Abstract
Background:
Improved outcome measures are necessary to reduce sample size and increase power in amyotrophic lateral sclerosis (ALS) clinical trials. Motor unit number estimation (MUNE) is a potentially attractive tool. MUNE methods previously employed in multicenter trials exhibited excessive variability and were prone to artifact.
Objective:
To evaluate a modification of standard incremental MUNE in a multicenter natural history study of subjects with ALS.
Methods:
Fifty healthy subjects were evaluated twice and 71 subjects with ALS were studied repeatedly for up to 500 days. Side and nerve studied was based on clinical examination findings. Nerves were stimulated at 3 specified locations and 3 increments were obtained at each location. Average single motor unit action potential (SMUP) amplitude was calculated by adding the amplitude of the third increment at each location and dividing by 9; SMUP was divided into maximum CMAP amplitude to determine the MUNE.
Results:
Test-retest variability was 9% in normal subjects. Average MUNE for normal subjects was 225 (±87), and was 41.9 (±39) among subjects with ALS at baseline. Subjects with ALS showed clear decrements over time, with an overage rate of decline of approximately 9% per month. SMUP amplitude increased with time in a fashion consistent with the known pathophysiology of ALS.
Conclusion:
Multipoint incremental MUNE has a number of attributes that make it attractive as an outcome measure in ALS and other diseases characterized by motor unit loss. It can be rapidly performed on any EMG machine and has repeatability and rates of decline that favorably compare to other previously described methods.
The search for effective therapies for patients with amyotrophic lateral sclerosis (ALS) is limited by the lack of sensitive outcome measures. Recent phase II and phase III trials have almost exclusively used a functional outcome, the ALS Functional Rating Scale–Revised (ALSFRS-R), as the primary measure.1–3 The ALSFRS-R is rapidly performed, reproducible, and easily standardized. However, the rate of decline and the high intersubject variability requires large sample sizes to detect a modest reduction in the rate of disease progression. In addition, the scale combines many patient attributes, such that a change in rate of decline in ALSFRS-R does not have intuitive meaning to patients or clinicians.
Motor unit number estimation (MUNE) has long been of interest as a measure that might sensitively assess lower motor neuron loss.4–6 In the hands of single investigators, MUNE conducted using a variety of methods has shown great promise. However, attempts to apply a specific technique (statistical method) to a multicenter trial proved problematic. Ideally, a MUNE technique should be easy to standardize, be performed rapidly on any EMG machine, have excellent test-retest reproducibility, and decline rapidly in subjects with ALS. As part of a multicenter study of novel outcome measures in ALS, a simple combination of both incremental and multipoint MUNE methods, which we have termed multipoint incremental MUNE, was employed. We report data on reliability of this technique in normal subjects and the rate of decline in subjects with ALS, to determine whether this measure has properties that would make it an attractive outcome measure in future ALS trials.
METHODS
Subjects.
Subjects were recruited at 8 US sites. Fifty normal control subjects were recruited with at least 4 subjects at each site for evaluation of test-retest reliability. Average age of control subjects was 32 years (SD 9.4); there were 19 men and 31 women. The ulnar nerve was studied in 17 subjects and the median nerve in 33 subjects. Subjects with ALS carried a diagnosis of probable or definite ALS as defined by the modified El Escorial Criteria.7 Subjects were between 18 and 85 years of age, had average muscle strength greater than 3.5 on a modified manual muscle testing scale, and were not ventilator dependent. Subjects with implanted electrical devices were excluded. Patients were also excluded if they had another disease that could impact assessment of peripheral motor neuron loss due to ALS. Participation in ALS treatment trials was allowed.
Standard protocol approvals, registrations, and patient consents.
This study was approved by the Institutional Review Boards of all the participating sites.
MUNE method.
