). Taken together with the above comparison, these data indicate that classic iron-bound Fur regulation in H. pylori is able to be partially complemented by Fur from C. jejuni, E. coli, and V. cholerae but not by D. vulgaris or P. aeruginosa Fur.
Comparison of apo-Fur complementation
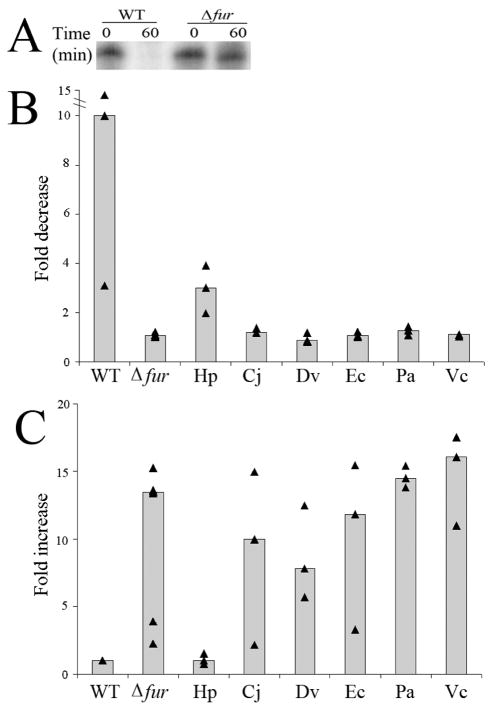
Despite the identity and similarity among the Fur proteins (Table 3, Fig. 1), apo-Fur regulation has thus far only been definitively identified in H. pylori (Spiegelhalder et al. 1993; Delany et al. 2001; Ernst et al. 2005). To determine whether the individual heterologous Fur proteins could complement apo-Fur regulation in the Δfur G27 strain, changes in the transcription of pfr were monitored in response to iron availability; pfr, encodes a prokaryotic nonheme iron-containing ferritin that is repressed by apo-Fur (Delany et al. 2001). As shown in Figure 3A, addition of dpp to the wild type strain resulted in a large decrease in pfr expression (10.0 fold). However, this decrease is lost in the Δfur strain (1.1 fold). These results are in accordance with pfr being repressed by the apo-Fur (Delany et al. 2001; Ernst et al. 2005); in the absence of iron, apo-Fur binds to the Fur box and represses expression of pfr. Once again, for each of the heterologous strains, three to four biological repeats of the chelation and RPAs were performed and the fold change relative to t0 calculated. As expected (Carpenter et al. 2007), decreased pfr expression in response to iron chelation was partially restored (3.0 fold) in the strain expressing G27 Fur in the context of pTM117 (Fig. 3B). Analysis of pfr expression in the strains carrying the heterologous Fur constructs showed the following changes: C. jejuni (1.2 fold), D. vulgaris (0.9 fold), E. coli (1.1 fold), P. aeruginosa (1.3 fold), and V. cholerae (1.1 fold).
Figure 3.
Determination of the ability of the heterologous constructs to complement apo-Fur regulation of pfr. Wild type H. pylori (WT), Δfur H. pylori (Δfur), and Δfur H. pylori carrying the heterologous Fur constructs from C. jejuni (Cj), D. vulgaris (Dv), E. coli (Ec), H. pylori (Hp), P. aeruginosa (Pa), and V. cholerae (Vc) were grown to exponential phase in iron replete liquid media. On the subsequent day, one half was used for RNA isolation (t0). The other half was exposed to iron deplete conditions for one hour by the addition of 200 μM dpp prior to isolation of the RNA (t60). Each triangle represents a biologically independent set of RNA. Median fold change is represented as a bar for each strain. (A) RPA using a pfr riboprobe showed apo-Fur dependent changes in pfr expression. (B) Graphic depiction of the fold decrease in expression of pfr calculated by comparing the relative amount of protected riboprobe in the iron replete (t0) condition to the iron deplete condition (t60). (C) Basal level of repression of pfr at t60 in each of the heterologous strains as compared to WT.
