Abstract
Objective:
To test the hypothesis that level of hemoglobin is associated with incident Alzheimer disease (AD).
Methods:
A total of 881 community-dwelling older persons participating in the Rush Memory and Aging Project without dementia and a measure of hemoglobin level underwent annual cognitive assessments and clinical evaluations for AD.
Results:
During an average of 3.3 years of follow-up, 113 persons developed AD. In a Cox proportional hazards model adjusted for age, sex, and education, there was a nonlinear relationship between baseline level of hemoglobin such that higher and lower levels of hemoglobin were associated with AD risk (hazard ratio [HR] for the quadratic of hemoglobin 1.06, 95% confidence interval [CI] 1.01–1.11). Findings were unchanged after controlling for multiple covariates. When compared to participants with clinically normal hemoglobin (n = 717), participants with anemia (n = 154) had a 60% increased hazard for developing AD (95% CI 1.02–2.52), as did participants with clinically high hemoglobin (n = 10, HR 3.39, 95% CI 1.25–9.20). Linear mixed-effects models showed that lower and higher hemoglobin levels were associated with a greater rate of global cognitive decline (parameter estimate for quadratic of hemoglobin = −0.008, SE −0.002, p < 0.001). Compared to participants with clinically normal hemoglobin, participants with anemia had a −0.061 z score unit annual decline in global cognitive function (SE 0.012, p < 0.001), as did participants with clinically high hemoglobin (−0.090 unit/year, SE 0.038, p = 0.018).
Conclusions:
In older persons without dementia, both lower and higher hemoglobin levels are associated with an increased hazard for developing AD and more rapid cognitive decline.
Alzheimer disease (AD) is the leading cause of dementia in older persons, but its underlying biology is poorly understood. AD affects about 5.3 million persons in the United States1 and is anticipated to affect 13.5 million individuals by 2050.2 Prevention of AD requires identifying risk factors for the development of AD, especially factors amenable to intervention. Hemoglobin abnormalities are common in the elderly3,4 and have been associated with increased mortality.5 Some cross-sectional studies have found relations between anemia and a lower level of cognition,6,7 and we previously reported that both lower and higher hemoglobin levels are associated with worse performance on cognitive tests.8 Currently, it is unclear whether hemoglobin level is related to developing AD. A historical cohort study found that older persons with anemia were not more likely to develop AD9 while another prospective cohort study pointed to anemia having a higher hazard for incident dementia, including AD.10 A recent meta-analysis highlighted the lack of studies examining the effects of high hemoglobin levels and AD.11
In this study, we examined the relationship of hemoglobin levels to incident AD utilizing data from almost 900 community-dwelling persons with hemoglobin assessment and annual detailed clinical evaluations for up to 5 years in the Rush Memory and Aging Project.12 A complementary analysis was conducted to examine the relation of hemoglobin level to the annual rate of cognitive decline.
METHODS
Participants.
All participants were older, community-dwelling individuals who agreed as part of the Memory and Aging Project to annual clinical evaluations and brain donation at the time of death.12 They come from more than 40 groups in the Chicago, IL, vicinity.
The Memory and Aging Project began in 1997, is still ongoing with rolling enrollment, and has an overall follow-up rate of about 95% of survivors. Because of the rolling admission and mortality, the length of follow-up and number of examinations varies across participants. Blood collection was started in February 2003. To maintain the temporal relation between hemoglobin measures and dementia assessments, the first evaluation of hemoglobin level and the associated cognitive testing and clinical evaluation defined “baseline” for this report. All subsequent clinical evaluations and cognitive testing available for each participant were used to estimate the hazard for incident AD and the rate of change in cognition, respectively. Inclusion in these analyses required a valid hemoglobin level, absence of dementia at the visit associated with the hemoglobin measurement, and one or more follow-up clinical evaluations to determine incident AD.
Standard protocol approval, registration, and patient consents.
The study was approved by the Institutional Review Board of Rush University Medical Center. Written informed consent was obtained from all study participants.
Assessment of cognitive function and AD diagnosis.