Either the ulnar or median nerve of the right or left hand was studied (see below). Recording electrodes were placed on either the ulnar nerve innervated abductor digiti minimi (ADM) or the median nerve innervated abductor pollicis brevis (APB) muscle, using the standard belly-tendon montage. There were 3 stimulus locations used for each muscle; for the median nerve, stimulus locations were 2 cm proximal to the wrist crease, 4 cm proximal to the first stimulation site, and in the cubital fossa. For the ulnar nerve, locations were 2 cm proximal to the wrist crease, 4 cm proximal to the first site, and 1 cm proximal to the ulnar groove at the elbow.
Filter settings were 10 Hz–10 KHz. For each stimulation site, optimum stimulus location was determined using a submaximal stimulus and moving the stimulator to evoke the greatest response. The location was marked, and stimulating electrodes applied; self-adhesive circular motor electrodes were employed. For the most distal site, a maximal response was obtained. Amplifier settings were than changed to 50 μV/division, and stimulus control increased to the maximum allowable; gradation in at least tenths of milliamps was necessary. A standard 3-site motor conduction program was used, with traces set to superimpose. Subthreshold stimuli were applied at a rate of approximately 1/second, with stimulus intensity slowly increased until an all-or-nothing initial response was obtained. Baseline to peak amplitude was measured. For both initial and subsequent incremental responses, the minimum negative peak amplitude considered to be acceptable for recording was 25 μV. Tracings with an initial positive component were measured from baseline to negative peak as well, disregarding the positive portion of the response. The initial response was recorded on trace 1, after which stimulus intensity was increased until a clearly defined incremental response (of more than 25 μV incremental amplitude) was obtained. This response was recorded on trace 2, and a second increment obtained with further slight increase in stimulus intensity. The final potential was recorded on trace 3. The negative peak amplitude of the third response was recorded. The tracings were printed, and stimulus location moved to the second site, where the same procedure of incremental stimulation was repeated. Stimulation at the third location was identical to the first and second.
Calculation of MUNE and single motor unit action potential amplitude.
The amplitude of the third response at each site was summed, then divided by 9 to yield the average single motor unit action potential (SMUP) amplitude. This amplitude was divided into the maximum compound motor unit action potential (CMAP) amplitude to yield the MUNE. For evaluation of rate of decline, change from baseline was calculated.
Time intervals and nerve selection.
The goal was to study subjects at 3-month intervals; if, however, they were participating in a clinical trial that required different intervals, the schedule was modified to match that of the clinical trial. This resulted in a wide distribution of intervals among subjects. At the first visit, the upper extremities were evaluated clinically. For patients with clinically detectable weakness in both upper extremities, the stronger of the 2 hands was chosen. If there was weakness only in one extremity, that extremity was studied. For that hand, motor and sensory nerve conduction studies of the median nerve were performed using standard techniques, to rule out the presence of median neuropathy at the wrist. If either a median neuropathy was detected or the CMAP amplitude was smaller than 5 mV, sensory and motor studies of the ulnar nerve were performed. If a significant ulnar neuropathy at the elbow or wrist was detected, or the CMAP amplitude was less than 5 mV, the other hand was studied in similar fashion. The underlying goal was to choose a nerve/muscle not affected by focal neuropathy and with a CMAP amplitude in the low normal range. If all nerves studied had CMAPs reduced in amplitude, the nerve with the largest motor response was chosen for study.
Training, validation, and quality assurance.
Prior to study onset, an in-person training session was held for all investigators. Following the training session, each investigator was required to study 4 normal subjects twice at their home institution with the interval between study being at least 3 hours and with the requirement that all electrodes be removed prior to restudy. Evaluators did not calculate the MUNE on site, but sent the tracings for all studies to the central MUNE coordination site (SUNY Upstate Medical University). A single reviewer (J.M.S.) evaluated all tracings for quality and correct cursor placement, and calculated the MUNE for each study. Test-retest variability was calculated; evaluators were certified as being capable of performing the study if variability was less than 20% for all subjects. If variability was greater than this criterion for any subject, the evaluator repeated the studies either on the same subject or on another. The data reported in this article reflect the original studies performed by each evaluator.