Given that the Δfur strain showed an increased level of expression of pfr (8.6 fold) in the absence of iron (Fig. 3A), we additionally asked whether there was a difference in the relative level of expression of pfr between strains at the t60 time point, since this would also be an indication of complementation. For this analysis, the level of pfr for each of the heterologous constructs at t60 was calculated relative to the level expressed in the wild type at t60. As expected (Carpenter et al. 2007), basal level expression of pfr in the strain expressing G27 Fur in the context of pTM117 was similar to wild type (1.0 fold), thus indicating that Fur carried in the context of pTM117 is able to complement a chromosomal fur mutation (Fig. 3C). Analysis of pfr basal level expression in the absence of iron in strains carrying the heterologous Fur constructs showed the following changes (Fig. 3C): C. jejuni (10.0 fold), D. vulgaris (7.8 fold), E. coli (11.9 fold), P. aeruginosa (14.5 fold) and V. cholerae (16.0 fold). Thus, all of the heterologous fusions exhibited a Δfur phenotype for apo-Fur regulation. Taken together with the above comparison, these data indicate that apo-Fur regulation in H. pylori is unable to be complemented by Fur from C. jejuni, D. vulgaris, E. coli, P. aeruginosa, or V. cholerae. This may suggest that apo-Fur regulation depends on unique structural features of H. pylori Fur that are absent in the other Fur proteins.
Confirmation of expression and translation of fur transcript
Since iron-bound complementation was not observed for all of the heterologous constructs and apo-Fur complementation was only observed in the control Δfur strain expressing H. pylori Fur on pTM117, we next confirmed that these results were not biased by an inability of the heterologous fur to be transcribed or for transcript to be stably maintained in H. pylori. To address these concerns, a riboprobe specific for each heterologous Fur species was generated using the primer pairs indicated in Table 2, and RPAs were conducted to detect each fur transcript. fur expression was detected in each strain (data not shown) therefore, lack of gene expression or instability of the heterologous mRNA is not responsible for the lack of complementation of Fur regulation.
Finally, given that we could detect transcript for each heterologous Fur species, we asked whether or not we could also detect each of the Fur proteins. As shown in Figure 4A, the P. aeruginosa Fur antibody was able to detect E. coli, P. aeruginosa, and V. cholerae Fur expression within the context of the H. pylori Δfur strain. The C. jejuni Fur antibody was able to detect expression of C. jejuni, E. coli, P. aeruginosa, and V. cholerae Fur (Fig. 4B) and the H. pylori antibody was able to detect expression of the H. pylori, C. jejuni, E. coli, D. vulgaris, and P. aeruginosa Fur proteins (Fig. 4C). Taken together, these data indicate that each of the heterologous Fur species is translated and accumulated within the context of the Δfur H. pylori strain (Fig. 4). Furthermore, since each of the various polyclonal Fur antibodies were unable to detect all Fur species, these results imply that despite the identity and similarity among the proteins (Table 1), there must be considerable Fur structural differences among the various species.
Figure 4.
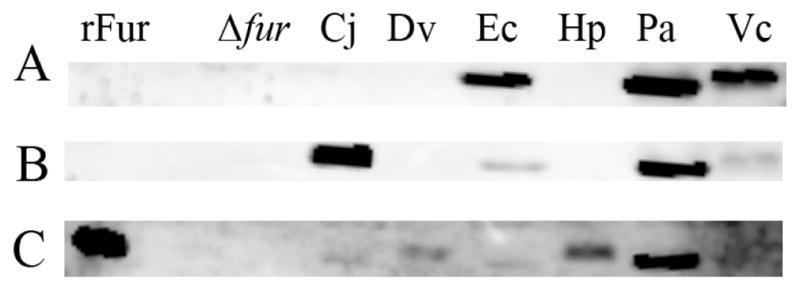
Anti-Fur Western blot. Purified recombinant H. pylori G27 Fur (rFur), and equal concentrations of lysates from Δfur H. pylori (Δfur) and Δfur H. pylori carrying the Fur constructs from C. jejuni (Cj), D. vulgaris (Dv), E. coli (Ec), H. pylori (Hp), P. aeruginosa (Pa), and V. cholerae (Vc) were subjected to Western blot analysis. (A) Martha 2472 rabbit polyclonal anti-P. aeruginosa Fur antibody was used to detect E. coli, P. aeruginosa, and V. cholerae Fur. (B) Polyclonal rabbit anti-C. jejuni Fur antibody was used to detect C. jejuni, E. coli, P. aeruginosa, and V. cholerae Fur proteins. (C) Polyclonal rabbit anti-H. pylori Fur antibody was used to detect recombinant H. pylori Fur, C. jejuni, D. vulgaris, E. coli, H. pylori and P. aeruginosa Fur. These data are representative of multiple independent experiments.