Participants underwent a uniform structured clinical evaluation that included a medical history, neurologic examination, and cognitive performance testing. Clinical diagnoses were made using a multistep process.13 A battery of 21 cognitive function tests was administered in an approximately 1-hour session. After cognitive test data were reviewed by an experienced neuropsychologist who determined if cognitive impairment was present, participants were evaluated in person by an experienced clinician who used all available current year cognitive and clinical testing results to diagnose dementia and AD using the criteria of the National Institute of Neurologic and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association.14 Mild cognitive impairment (MCI) was defined as having impairment on cognitive evaluation but not meeting AD diagnostic criteria.15 Using the mean and SD from the baseline evaluation of all participants, raw scores from 19 individual cognitive tests were converted to z scores which were averaged to construct a global cognitive function summary score along with 5 specific cognitive domain scores for episodic memory, semantic memory, working memory, visuospatial ability, and perceptual speed.16 A summary score was considered as missing if less than half of its component raw test scores were available. All follow-up evaluations were performed by examiners blinded to data collected in prior years.
Measurement of hemoglobin.
Phlebotomists and nurses skilled in venipuncture collected blood in a 2-mL EDTA tube. Complete blood count analyses were performed using a Beckman/Coulter LH750 automated processor (Quest Laboratories, Wood Dale, IL).8 Clinically low hemoglobin (anemia) was defined as having a hemoglobin level less than 12 g/dL for women and less than 13 g/dL for men.17 Clinically high hemoglobin was defined as having a hemoglobin level greater than 15.5 g/dL for women and greater than 17.5 g/dL for men.
Comorbidities and other covariates.
Individuals were asked for demographic information including date of birth, sex, and highest number of years of education completed. Mean corpuscular volume and red cell distribution width were determined using a Beckman/Coulter LH750 automated processor.8 Body mass index was calculated by dividing the measured weight converted to kilograms by the square of the measured height expressed in meters. Glomerular filtration rate was calculated using the 4-variable formula derived from the Modification of Diet in Renal Disease Study.18 The number of chronic medical conditions at study baseline was determined based on self-report for cancer, congestive heart failure, coronary artery disease, head injury, hip fracture, smoking, and thyroid disease; self-report along with review of medications for diabetes and hypertension; and self-report along with clinician assessment for depression, Parkinson disease, and stroke. Forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1) raw scores were averaged from 2 trials with a hand-held spirometer and converted to z scores.19,20 Physical activity was based on self-report on the number of hours of physical activities engaged in during the 2 weeks prior to the evaluation.21,22 Current frequency of participation in cognitively stimulating activities was assessed with a structured, 9-item questionnaire.21 Depressive symptoms experienced in the previous week were assessed using a 10-item version of the Center for Epidemiologic Studies Depression Scale.23 Social network size was the number of children, family, and friends seen at least once per month.24 APOE genotype was determined by high throughput sequencing of codon 112 (position 3937) and codon 158 (position 4075) of exon 4 of the APOE gene on chromosome 19 (Agencourt Bioscience Corporation, Beverly, MA).25
Statistical analysis.
We compared the bivariate associations of baseline hemoglobin levels with demographic variables and other covariates. We divided the cohort into those who did and who did not develop AD during the course of the study and compared their baseline demographic and covariate measures. Discrete time Cox proportional hazards models26 were used to examine the hazard for developing AD associated with baseline hemoglobin. Previous cross-sectional analyses in this cohort8 showed a nonlinear relationship between hemoglobin and cognitive function. Therefore, our core model for these analyses included both linear and quadratic terms for hemoglobin, together with terms adjusting for age, sex, and education. Then, we replaced the hemoglobin terms with terms comparing the hazard for developing incident AD for participants with anemia or clinically high hemoglobin as compared to participants with clinically normal hemoglobin levels. To examine if the relationship of hemoglobin levels to incident AD was modified by demographic variables, our core model was repeated 3 times with interactions for linear and quadratic terms for hemoglobin and age, sex, and education, respectively. Next, we repeated the core model adding several covariates which might affect the association of baseline hemoglobin with developing AD. We added each covariate individually into the core model (result not shown) and then all the covariates together in the same model. In a sensitivity analysis, we repeated our core model after excluding individuals with MCI. We conducted a complementary set of analyses using mixed-effects, repeated measures models27 to examine the relationship of hemoglobin to decline in cognitive function over time, the clinical hallmark of AD, and to control for baseline cognitive function. We included random person-specific intercepts and slopes, with coefficients for linear and quadratic terms in hemoglobin levels as well as with their interactions with time in study. Terms for age, sex, and education were included in all models along with their interactions with time in study. We repeated the mixed effects model 5 times with each specific cognitive domain as the outcome. Model assumptions of normality, independence, and constant variance of errors were adequately met. Analyses were carried out in SAS®, version 9.1.8 (SAS Institute Inc., Cary, NC).