Throughout the study, all tracings for all subject MUNE assessments were sent to the central MUNE coordination center. Studies were reviewed and feedback was given to maintain a high level of quality of study performance. The MUNE assessor (J.M.S.) evaluated each study without information regarding results of prior studies for that subject.
RESULTS
Fifty normal subjects were studied at 8 sites. Although each site was required to submit 4 normal subjects for reliability assessment, multiple evaluators at several sites necessitated additional subjects. Mean MUNE (±SD) was 225 (±87) for 33 median nerves, and 220 (±81) for 17 ulnar nerves. Given that the mean MUNEs for both nerves were so similar, and the focus of this study is to develop a single measure, data from both nerves were analyzed together. Overall normal values for MUNE were 223, 84.9, 0.70, and 219 for mean, SD, skew, and median, 55.2, 20.5, 1.11, and 50.7 for SMUP amplitude, and 11.0, 2.54, 0.62, and 10.2 for CMAP amplitude. Test-retest reliability for MUNE was 0.905 for all subjects studied. All sites had test-retest reliability greater than 80%.
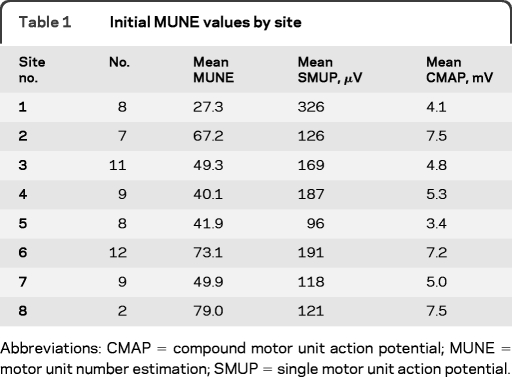
Seventy-one subjects with ALS were studied at the 8 sites, with 64 subjects studied at least twice, with a minimum interval of 37 days. Number of subjects studied was chosen on the basis of expected variability of another exploratory measure of ALS disease progression, and not on the basis of expected MUNE results. The median nerve was studied in 45 subjects, and the ulnar nerve in 26 subjects. Initial mean MUNE recorded from median nerve was 45.5 (SD 44.8, skew 1.56, median 28.0) and 73.3 (SD 47.2, skew 0.49, median 69.0) for the ulnar nerve. Numbers of subjects studied and initial values across sites are presented in table 1. For subjects studied at least twice, the interval between first and last study ranged from 37 to 500 days. Initial MUNE ranged from 192 to 2 with a mean of 41.9 (±39.4) for all nerves studied.
Table 1.
Initial MUNE values by site
Abbreviations: CMAP = compound motor unit action potential; MUNE = motor unit number estimation; SMUP = single motor unit action potential.