Discussion
Fur has been characterized in a diverse number of bacterial species, and shown to play a crucial role in iron homeostasis (Ernst et al. 1978; Hantke 1984; Ochsner et al. 1995; Horsburgh et al. 2001; van Vliet et al. 2002; Fiorini et al. 2008). Typically, Fur only acts as a repressor when bound to iron. Despite extensive study, to date, H. pylori Fur holds the distinction of being the only Fur definitively shown to repress in the absence of its iron cofactor (Bereswill et al. 2000; Ernst et al. 2005). Though plasmid complementation systems are often not as efficient as chromosomal borne systems, overall, our data indicate that both iron-bound and apo-Fur regulation can be partially complemented by H. pylori Fur carried on a plasmid vector and expressed in the Δfur strain (Fig. 2C, 2B). Additionally, iron-bound Fur regulation can be partially complemented by expression of C. jejuni, E. coli, and V. cholerae Fur in H. pylori Δfur. However, apo-Fur regulation is unable to be complemented by any of the examined Fur proteins from the five other bacterial species. This strongly suggests that H. pylori Fur contains unique structure/function features in comparison to Fur from other bacterial species. In turn, these features likely affect the ability of Fur to recognize and bind its DNA target. H. pylori is an A/T-rich organism (approximately 60%) (Alm et al. 1999; Baltrus et al. 2009), and the Fur box consensus sequence appears less conserved among the iron-bound Fur regulated H. pylori genes than the consensus sequences within these other organisms (Merrell et al. 2003). Indeed, previous studies have suggested that iron-bound H. pylori Fur recognizes a poorly defined conserved A/T-rich consensus Fur box sequence (AATAATNNTNA) (Merrell et al. 2003), which is quite different from the E. coli Fur box (GATAATGAT[A/T]ATCATTATC) (de Lorenzo et al. 1987). Interestingly, however, Bereswill, et al. observed that H. pylori Fur is able to complement an E. coli fur mutant strain (Bereswill et al. 1999), and herein we found that E. coli Fur provided the most efficient heterologous complementation in the H. pylori Δfur strain (Fig. 2). Studies directed at understanding the Fur box recognized by iron-bound H. pylori Fur may reveal greater conservation than previously appreciated. Additionally, given that the current binding sequence for apo-Fur (TTNNNNNNNANNTNNNNNAATNNTNNNANNN) (Delany et al. 2001) is even less well defined, there is clearly much to learn about how H. pylori Fur identifies its target genes.
Interestingly, despite the relatively high degree of conservation among bacterial Fur species, this conservation does not necessarily translate into the individual Fur species showing compatible binding and functional capabilities. Indeed this may be due to subtle but important structural differences among the various protein species. For instance, even though V. cholerae and P. aeruginosa share 51.3% identity and 70.7% similarity, recent crystal structures of each protein revealed that their DNA binding regions show very different orientations (Pohl et al. 2003; Sheikh and Taylor 2009), which likely greatly affects Fur function and DNA recognition. Additionally, regions that are implicated for being necessary for metal binding in one species (V. cholerae) appear to be nonessential in a closely related species with 96% identity (Vibrio harveyi) (Sun et al. 2008). Therefore, while Fur may be found in many Gram positive and Gram negative bacterial species and regulate many similar types of genes, conservation of motifs and domains does not guarantee conservation of function.