RESULTS
As of October 2008, 1,059 participants had a valid baseline hemoglobin level. Of these participants, 96 had clinical dementia and 39 were not eligible for follow-up examination (10 persons died prior to their first follow-up and 29 had not yet reached their first follow-up). Of the 924 participants who were eligible for follow-up examination, 43 had missing follow-up data, yielding a participation rate of >95%. The remaining 881 participants had an average 3.3 years of follow-up (SD 1.4 years, range 1–5 years), 75% were female, the mean age was 80.6 (SD 7.4) years, and the mean education level was 14.4 (SD 3.0) years.
Descriptive properties of hemoglobin.
Baseline hemoglobin level was symmetrically distributed with a mean of 13.3 g/dL (SD 1.3, range 8.7–18.0, skewness = −0.22). Hemoglobin levels were weakly correlated with age (r = −0.078, p = 0.02) and education level (r = 0.128, p < 0.001). Hemoglobin levels were higher in men than women (13.9 g/dL [SD 1.4] vs 13.2 g/dL [SD 1.2] [t(df = 335] = 7.36, p < 0.001). Hemoglobin level was correlated with mean corpuscular volume (r = 0.234, p < 0.001), red cell distribution width (r = −0.276, p < 0.001), estimated glomerular filtration rates (r = 0.217, p < 0.001), and pulmonary function tests measured by forced expiratory volume (r = 0.246, p < 0.001) and vital capacity (r = 0.233, p < 0.001). Hemoglobin levels had a weak correlation with report of chronic medical conditions (r = −0.095, p = 0.01). Baseline characteristics for participants with clinically low, normal, and high hemoglobin levels are shown in table e-1 on the Neurology® Web site at www.neurology.org.
Hemoglobin and incident AD.
Over an average of 3.3 years of follow-up, 113 participants developed AD. At baseline, individuals who developed AD were older and had lower Mini-Mental State Examination scores, lower body mass index, lower cognitive activity, more depressive symptoms, and lower social network contacts as compared to individuals who did not (table 1).
Table 1.
Baseline participant characteristics by development of Alzheimer disease
Abbreviation: AD=Alzheimer disease.
The Mini-Mental State Examination score has a maximum value of 30 with higher scores indicating better performance.
Chronic medical conditions determined by report of cancer, congestive heart failure, coronary artery disease, depression, diabetes, head injury, hip fracture, hypertension, smoking, stroke, Parkinson disease, and thyroid disease.
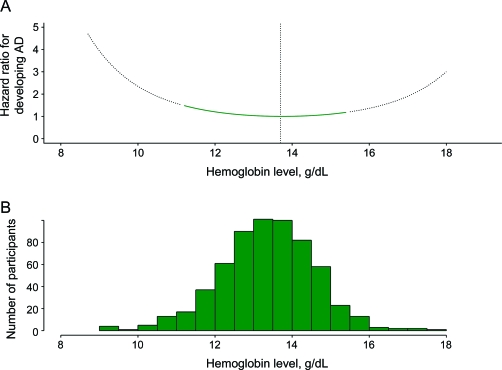
Using a Cox proportional hazards model adjusted for age, sex, and education, both linear and quadratic terms for hemoglobin were associated with incident AD (table 2, model A). As shown in figure 1, the incident AD hazard ratios (HRs) increased with hemoglobin levels lower or higher than 13.7 g/dL. In a model comparing the hazard for developing AD for participants with anemia or clinically high hemoglobin to participants with clinically normal hemoglobin, anemia was associated with a 60% increased hazard ratio (95% CI 1.02–2.52). Having a clinically high hemoglobin level also was associated with an increased hazard for developing AD (HR 3.39; 95% CI 1.25–9.20).