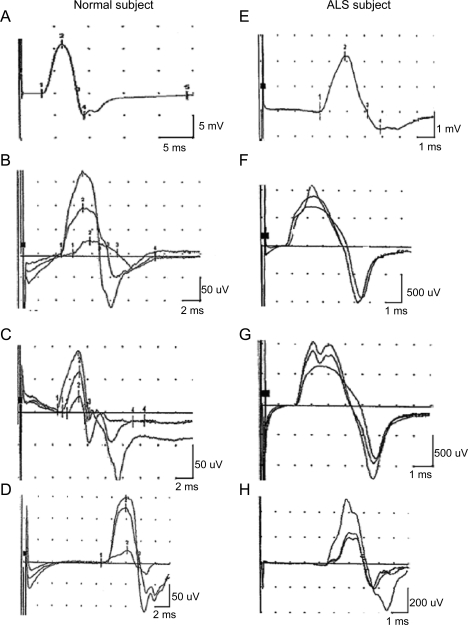
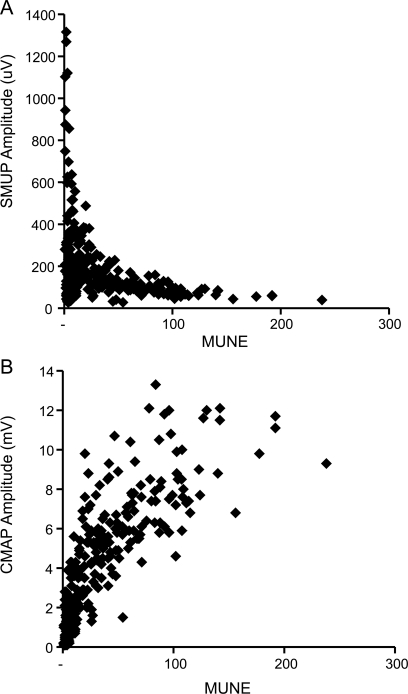
Examples of tracings obtained from each stimulation site are shown in figure 1, for both a normal subject and a subject with ALS. As expected, SMUP amplitude varied as a function of the calculated MUNE (figure 2A). For higher MUNE values, SMUP amplitude was near normal values, with amplitude increasing as MUNE declined. However, for end-stage muscles, SMUP amplitude often declined; this is reflected in the number of small amplitude motor units seen for very small MUNE values in figure 2A. Across all subjects with ALS studied, there were 15 studies in which average SMUP amplitude was less than 50 μV. Mean MUNE for those studies was 38.7; more strikingly, in 10/15 of these studies, MUNE was less than 10. CMAP amplitude also decreased with decreasing MUNE values (figure 2B); the slope of the decline in CMAP amplitude was greatest at small MUNE values, suggesting CMAP amplitude was relatively preserved when MUNE was beginning to decline.
Figure 1. Examples of tracings for motor unit number estimation studies on a normal subject (left) and a subject with amyotrophic lateral sclerosis (ALS) (right).
Top panels show maximum compound motor unit action potential, with lower panels showing 3 increments at wrist, 4 cm proximal to the wrist, and at the elbow. Panels A–D represent data from a normal subject; panels E–H represent data from a subject with ALS.
Figure 2. Comparison of single motor unit action potential (SMUP) (A) and compound motor unit action potential (CMAP) (B) amplitude with motor unit number estimation (MUNE) for 71 subjects with amyotrophic lateral sclerosis, at all timepoints studied.
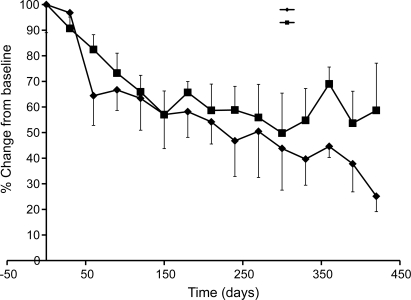
The rate of decline in MUNE may vary as a function of absolute motor unit number; to determine whether this was a factor in the current study, rate of decline of MUNE was calculated separately for the 10 subjects with initial MUNE values of 10 or less, the 42 subjects with MUNE values between 10 and 100, and the 10 subjects with MUNE values of greater than 100. MUNE, expressed as % change from baseline, declined by 2.94% (±15.24) per month for the <10 group, 8.78% (±5.16) for the 10–100 group, and 8.60% (±11.42) for the greater than 100 group. Given the similarity in rates of decline for subjects with initial MUNE greater than 10, data from the 52 subjects studied at least twice and who had an initial MUNE of 10 or greater at the first measurement session were combined and analyzed in greater detail. For these subjects, decline in MUNE and CMAP amplitude, expressed as % change from baseline, is shown in figure 3. For each of these subjects, slope was calculated using a linear regression model. The average rate of decline of MUNE was 8.94% (±7.92) per month, while CMAP declined at a rate of 6.32% (±8.05) per month. SMUP amplitude increased over time, at a rate of 6.9% (±25.61) per month. The difference between rates of decline in MUNE and CMAP amplitude appear to increase with time; this may reflect increased motor unit remodeling with time.