Given its capacity for chronic infection, H. pylori has clearly evolved to exist in the dynamic gastric niche. However, interestingly the bacterium encodes few two component systems (Wang et al. 2006), a paucity of general transcriptional regulators, and, to date, only four identified sRNAs (Xiao et al. 2009; Xiao et al. 2009; Xiao et al. 2009). Given this regulatory deficit, to successfully respond to the environmental stressors found in the stomach, the transcriptional regulators encoded by H. pylori may have evolved to assume more complex mechanisms of regulation to compensate for their limited numbers. For example, while apo-Fur regulation has not been identified in other species, certain genes in E. coli, P. aeruginosa, and V. cholerae are known to be repressed in a Fur-dependent manner when iron is depleted (Litwin and Calderwood 1994; Wilderman et al. 2004; Masse et al. 2007). However, in these organisms, this regulation is mediated by the Fur-regulated sRNA RyhB (Masse and Gottesman 2002). Similar to apo-Fur regulation of sodB and pfr in H. pylori (Ernst et al. 2005), RyhB has been shown to regulate sodB and ferritin expression in E. coli (Dubrac and Touati 2000; Masse and Gottesman 2002; Masse et al. 2007), P. aeruginosa (Wilderman et al. 2004), and V. cholerae (Mey et al. 2005). However, to date, no RhyB homolog has been identified in H. pylori (Delany et al. 2001). Thus, perhaps in an effort to compensate for the lack of ryhB, in H. pylori Fur may have evolved to acquire dual iron-bound and apo-Fur regulatory functions. Conversely, one could predict that those organisms with RhyB would not need to acquire apo-Fur regulation. Thus, the unique ability of H. pylori Fur to function as an apo-regulator in the absence of its iron cofactor may be a sign of this evolution. The data presented here support this idea since none of the heterologous Fur proteins were able to complement apo-Fur regulation despite a moderate degree of identity and similarity. While the regions of H. pylori Fur that impart the unique ability for apo-regulation are not immediately evident, Carpenter and Merrell recently showed that mutations in E90 and H134, which lie in residues predicted to be H. pylori Fur metal binding sites, result in an altered apo-Fur phenotype (Carpenter and Merrell, unpublished data). These residues are completely conserved within C. jejuni, D. vulgaris, E. coli, P. aeruginosa, and V. cholerae (Figure 1), suggesting that the presence of these sites alone does not confer apo-Fur regulation. In all, these data highlight how much remains to be understood about apo-Fur regulation and the need for continued study of this unique regulatory mechanism in this medically important pathogen.
Acknowledgments
Research in the laboratory of D. Scott Merrell is supported by AI065529 from the NIH. We thank members of the Merrell lab for useful discussions, M. Vasil for providing Martha 2472 P. aeruginosa polyclonal anti-Fur antibody, A. Stintzi for providing C. jejuni polyclonal anti-Fur antibody and D. Hendrixson, J. Wall, K. Keller, A. O’Brien, L. Teele, V. Lee, and A. Camilli for providing template DNA used in these studies. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official view of the NIH or DOD.
References
- Alm RA, Ling LS, Moir DT, King BL, Brown ED, Doig PC, Smith DR, Noonan B, Guild BC, deJonge BL, Carmel G, Tummino PJ, Caruso A, Uria-Nickelsen M, Mills DM, Ives C, Gibson R, Merberg D, Mills SD, Jiang Q, Taylor DE, Vovis GF, Trust TJ. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature. 1999;397(6715):176–180. doi: 10.1038/16495. [DOI] [PubMed] [Google Scholar]
- Andrews SC, Robinson AK, Rodriguez-Quinones F. Bacterial iron homeostasis. FEMS Microbiol Rev. 2003;27(2–3):215–237. doi: 10.1016/S0168-6445(03)00055-X. [DOI] [PubMed] [Google Scholar]