Table 2.
Relationship of baseline hemoglobin with incident Alzheimer disease
Abbreviation: CI=confidence interval.
From proportional hazards model adjusted for age, sex, and education.
From proportional hazards model which included all the terms in model A as well as terms for the following covariates: linear and nonlinear terms for mean corpuscular volume, red cell distribution width, linear and nonlinear terms for body mass index, estimated glomerular filtration rate, forced expiratory volume, forced vital capacity, number of 12 common chronic conditions reported, physical activity, cognitive activity, depressive symptoms, social networks, and the presence of an APOE ϵ4 allele.
The effect of a 1 g/dL difference in hemoglobin.
Figure 1. Hazard ratio for incident Alzheimer disease (AD) as a function of baseline hemoglobin level.
(A) The curve is generated from a proportional hazards model with age, sex, education, and linear and quadratic terms for hemoglobin. Reference hazard ratio was for the hemoglobin level associated with the lowest hazard ratio (13.7 g/dL). (B) The distribution of hemoglobin for the cohort depicts data available for interpreting the relationship of baseline level to risk of AD.
When we repeated the core model to determine if the association between baseline hemoglobin level and incident AD varied by demographic variables, no interactions were found (results not shown). When we repeated the core model by adding terms for covariates potentially associated with hemoglobin levels (linear and quadratic terms for mean corpuscular volume, red cell distribution width, linear and quadratic terms for body mass index, glomerular filtration rate, common chronic medical conditions, and pulmonary function measures) and potentially associated with cognitive function (physical activity, cognitive activity, depressive symptoms, social networks, and presence of an APOE ϵ4 allele), the association between baseline hemoglobin and incident AD was unchanged (table 2, model B). When we repeated the core model excluding participants with MCI, the HR for developing AD was unchanged but no longer significant (HR for quadratic of hemoglobin 1.08; 95% CI 0.99–1.17).
Hemoglobin and rate of change in cognition.
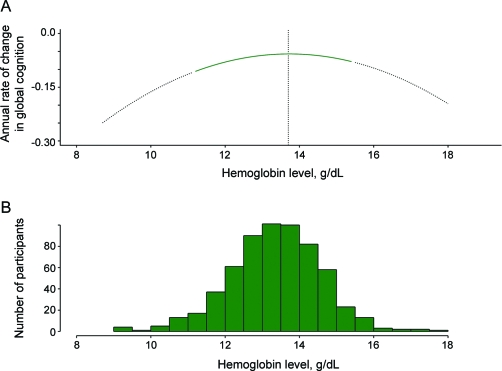
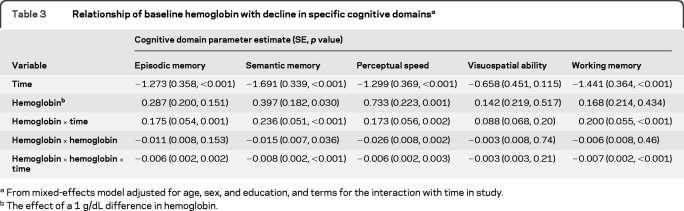
To ensure that our findings were not an artifact of diagnostic classification and to control for baseline global cognitive function, we examined the relation of hemoglobin level with rate of cognitive decline. Baseline global cognition z scores ranged from −1.81 to 1.43, with higher scores indicating better function. Linear mixed-effects models adjusted for age, sex, education, and baseline level of cognition showed that lower and higher hemoglobin levels were associated with a greater annual rate of global cognitive decline (parameter estimate for quadratic of hemoglobin = −0.008, SE −0.002, p < 0.001). The lowest rate of decline was associated with a hemoglobin level of 13.7 g/dL (figure 2). Compared to participants with clinically normal hemoglobin, participants with anemia had a more rapid cognitive decline (parameter estimate = −0.061, SE 0.012, p < 0.001) as did participants with clinically high hemoglobin (parameter estimate = −0.090, SE 0.038, p = 0.018). Since baseline age was associated with cognitive decline in this model, having anemia can be contextualized as being associated with an equivalent rate of cognitive decline associated with a participant approximately being 12 years older (anemia × time, −0.061 = age × time, −0.005 × 12 years). Having clinically high hemoglobin was associated with an equivalent rate of cognitive decline for a participant approximately being 18 years older. Hemoglobin was nonlinearly associated with all specific cognitive domains except visuospatial ability (table 3).