Figure 3. Decline in motor unit number estimation (MUNE) and compound motor unit action potential (CMAP) amplitude, expressed as change from baseline, for 52 subjects with amyotrophic lateral sclerosis with a minimum of MUNE of 10 at first evaluation.
Squares represent CMAP data, diamonds represent MUNE data. Error bars denote the standard error for each data point.
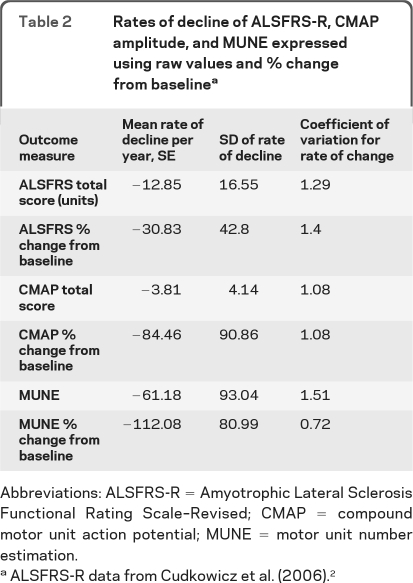
In order to assess the behavior of MUNE and CMAP amplitude as outcome measures, the coefficient of variation for rate of change was calculated for all subjects with initial MUNE >10. Slopes were calculated using raw data and % change from baseline. Coefficient of variation for the rate of change was calculated by dividing the SD of individual slopes by the mean (table 2). As can be seen, the multipoint technique provides the lowest coefficient of variation of the rate of change, supporting its potential to be a more efficient outcome measure than standard approaches such as the ALSFRS-R.
Table 2.
Rates of decline of ALSFRS-R, CMAP amplitude, and MUNE expressed using raw values and % change from baselinea
Abbreviations: ALSFRS-R = Amyotrophic Lateral Sclerosis Functional Rating Scale–Revised; CMAP = compound motor unit action potential; MUNE = motor unit number estimation.
ALSFRS-R data from Cudkowicz et al. (2006).2
DISCUSSION
This study shows that multipoint incremental MUNE produces a value that is highly reproducible and declines faster than other commonly employed outcome measures used in ALS trials. At 1 year, MUNE dropped by approximately 60%. Test-retest variability in normal subjects matched or exceeded that seen in single-center studies using other MUNE techniques, and was superior to prior multiple-center studies using the statistical technique.8–12 A similar technique adapted to evaluating motor units in the rat tail also showed excellent reproducibility.13 In humans, a technique called adaptive multipoint MUNE also involved obtaining several incremental responses at multiple points along a nerve14–16; however, locations and number of points studied were not standardized, increasing the variability of the measurement.
Previous MUNE studies in ALS have for the most part been conducted at single sites. Using the multipoint method, % change of MUNE was found to be greater than change in strength, pulmonary function, or the Appel Rating Scale over a 1-year period,17 approaching 70% on average. A similar decline in multipoint MUNE in another natural history study of patients with ALS was found more recently.17 A study employing an entirely different technique, the incremental method,18 identified virtually the same rate of decline.