- Baltrus DA, Amieva MR, Covacci A, Lowe TM, Merrell DS, Ottemann KM, Stein M, Salama NR, Guillemin K. The complete genome sequence of Helicobacter pylori strain G27. J Bacteriol. 2009;191(1):447–448. doi: 10.1128/JB.01416-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender KS, Yen HC, Hemme CL, Yang Z, He Z, He Q, Zhou J, Huang KH, Alm EJ, Hazen TC, Arkin AP, Wall JD. Analysis of a ferric uptake regulator (Fur) mutant of Desulfovibrio vulgaris Hildenborough. Appl Environ Microbiol. 2007;73(17):5389–5400. doi: 10.1128/AEM.00276-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereswill S, Greiner S, van Vliet AH, Waidner B, Fassbinder F, Schiltz E, Kusters JG, Kist M. Regulation of ferritin-mediated cytoplasmic iron storage by the ferric uptake regulator homolog (Fur) of Helicobacter pylori. J Bacteriol. 2000;182(21):5948–5953. doi: 10.1128/jb.182.21.5948-5953.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereswill S, Lichte F, Greiner S, Waidner B, Fassbinder F, Kist M. The ferric uptake regulator (Fur) homologue of Helicobacter pylori: functional analysis of the coding gene and controlled production of the recombinant protein in Escherichia coli. Med Microbiol Immunol. 1999;188(1):31–40. doi: 10.1007/s004300050102. [DOI] [PubMed] [Google Scholar]
- Bijlsma JJ, Lie ALM, Nootenboom IC, Vandenbroucke-Grauls CM, Kusters JG. Identification of loci essential for the growth of Helicobacter pylori under acidic conditions. J Infect Dis. 2000;182(5):1566–1569. doi: 10.1086/315855. [DOI] [PubMed] [Google Scholar]
- Bijlsma JJ, Waidner B, Vliet AH, Hughes NJ, Hag S, Bereswill S, Kelly DJ, Vandenbroucke-Grauls CM, Kist M, Kusters JG. The Helicobacter pylori homologue of the ferric uptake regulator is involved in acid resistance. Infect Immun. 2002;70(2):606–611. doi: 10.1128/iai.70.2.606-611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser MJ. Helicobacter pylori and gastric diseases. BMJ. 1998;316(7143):1507–1510. doi: 10.1136/bmj.316.7143.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bury-Mone S, Thiberge JM, Contreras M, Maitournam A, Labigne A, De Reuse H. Responsiveness to acidity via metal ion regulators mediates virulence in the gastric pathogen Helicobacter pylori. Mol Microbiol. 2004;53(2):623–638. doi: 10.1111/j.1365-2958.2004.04137.x. [DOI] [PubMed] [Google Scholar]
- Campanella JJ, Bitincka L, Smalley J. MatGAT: an application that generates similarity/identity matrices using protein or DNA sequences. BMC Bioinformatics. 2003;4:29. doi: 10.1186/1471-2105-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter B, Gancz H, Benoit S, Evans S, Michel PSJ, Maier R, Merrell DS. Mutagenesis of Conserved Amino Acids of Helicobacter pylori. Fur Reveals Residues Important for Function. 2010 doi: 10.1128/JB.00198-10. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter BM, McDaniel TK, Whitmire JM, Gancz H, Guidotti S, Censini S, Merrell DS. Expanding the Helicobacter pylori genetic toolbox: modification of an endogenous plasmid for use as a transcriptional reporter and complementation vector. Appl Environ Microbiol. 2007;73(23):7506–7514. doi: 10.1128/AEM.01084-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter BM, Whitmire JM, Merrell DS. This is not your mother’s repressor: the complex role of fur in pathogenesis. Infect Immun. 2009;77(7):2590–2601. doi: 10.1128/IAI.00116-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lorenzo V, Wee S, Herrero M, Neilands JB. Operator sequences of the aerobactin operon of plasmid ColV-K30 binding the ferric uptake regulation (fur) repressor. J Bacteriol. 1987;169(6):2624–2630. doi: 10.1128/jb.169.6.2624-2630.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delany I, Ieva R, Soragni A, Hilleringmann M, Rappuoli R, Scarlato V. In vitro analysis of protein-operator interactions of the NikR and fur metal-responsive regulators of coregulated genes in Helicobacter pylori. J Bacteriol. 2005;187(22):7703–7715. doi: 10.1128/JB.187.22.7703-7715.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delany I, Pacheco AB, Spohn G, Rappuoli R, Scarlato V. Iron-dependent transcription of the frpB gene of Helicobacter pylori is controlled by the Fur repressor protein. J Bacteriol. 2001;183(16):4932–4937. doi: 10.1128/JB.183.16.4932-4937.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delany I, Spohn G, Rappuoli R, Scarlato V. The Fur repressor controls transcription of iron-activated and -repressed genes in Helicobacter pylori. Mol Microbiol. 2001;42(5):1297–1309. doi: 10.1046/j.1365-2958.2001.02696.x. [DOI] [PubMed] [Google Scholar]