Figure 2. Rate of annual cognitive decline as a function of baseline hemoglobin level.
(A) The curve is generated from a mixed-effects model with time, age, sex, education, linear and quadratic terms for hemoglobin, and each term's interaction with time. Y-axis shows the annual rate of change in cognition with a more negative value associated with a more rapid rate of decline. Lowest annual rate of cognitive decline was associated with hemoglobin of 13.7 g/dL. (B) The distribution of hemoglobin for the cohort depicts data available for interpreting the relationship of baseline level to annual rate of cognitive decline.
Table 3.
Relationship of baseline hemoglobin with decline in specific cognitive domainsa
From mixed-effects model adjusted for age, sex, and education, and terms for the interaction with time in study.
The effect of a 1 g/dL difference in hemoglobin.
DISCUSSION
In nearly 900 older persons without dementia examined annually for up to 5 years, low and high hemoglobin levels were associated with incident AD. Our complementary finding that low and high hemoglobin levels were associated with the rate of cognitive decline in analyses that controlled for baseline level of cognition suggests that the association of hemoglobin with incident AD is not likely the result of diagnostic misclassification. The results point to the possibility of common pathophysiologic processes between hemoglobin abnormalities and brain dysfunction in elders.
A novel feature of this study is the ability to examine the effects of the entire range of hemoglobin levels on hazard of developing AD. To our knowledge, prior studies only examined clinically low hemoglobin levels (anemia) and found mixed results. A retrospective cohort study of persons over age 65 with anemia found no increased hazard for AD over 5 years of follow-up9 while a prospective study in an older Swedish cohort found that anemia was associated with a 2-fold increased hazard for developing AD over 3 years.10 Our work is consistent with the prior prospective cohort finding with anemia. The current study extends prior work in 2 important ways. First, by measuring the full range of hemoglobin, the current study found that each unit of hemoglobin lower and higher than 13.7 g/dL was nonlinearly associated with an increased risk of incident AD. Second, the current study found that there is also a nonlinear relationship between hemoglobin and the rate of cognitive decline, consistent with our prior cross-sectional data.8 As hemoglobin is frequently measured in current clinical practice, these results may have important translational consequences for identifying older persons at increased risk for developing AD and cognitive decline in our aging population.