The protocol for performing this method of motor unit estimation was designed to be as simple as possible, so that the technique could be performed on any EMG machine and in a uniform fashion by multiple evaluators at different study sites. For this reason, amplitude was chosen as the attribute measured rather than area, which requires more judgments on the part of the evaluator with respect to cursor placement. Using baseline to peak amplitude also eliminates the need to make judgments as to whether waveforms with prominent positive dips should be excluded, as the only decision point is whether a given waveform is more than 25 μV greater than its predecessor. We recognize that some units can change the overall waveform area without affecting amplitude; however, our data suggest that reliable data can be obtained using strict amplitude criteria. Another criterion that was strictly followed for both normal subjects and subjects with ALS was to not include any units with negative peak amplitude of less than 25 μV; this criterion was applied both to initial waveforms and subsequent increments. While we cannot eliminate the possibility that normal muscle does in fact contain units smaller than 25 μV, prior studies with a variety of techniques suggest that such units are rare.10,12,19
The ALSFRS-R is commonly used as the primary outcome in recent ALS trials. The rates of decline of MUNE, CMAP amplitude, and ALSFRS-R are shown in table 2, using both raw values and % change from baseline. The data for ALSFRS-R are taken from the placebo group of a recently reported clinical trial of celecoxib in ALS.2 The coefficient of variation for rate of change combines both the rate of change and its variability from all sources and is thus a good way to compare measures. Multipoint incremental MUNE compares favorably in this regard to both CMAP amplitude and ALSFRS-R, when expressed as % change from baseline.
There are several attractive practical aspects to this form of MUNE worth highlighting. First, it is relatively easy to perform, even in patients with a large number of motor unit potentials, and the evaluators at each of the centers were typically able to complete a measurement session within about 20 minutes. Second, training of evaluators is straightforward, in part because the decisions regarding suitability of a given waveform are similar to those made in performing routine nerve conduction studies. Third, specialized equipment is not necessary to perform the measurements. Finally, it is also well-tolerated by patients; in addition to being rapidly performed, it requires relatively low stimulus intensities. This method could also be applied to muscles of the foot, although stimulus intensities required for nerve stimulation at some stimulus locations may make the procedure somewhat more uncomfortable. However, the data presented here suggest that limiting investigation to the upper extremities still yields data that compare well to other outcome measures that evaluate more global deficits.
As with all MUNE methods, this technique is vulnerable to bias. First, sampling is limited to units near electrical threshold; this potentially could bias the sample toward larger units. Second, using amplitude as the measure of interest may lead to errors in estimation when summation of units is not linear. It is also possible that the same unit may be sampled at different locations, acting to further reduce the sample on which MUNE is estimated. Neither this method nor any other MUNE method has been specifically validated against an objective assessment of motor unit number; indeed, it is hard to conceive of such a study being performed in humans.
Despite this, MUNE using a variety of techniques has been shown to predict meaningful clinical outcomes including survival.5,20,21 Thus, MUNE should be considered a surrogate marker of disease progression in ALS rather than a quantitative estimate of an underlying biological process.
For these reasons, multipoint incremental MUNE likely should not be employed as a primary outcome measure in a phase III trial, for which clinically meaningful or biologically relevant changes must be demonstrated. Multipoint incremental MUNE does, however, have great potential in phase II studies, where the goal is to determine whether an experimental agent is active at the hypothesized target. In addition, as a secondary measure, this technique may be extremely useful in phase III trials by way of providing insight into the physiology by which a new therapy is exerting its effect.
Editorial, page 208
- ADM
- abductor digiti minimi
- ALS
- amyotrophic lateral sclerosis
- ALSFRS-R
- Amyotrophic Lateral Sclerosis Functional Rating Scale–Revised
- APB
- abductor pollicis brevis
- CMAP
- compound motor unit action potential
- MUNE
- motor unit number estimation
- SMUP
- single motor unit action potential
AUTHOR CONTRIBUTIONS
Dr. Shefner: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, statistical analysis, study supervision, obtaining funding. M.L. Watson: analysis or interpretation of data, acquisition of data, study supervision. Dr. Simionescu: drafting/revising the manuscript, analysis or interpretation of data, acquisition of data. Dr. Caress: drafting/revising the manuscript, analysis or interpretation of data, acquisition of data. Dr. Burns: drafting/revising the manuscript, acquisition of data. Dr. Maragakis: drafting/revising the manuscript, analysis or interpretation of data, acquisition of data. Dr. Benatar: drafting/revising the manuscript, acquisition of data, study supervision. Dr. David: drafting/revising the manuscript, analysis or interpretation of data, acquisition of data, study supervision. Dr. Sharma: drafting/revising the manuscript, study concept or design, acquisition of data. Dr. Rutkove: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, obtaining funding.