- Dubrac S, Touati D. Fur positive regulation of iron superoxide dismutase in Escherichia coli: functional analysis of the sodB promoter. J Bacteriol. 2000;182(13):3802–3808. doi: 10.1128/jb.182.13.3802-3808.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst FD, Bereswill S, Waidner B, Stoof J, Mader U, Kusters JG, Kuipers EJ, Kist M, van Vliet AH, Homuth G. Transcriptional profiling of Helicobacter pylori Fur- and iron-regulated gene expression. Microbiology. 2005;151(Pt 2):533–546. doi: 10.1099/mic.0.27404-0. [DOI] [PubMed] [Google Scholar]
- Ernst FD, Homuth G, Stoof J, Mader U, Waidner B, Kuipers EJ, Kist M, Kusters JG, Bereswill S, van Vliet AH. Iron-responsive regulation of the Helicobacter pylori iron-cofactored superoxide dismutase SodB is mediated by Fur. J Bacteriol. 2005;187(11):3687–3692. doi: 10.1128/JB.187.11.3687-3692.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst JF, Bennett RL, Rothfield LI. Constitutive expression of the iron-enterochelin and ferrichrome uptake systems in a mutant strain of Salmonella typhimurium. J Bacteriol. 1978;135(3):928–934. doi: 10.1128/jb.135.3.928-934.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorini F, Stefanini S, Valenti P, Chiancone E, De Biase D. Transcription of the Listeria monocytogenes fri gene is growth-phase dependent and is repressed directly by Fur, the ferric uptake regulator. Gene. 2008;410(1):113–121. doi: 10.1016/j.gene.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Gancz H, Censini S, Merrell DS. Iron and pH homeostasis intersect at the level of Fur regulation in the gastric pathogen Helicobacter pylori. Infect Immun. 2006;74(1):602–614. doi: 10.1128/IAI.74.1.602-614.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge JM, Quinlan GJ, Evans TW. The iron paradox of heart and lungs and its implications for acute lung injury. Free Radic Res. 2001;34(5):439–443. doi: 10.1080/10715760100300381. [DOI] [PubMed] [Google Scholar]
- Hantke K. Cloning of the repressor protein gene of iron-regulated systems in Escherichia coli K12. Mol Gen Genet. 1984;197(2):337–341. doi: 10.1007/BF00330982. [DOI] [PubMed] [Google Scholar]
- Hantke K. Iron and metal regulation in bacteria. Curr Opin Microbiol. 2001;4(2):172–177. doi: 10.1016/s1369-5274(00)00184-3. [DOI] [PubMed] [Google Scholar]
- Heidelberg JF, Eisen JA, Nelson WC, Clayton RA, Gwinn ML, Dodson RJ, Haft DH, Hickey EK, Peterson JD, Umayam L, Gill SR, Nelson KE, Read TD, Tettelin H, Richardson D, Ermolaeva MD, Vamathevan J, Bass S, Qin H, Dragoi I, Sellers P, McDonald L, Utterback T, Fleishmann RD, Nierman WC, White O, Salzberg SL, Smith HO, Colwell RR, Mekalanos JJ, Venter JC, Fraser CM. DNA sequence of both chromosomes of the cholera pathogen. Vibrio cholerae Nature. 2000;406(6795):477–483. doi: 10.1038/35020000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidelberg JF, Seshadri R, Haveman SA, Hemme CL, Paulsen IT, Kolonay JF, Eisen JA, Ward N, Methe B, Brinkac LM, Daugherty SC, Deboy RT, Dodson RJ, Durkin AS, Madupu R, Nelson WC, Sullivan SA, Fouts D, Haft DH, Selengut J, Peterson JD, Davidsen TM, Zafar N, Zhou L, Radune D, Dimitrov G, Hance M, Tran K, Khouri H, Gill J, Utterback TR, Feldblyum TV, Wall JD, Voordouw G, Fraser CM. The genome sequence of the anaerobic, sulfate-reducing bacterium Desulfovibrio vulgaris Hildenborough. Nat Biotechnol. 2004;22(5):554–559. doi: 10.1038/nbt959. [DOI] [PubMed] [Google Scholar]