The mechanisms linking hemoglobin levels to incident AD and cognitive decline are not understood. The association of hemoglobin levels and AD may be due to both being markers for frailty in older persons. Low hemoglobin level may be a marker for ischemia associated with cerebrovascular disease, hypoxia-associated changes in hypoxia inducible factor and erythropoietin levels, or oxidative stress–associated changes in heme regulation. Our finding that hemoglobin levels are associated with cognitive decline in other domains than episodic memory hints at a potential vascular cause. In older, community-dwelling persons, anemia has been associated with increased risk of white matter disease progression on neuroimaging.28 Second, chronic kidney disease (associated with low hemoglobin levels) could result in cerebral hypoxia. Initial studies mainly in animal models point to chronic kidney disease29 being associated with decreased production of hypoxia inducible factor, which may reduce erythropoietin production. As erythropoietin receptors have been localized in the brain30 and seem to have a neuroprotective effect in animal models of stroke or hypoxia,31,32 lower erythropoietin levels may increase the risk of neuronal degeneration in certain cognitive pathways. Finally, greater red cell fragility in conditions associated with lower hemoglobin levels may lead to brain astroglia having to process more heme molecules crossing the blood–brain barrier. Heme may upregulate the production of hemo-oxygenase-1 resulting in increased sterol dysregulation and oxidative stress damage, especially in individuals already with subclinical AD pathology.33 Our finding of high hemoglobin levels being associated with AD and cognitive decline warrant further investigation given the limited number of cases with clinically high hemoglobin levels. High hemoglobin levels may be associated with cognitive decline via ischemic and hypoxic mechanisms. Polycythemia vera has been associated with an increased risk of cerebral thrombosis.34 In limited studies, chronic obstructive pulmonary disease (associated with high hemoglobin levels) has been associated with cognitive decline in older persons35 and with decreased frontal and parietal lobe perfusion on brain imaging.36 Further studies are needed to determine the biologic basis for the association between hemoglobin, cognitive decline, and AD in elders.
Strengths of our study include detailed annual cognitive and clinical evaluations on a large, community-based cohort. We also were able to adjust for some important comorbidities associated with hemoglobin levels and AD. Our study has limitations. Although we were able to find an association between hemoglobin and incident AD, our study design limits our ability to determine whether hemoglobin alone causes AD or whether hemoglobin levels and AD may share a common cause. Also, due to the multiple risk factors that may be associated with both hemoglobin levels and AD, we were not able to adjust for other factors that may be associated with both items including but not limited to macronutrient and micronutrient deficiencies.
Our findings suggest that low hemoglobin levels may need to be considered as a potential contributing factor to the development of AD in older persons. Before tests of specific interventions in the elderly to correct hemoglobin abnormalities on reducing the hazard of developing AD are initiated, further confirmation of the relationship between hemoglobin and AD will be needed from other longitudinal cohort studies.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the participants of the Rush Memory and Aging Project. They also thank Traci Colvin and Tracey Nowakowski for project coordination; Barbara Eubeler, Karen Lowe Graham, and Mary Futrell for participant recruitment; John Gibbons and Greg Klein for data management; Lei Yu, PhD, and Wenqing Fan, MS, for statistical programming; and the staff of the Rush AD Center.
Editorial, page 206
Supplemental data at www.neurology.org
 Scan this code with your smartphone to access this feature
Scan this code with your smartphone to access this feature
- AD
- Alzheimer disease
- CI
- confidence interval
- FEV1
- forced expiratory volume in 1 second
- FVC
- forced vital capacity
- HR
- hazard ratio
- MCI
- mild cognitive impairment
AUTHOR CONTRIBUTIONS
Dr. Shah: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, statistical analysis. Dr. Buchman: drafting/revising the manuscript, analysis or interpretation of data, acquisition of data, study supervision, obtaining funding. Dr. Wilson: drafting/revising the manuscript, analysis or interpretation of data. Dr. Leurgans: drafting/revising the manuscript, analysis or interpretation of data, statistical analysis. Dr. Bennett: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, study supervision, obtaining funding.
DISCLOSURE
Dr. Shah receives research support from Ceregene, Danone Research B.V., Eisai Inc., Elan Corporation, Merck & Co., Inc., Orasi Medical, Inc., Pamlab, L.L.C., and Pfizer Inc; and receives research support from the NIH and the Illinois Department of Public Aid Alzheimer's Disease Assistance Center. Dr. Buchman receives research support from the NIH. Dr. Wilson serves as a Consulting Editor for Aging, Neuropsychology, and Cognition and Psychology and Aging and receives research support from the NIH/NIA. Dr. Leurgans receives research support from the NIH (NIA, NINDS). Dr. Bennett serves on the editorial boards of Neurology®, Neuroepidemiology, and Current Alzheimer's Research; serves on the scientific advisory board for Vigorous Minds; serves/has served as a consultant to Schering-Plough Corp., Double Helix Development, Medivation, Inc., Danone Research B.V., and Gerson Lehrman Group; and receives research support from Danone Inc, the NIH, the Illinois Department of Public Health, and the Robert C. Borwell Endowment Fund.