DISCLOSURE
Dr. Shefner serves on a DSMB for the NIH; serves as Neuromuscular Section Editor for and receives publishing royalties from UpToDate; has served as a consultant for Teva Pharmaceutical Industries Ltd., Gilead Sciences, Inc., GlaxoSmithKline, Trophos, and BrainGate; and receives research support from Isis Pharmaceuticals, Inc., Neuralstem, Inc., GlaxoSmithKline, sanofi-aventis, Teva Pharmaceutical Industries Ltd., Knopp Neurosciences Inc., Cytokinetics Inc., the NIH, and the ALS Association. M.L. Watson reports no disclosures. Dr. Simionescu receives research support from the NIH/NINDS. Dr. Caress has served as a consultant for ALS Biopharma, LLC and has received research support from GlaxoSmithKline, Talecris Biotherapeutics, the NIH/NINDS, and the ALS Association. Dr. Burns serves as Podcast Section Editor for Neurology®; has served as a consultant for Bayhill Therapeutics; and has received research support from Knopp Neurosciences Inc. and the Myasthenia Gravis Foundation of America. Dr. Maragakis serves on a scientific advisory board for Q Therapeutics, Inc.; received support from the Michael S. Ansari Gift Fund for basic science research; serves as a contributor to UpToDate; has served as a consultant for California Institute for Regenerative Medicine (CIRM); and receives/has received research support from Cytokinetics Inc., Sangamo BioSciences, Inc., TEDCO-Maryland Stem Cell Research Fund, NIH/NINDS, U.S. Department of Defense, and the ALS Association Packard Center for ALS Research at Johns Hopkins. Dr. Benatar has served as a consultant for Bayhill Therapeutics and Cytokinetics Inc.; receives publishing royalties for Neuromuscular Disease: Evidence and Analysis in Clinical Neurology (Humana Press, 2006), BluePrints in Neurology, (Lippincott Williams & Wilkins, 2002), and Field of Vision: A Manual and Atlas of Perimetry (Humana Press, 2010); receives/has received research support from CytRx Corporation, the Muscular Dystrophy Association, the ALS Association, the Food & Drug Administration, and the Centers for Disease Control and Prevention, the Woodruff Health Sciences Center (Emory University), and the NIH; and has participated in medico-legal cases. Dr. David has served as a consultant for Apnex Medical™, Inc. and Allergan, Inc. Dr. Sharma reports no disclosures. Dr. Rutkove has 2 patents pending re: Electrical impedance myography; receives royalties from UpToDate, Inc. and for the publication of The Clinical Neurophysiology Primer (Humana Press, 2007); serves as a consultant for and holds stock in Convergence Medical Devices, Inc.; serves as a consultant to Neuralstem, Inc.; and receives research support from the NIH/NINDS, the SMA Foundation, and the ALS Association.