- Holmes K, Mulholland F, Pearson BM, Pin C, McNicholl-Kennedy J, Ketley JM, Wells JM. Campylobacter jejuni gene expression in response to iron limitation and the role of Fur. Microbiology. 2005;151(Pt 1):243–257. doi: 10.1099/mic.0.27412-0. [DOI] [PubMed] [Google Scholar]
- Horsburgh MJ, Ingham E, Foster SJ. In Staphylococcus aureus, fur is an interactive regulator with PerR, contributes to virulence, and is necessary for oxidative stress resistance through positive regulation of catalase and iron homeostasis. J Bacteriol. 2001;183(2):468–475. doi: 10.1128/JB.183.2.468-475.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton RM, Ho SN, Pullen JK, Hunt HD, Cai Z, Pease LR. Gene splicing by overlap extension. Methods Enzymol. 1993;217:270–279. doi: 10.1016/0076-6879(93)17067-f. [DOI] [PubMed] [Google Scholar]
- Litwin CM, Calderwood SB. Analysis of the complexity of gene regulation by fur in Vibrio cholerae. J Bacteriol. 1994;176(1):240–248. doi: 10.1128/jb.176.1.240-248.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masse E, Gottesman S. A small RNA regulates the expression of genes involved in iron metabolism in Escherichia coli. Proc Natl Acad Sci U S A. 2002;99(7):4620–4625. doi: 10.1073/pnas.032066599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masse E, Salvail H, Desnoyers G, Arguin M. Small RNAs controlling iron metabolism. Curr Opin Microbiol. 2007;10(2):140–145. doi: 10.1016/j.mib.2007.03.013. [DOI] [PubMed] [Google Scholar]
- McArthur KE, Feldman M. Gastric acid secretion, gastrin release, and gastric emptying in humans as affected by liquid meal temperature. Am J Clin Nutr. 1989;49(1):51–54. doi: 10.1093/ajcn/49.1.51. [DOI] [PubMed] [Google Scholar]
- Merrell DS, Goodrich ML, Otto G, Tompkins LS, Falkow S. pH-regulated gene expression of the gastric pathogen Helicobacter pylori. Infect Immun. 2003;71(6):3529–3539. doi: 10.1128/IAI.71.6.3529-3539.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrell DS, Thompson LJ, Kim CC, Mitchell H, Tompkins LS, Lee A, Falkow S. Growth phase-dependent response of Helicobacter pylori to iron starvation. Infect Immun. 2003;71(11):6510–6525. doi: 10.1128/IAI.71.11.6510-6525.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mey AR, Craig SA, Payne SM. Characterization of Vibrio cholerae RyhB: the RyhB regulon and role of ryhB in biofilm formation. Infect Immun. 2005;73(9):5706–5719. doi: 10.1128/IAI.73.9.5706-5719.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner UA, Vasil AI, Vasil ML. Role of the ferric uptake regulator of Pseudomonas aeruginosa in the regulation of siderophores and exotoxin A expression: purification and activity on iron-regulated promoters. J Bacteriol. 1995;177(24):7194–7201. doi: 10.1128/jb.177.24.7194-7201.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhill J, Wren BW, Mungall K, Ketley JM, Churcher C, Basham D, Chillingworth T, Davies RM, Feltwell T, Holroyd S, Jagels K, Karlyshev AV, Moule S, Pallen MJ, Penn CW, Quail MA, Rajandream MA, Rutherford KM, van Vliet AH, Whitehead S, Barrell BG. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature. 2000;403(6770):665–668. doi: 10.1038/35001088. [DOI] [PubMed] [Google Scholar]
- Perna NT, Plunkett G, 3rd, Burland V, Mau B, Glasner JD, Rose DJ, Mayhew GF, Evans PS, Gregor J, Kirkpatrick HA, Posfai G, Hackett J, Klink S, Boutin A, Shao Y, Miller L, Grotbeck EJ, Davis NW, Lim A, Dimalanta ET, Potamousis KD, Apodaca J, Anantharaman TS, Lin J, Yen G, Schwartz DC, Welch RA, Blattner FR. Genome sequence of enterohaemorrhagic Escherichia coli O157:H7. Nature. 2001;409(6819):529–533. doi: 10.1038/35054089. [DOI] [PubMed] [Google Scholar]
- Pohl E, Haller JC, Mijovilovich A, Meyer-Klaucke W, Garman E, Vasil ML. Architecture of a protein central to iron homeostasis: crystal structure and spectroscopic analysis of the ferric uptake regulator. Mol Microbiol. 2003;47(4):903–915. doi: 10.1046/j.1365-2958.2003.03337.x. [DOI] [PubMed] [Google Scholar]
- Ratledge C, Dover LG. Iron metabolism in pathogenic bacteria. Annu Rev Microbiol. 2000;54:881–941. doi: 10.1146/annurev.micro.54.1.881. [DOI] [PubMed] [Google Scholar]