REFERENCES
- 1. Alzheimer's Association 2009 Alzheimer's disease facts and figures. Alzheimers Dement 2009;5:234–270 [DOI] [PubMed] [Google Scholar]
- 2. Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer's disease in the U.S. population: prevalence estimates using the 2000 census. Arch Neurol 2003;60:1119–1122 [DOI] [PubMed] [Google Scholar]
- 3. Woodman R, Ferrucci L, Guralnik J. Anemia in older adults. Curr Opin Hematol 2005;12:123–128 [DOI] [PubMed] [Google Scholar]
- 4. Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood 2004;104:2263–2268 [DOI] [PubMed] [Google Scholar]
- 5. Zakai NA, Katz R, Hirsch C, et al. A prospective study of anemia status, hemoglobin concentration, and mortality in an elderly cohort: the Cardiovascular Health Study. Arch Intern Med 2005;165:2214–2220 [DOI] [PubMed] [Google Scholar]
- 6. Denny SD, Kuchibhatla MN, Cohen HJ. Impact of anemia on mortality, cognition, and function in community-dwelling elderly. Am J Med 2006;119:327–334 [DOI] [PubMed] [Google Scholar]
- 7. Chaves PH, Carlson MC, Ferrucci L, Guralnik JM, Serba R, Fried LP. Association between mild anemia and executive function impairment in community-dwelling older women: The Women's Health and Aging Study II. J Am Geriatr Soc 2006;54:1429–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shah RC, Wilson RS, Tang Y, Dong X, Murray A, Bennett DA. Relation of hemoglobin to level of cognitive function in older persons. Neuroepidemiology 2009;32:40–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beard CM, Kokmen E, O'Brien PC, Ania BJ, Melton LJ., 3rd Risk of Alzheimer's disease among elderly patients with anemia: population-based investigations in Olmsted County Minnesota. Ann Epidemiol 1997;7:219–224 [DOI] [PubMed] [Google Scholar]
- 10. Atti AR, Palmer K, Volpato S, Zuliani G, Winblad B, Fratiglioni L. Anemia increases the risk of dementia in cognitively intact elderly. Neurobiol Aging 2006;27:278–284 [DOI] [PubMed] [Google Scholar]
- 11. Peters R, Burch L, Warner J, Beckeet N, Poulter R, Bulpitt C. Hemoglobin, anemia, dementia and cognitive decline in the elderly, a systematic review. BMC Geriatr 2008;8:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bennett DA, Schneider JA, Buchman AS, Mendes de Leon C, Bienias JL, Wilson RS. The Rush Memory and Aging Project: study design and baseline characteristics of the study cohort. Neuroepidemiology 2005;25:163–175 [DOI] [PubMed] [Google Scholar]
- 13. Bennett DA, Schneider JA, Aggarwal NT, et al. Decision rules guiding the clinical diagnosis of Alzheimer's disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiology 2006;27:169–176 [DOI] [PubMed] [Google Scholar]
- 14. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology 1984;34:939–944 [DOI] [PubMed] [Google Scholar]
- 15. Aggarwal NT, Wilson RS, Beck TL, Bienias JL, Bennett DA. Mild cognitive impairment in different function domains and incident Alzheimer's disease. J Neurol Neurosurg Psychiatry 2005;76:1479–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wilson RS, Barnes LL, Krueger KR, Hoganson G, Bienias JL, Bennett DA. Early and late life cognitive activity and cognitive systems in old age. J Int Neuropsychol Soc 2005;11:400–407 [PubMed] [Google Scholar]
- 17. World Health Organization Nutritional Anemias: Report of a WHO Scientific Group: World Health Organization Tec Rep Ser 405. Geneva: WHO; 1968 [PubMed] [Google Scholar]
- 18. National Kidney Disease Educational Program GFR Calculators. Available at: http://www.nkdep.nih.gov/professionals/gfr_calculators/orig_con.htm Accessed February 21, 2007