REFERENCES
- 1. Aggarwal SP, Zinman L, Simpson E, et al. Safety and efficacy of lithium in combination with riluzole for treatment of amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 2010;9:481–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cudkowicz ME, Shefner JM, Schoenfeld DA, et al. Trial of celecoxib in amyotrophic lateral sclerosis. Ann Neurol 2006;60:22–31 [DOI] [PubMed] [Google Scholar]
- 3. Gordon PH, Moore DH, Miller RG, et al. Efficacy of minocycline in patients with amyotrophic lateral sclerosis: a phase III randomised trial. Lancet Neurol 2007;6:1045–1053 [DOI] [PubMed] [Google Scholar]
- 4. Felice KJ. A longitudinal study comparing thenar motor unit number estimates to other quantitative tests in patients with amyotrophic lateral sclerosis. Muscle Nerve 1997;20:179–185 [DOI] [PubMed] [Google Scholar]
- 5. Yuen EC, Olney RK. Longitudinal study of fiber density and motor unit number estimate in patients with amyotrophic lateral sclerosis. Neurology 1997;49:573–578 [DOI] [PubMed] [Google Scholar]
- 6. Shefner JM, Cudkowicz ME, Zhang H, Schoenfeld D, Jillapalli D. Revised statistical motor unit number estimation in the Celecoxib/ALS trial. Muscle Nerve 2007;35:228–234 [DOI] [PubMed] [Google Scholar]
- 7. Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 2000;1:293–299 [DOI] [PubMed] [Google Scholar]
- 8. Bromberg MB. Motor unit estimation: reproducibility of the spike-triggered averaging technique in normal and ALS subjects. Muscle Nerve 1993;16:466–471 [DOI] [PubMed] [Google Scholar]
- 9. Doherty TJ, Stashuk DW, Brown WF. Determinants of mean motor unit size: impact on estimates of motor unit number. Muscle Nerve 1993;16:1326–1331 [DOI] [PubMed] [Google Scholar]
- 10. Lomen-Hoerth C, Olney RK. Comparison of multiple point and statistical motor unit number estimation. Muscle Nerve 2000;23:1525–1533 [DOI] [PubMed] [Google Scholar]
- 11. Shefner JM, Cudkowicz ME, Zhang H, Schoenfeld D, Jillapalli D. The use of statistical MUNE in a multicenter clinical trial. Muscle Nerve 2004;30:463–469 [DOI] [PubMed] [Google Scholar]
- 12. Brown WF, Jaatoul N. Amyotrophic lateral sclerosis. Electrophysiologic study (number of motor units and rate of decay of motor units). Arch Neurol 1974;30:242–248 [DOI] [PubMed] [Google Scholar]
- 13. Kasselman LJ, Shefner JM, Rutkove SB. Motor unit number estimation in the rat tail using a modified multipoint stimulation technique. Muscle Nerve 2009;40:115–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang FC, Bouquiaux O, De Pasqua V, Delwaide PJ. Changes in motor unit numbers in patients with ALS: a longitudinal study using the adapted multiple point stimulation method. Amyotroph Lateral Scler Other Motor Neuron Disord 2002;3:31–38 [DOI] [PubMed] [Google Scholar]
- 15. Wang FC, Delwaide PJ. Number and relative size of thenar motor units estimated by an adapted multiple point stimulation method. Muscle Nerve 1995;18:969–979 [DOI] [PubMed] [Google Scholar]
- 16. Wang FC, Delwaide PJ. Number and relative size of thenar motor units in ALS patients: application of the adapted multiple point stimulation method. Electroencephalogr Clin Neurophysiol 1998;109:36–43 [DOI] [PubMed] [Google Scholar]
- 17. Mitsumoto H, Ulug AM, Pullman SL, et al. Quantitative objective markers for upper and lower motor neuron dysfunction in ALS. Neurology 2007;68:1402–1410 [DOI] [PubMed] [Google Scholar]
- 18. Dantes M, McComas A. The extent and time course of motoneuron involvement in amyotrophic lateral sclerosis. Muscle Nerve 1991;7:416–421 [DOI] [PubMed] [Google Scholar]
- 19. Shefner JM. Motor unit number estimation in human neurological diseases and animal models. Clin Neurophysiol 2001;112:955–964 [DOI] [PubMed] [Google Scholar]
- 20. Shefner JM, Cudkowicz M, Brown RH., Jr. Motor unit number estimation predicts disease onset and survival in a transgenic mouse model of amyotrophic lateral sclerosis. Muscle Nerve 2006;34:603–607 [DOI] [PubMed] [Google Scholar]
- 21. Armon C, Moses D. Linear estimates of rates of disease progression as predictors of survival in patients with ALS entering clinical trials. J Neurol Sci 1998;160(suppl 1):S37–S41 [DOI] [PubMed] [Google Scholar]