- Seyler RW, Jr, Olson JW, Maier RJ. Superoxide dismutase-deficient mutants of Helicobacter pylori are hypersensitive to oxidative stress and defective in host colonization. Infect Immun. 2001;69(6):4034–4040. doi: 10.1128/IAI.69.6.4034-4040.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh MA, Taylor GL. Crystal structure of the Vibrio cholerae ferric uptake regulator (Fur) reveals insights into metal co-ordination. Mol Microbiol. 2009;72(5):1208–1220. doi: 10.1111/j.1365-2958.2009.06718.x. [DOI] [PubMed] [Google Scholar]
- Spiegelhalder C, Gerstenecker B, Kersten A, Schiltz E, Kist M. Purification of Helicobacter pylori superoxide dismutase and cloning and sequencing of the gene. Infect Immun. 1993;61(12):5315–5325. doi: 10.1128/iai.61.12.5315-5325.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover CK, Pham XQ, Erwin AL, Mizoguchi SD, Warrener P, Hickey MJ, Brinkman FS, Hufnagle WO, Kowalik DJ, Lagrou M, Garber RL, Goltry L, Tolentino E, Westbrock-Wadman S, Yuan Y, Brody LL, Coulter SN, Folger KR, Kas A, Larbig K, Lim R, Smith K, Spencer D, Wong GK, Wu Z, Paulsen IT, Reizer J, Saier MH, Hancock RE, Lory S, Olson MV. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature. 2000;406(6799):959–964. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- Sun K, Cheng S, Zhang M, Wang F, Sun L. Cys-92, Cys-95, and the C-terminal 12 residues of the Vibrio harveyi ferric uptake regulator (Fur) are functionally inessential. J Microbiol. 2008;46(6):670–680. doi: 10.1007/s12275-008-0113-3. [DOI] [PubMed] [Google Scholar]
- Thompson LJ, Merrell DS, Neilan BA, Mitchell H, Lee A, Falkow S. Gene expression profiling of Helicobacter pylori reveals a growth-phase-dependent switch in virulence gene expression. Infect Immun. 2003;71(5):2643–2655. doi: 10.1128/IAI.71.5.2643-2655.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vliet AH, Stoof J, Poppelaars SW, Bereswill S, Homuth G, Kist M, Kuipers EJ, Kusters JG. Differential regulation of amidase- and formamidase-mediated ammonia production by the Helicobacter pylori fur repressor. J Biol Chem. 2003;278(11):9052–9057. doi: 10.1074/jbc.M207542200. [DOI] [PubMed] [Google Scholar]
- van Vliet AH, Stoof J, Vlasblom R, Wainwright SA, Hughes NJ, Kelly DJ, Bereswill S, Bijlsma JJ, Hoogenboezem T, Vandenbroucke-Grauls CM, Kist M, Kuipers EJ, Kusters JG. The role of the Ferric Uptake Regulator (Fur) in regulation of Helicobacter pylori iron uptake. Helicobacter. 2002;7(4):237–244. doi: 10.1046/j.1523-5378.2002.00088.x. [DOI] [PubMed] [Google Scholar]
- Wang G, Alamuri P, Maier RJ. The diverse antioxidant systems of Helicobacter pylori. Mol Microbiol. 2006;61(4):847–860. doi: 10.1111/j.1365-2958.2006.05302.x. [DOI] [PubMed] [Google Scholar]
- Wilderman PJ, Sowa NA, FitzGerald DJ, FitzGerald PC, Gottesman S, Ochsner UA, Vasil ML. Identification of tandem duplicate regulatory small RNAs in Pseudomonas aeruginosa involved in iron homeostasis. Proc Natl Acad Sci U S A. 2004;101(26):9792–9797. doi: 10.1073/pnas.0403423101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao B, Li W, Guo G, Li B, Liu Z, Jia K, Guo Y, Mao X, Zou Q. Identification of small noncoding RNAs in Helicobacter pylori by a bioinformatics-based approach. Curr Microbiol. 2009;58(3):258–263. doi: 10.1007/s00284-008-9318-2. [DOI] [PubMed] [Google Scholar]
- Xiao B, Li W, Guo G, Li BS, Liu Z, Tang B, Mao XH, Zou QM. Screening and identification of natural antisense transcripts in Helicobacter pylori by a novel approach based on RNase I protection assay. Mol Biol Rep. 2009;36(7):1853–1858. doi: 10.1007/s11033-008-9390-5. [DOI] [PubMed] [Google Scholar]
- Xiao B, Liu Z, Li BS, Tang B, Li W, Guo G, Shi Y, Wang F, Wu Y, Tong WD, Guo H, Mao XH, Zou QM. Induction of microRNA-155 during Helicobacter pylori infection and its negative regulatory role in the inflammatory response. J Infect Dis. 2009;200(6):916–925. doi: 10.1086/605443. [DOI] [PubMed] [Google Scholar]