- 19. Otulana BA, Higenbottam T, Ferrari L, Scott J, Igboaka G, Wallwork J. The use of home spirometry in detecting acute lung rejection and infection following heart-lung transplantation. Chest 1990;97:353–357 [DOI] [PubMed] [Google Scholar]
- 20. Buchman AS, Wilson RS, Boyle PA, Tang Y, Fleischman DA, Bennett DA. Physical activity and leg strength predict decline in mobility performance in older persons. J Am Geriatr Soc 2007;55:1618–1623 [DOI] [PubMed] [Google Scholar]
- 21. Wilson RS, Mendes de Leon CF, Barnes LL, et al. Participation in cognitively stimulating activities and risk of incident Alzheimer's disease. JAMA 2002;287:742–748 [DOI] [PubMed] [Google Scholar]
- 22. McPhillips JB, Pellettera KM, Barrett-Conner E, Wingard DL, Criqui MH. Exercise patterns in a population of older adults. Am J Prev Med 1989;5:65–72 [PubMed] [Google Scholar]
- 23. Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401 [Google Scholar]
- 24. Bennett DA, Schneider JA, Tang Y, Arnold SE, Wilson RS. The effect of social networks on the relation between Alzheimer's disease pathology and level of cognitive function in old people: a longitudinal cohort study. Lancet Neurol 2006;5:406–412 [DOI] [PubMed] [Google Scholar]
- 25. Buchman AS, Boyle PA, Wilson RS, Beck TL, Kelly JF, Bennett DA. Apolipoprotein E e4 allele is associated with more rapid motor decline in older persons. Alzheimer Dis Assoc Disord 2009;23:63–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cox DR. Regression models and life tables (with discussion). J R Stat Soc B 1972;74:187–220 [Google Scholar]
- 27. Laird N, Ware J. Random-effects models for longitudinal data. Biometrics 1982;38:963–974 [PubMed] [Google Scholar]
- 28. Inzitari M, Studenski S, Rosano C, et al. Anemia is associated with the progression of white matter disease in older adults with high blood pressure: the cardiovascular health study. J Am Geriatr Soc 2008;56:1867–1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nangaku M, Inagi R, Miyata T, Fujita T. Hypoxia and hypoxia-inducible factor in renal disease. Nephron Exp Nephrol 2008;110:e1–e7 [DOI] [PubMed] [Google Scholar]
- 30. Assaraf MI, Diaz Z, Liberman A, et al. Brain erythropoietin receptor expression in Alzheimer disease and mild cognitive impairment. J Neuropathol Exp Neurol 2007;66:389–398 [DOI] [PubMed] [Google Scholar]
- 31. Hasselblatt M, Ehrenreich H, Siren AL. The brain erythropoietin system and its potential for therapeutic exploitation in brain disease. J Neurosurg Anesthesiol 2006;18:132–138 [DOI] [PubMed] [Google Scholar]
- 32. Maiese K, Li F, Chong ZZ. New avenues of exploration for erythropoietin. JAMA 2005;293:90–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hascalovici JR, Vaya J, Khatib S, et al. Brain sterol dysregulation in sporadic AD and MCI: relationship to heme oxygenase-1. J Neurochem 2009;110:1241–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gruppo Italiano Studio Policitemia Polycythemia vera: the natural history of 1213 patients followed for 20 years. Ann Intern Med 1995;123:656–664 [DOI] [PubMed] [Google Scholar]
- 35. Hung WW, Wisnivesky JP, Siu AL, Ross JS. Cognitive decline among patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2009;180:134–137 [DOI] [PubMed] [Google Scholar]
- 36. Ortapamuk H, Naldoken S. Brain perfusion abnormalities in chronic obstructive pulmonary disease: comparison with cognitive impairment. Ann Nucl Med 2006;20:99–106 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.