Abstract
Effects of three-dimensional (3D) calcium phosphate (CaP) porous granules on the growth and odontogenic differentiation of human dental pulp stem cells (hDPSCs) were examined for dental tissue engineering. hDPSCs isolated from adult human dental pulps were cultured for 3-4 passages, and populated on porous granules. Cell growth on the culture dish showed an ongoing increase for up to 21 days, whereas the growth on the 3D granules decreased after 14 days. This reduction in proliferative potential on the 3D granules was more conspicuous under the osteogenic medium conditions, indicating that the 3D granules may induce the odontogenic differentiation of hDPSCs. Differentiation behavior on the 3D granules was confirmed by the increased alkaline phosphatase activity, up-regulation of odontoblast-specific genes, including dentin sialophosphoprotein (DSPP) and dentin matrix protein 1 (DMP1) by quantitative polymerase chain reaction, and greater level of dentin sialoprotein synthesis by western blot. Moreover, the cellular mineralization, as assessed by Alizarin red S and calcium quantification, was significantly higher in the 3D CaP granules than in the culture dish. Taken all, the 3D CaP porous granules should be useful for dental tissue engineering in combination with hDPSCs by providing favorable 3D substrate conditions for cell growth and odontogenic development.
1. Introduction
Human dental pulp stem cells (hDPSCs) have gained great importance for use in the regenerative treatment of defective dental tissues, particularly those in the dentin-pulp complex [1, 2]. Recent advances in stem-cell biology have revealed that progenitor cells are also present in dental pulp tissue [3, 4], which suggests the possible regenerative healing of dentin-pulp complex, as opposed to the conventional endodontic treatments [5]. DPSCs derived from adult pulp tissue maintain the characteristics of stem cells, including self-renewal and multipotency [6]. Studies have shown that hDPSCs can give rise to a variety of cells and tissues other than dentin, such as adipocytes, neural progenitor cells and myotubes [7–9].
Recent advances in the development of biomaterials have spurred the extended use of stem cells in tissue engineering [10–12]. When formulated within the 3D scaffolding substrate, stem cells can maintain the physiological stability and better perform the biological roles involved in the regeneration process [13, 14]. Some bioactive scaffolds have shown promise in the regeneration of dental tissues, including dentin-pulp complex. One of the most popular groups of materials are calcium phosphate (CaP) ceramics, which have generally been shown to induce appropriate osteoblastic differentiation of stem/progenitor cells in vitro and bone formation in vivo [15–17]. The granular form of bioactive ceramics has commonly been an attractive choice of materials to fill bone defects and deliver biosignals as an injectable system [18–22]. However, few studies have been conducted to examine the effects of these bone-regenerating materials on odontoblast behavior and dentin formation [23].
Previously, the authors produced 3D porous scaffolds of CaP using the polymer-reticulate method [24]. Because the porous structure of the granule and the surface bioactivity can provide effective cues and microenvironments for hard tissue development, it has the potential for use in dentin regeneration. An even more promising approach to utilization of the 3D granule is tissue engineering in combination with hDPSCs and biosignals to mimic the extracellular compartment of dentin ex vivo and then implant these materials within the degenerative oral cavity.
As a first step, we herein focus on identifying the in vitro performance of the 3D CaP bioactive granules in the population of hDPSCs and their differentiation into odontoblasts, which can further be used in dental tissue engineering. DPSCs isolated from human pulp tissues were grown on granular scaffolds, and the effects of the granules on the odontoblastic differentiation were then examined in terms of identification of markers at the gene and protein level.
2. Materials and Methods
2.1. hDPSCs Isolation and Culture
Human dental pulps were collected from the third molar teeth of adult patients with ages ranging from 19-to-25 years old at the Dental and Craniofacial Clinic of the School of Dentistry in Dankook University and used with the patients' informed consent. The primary DPSCs were cultured as described in a previous study conducted by Gronthos et al. [6]. Briefly, the pulp was separated from the crowns and roots, minced, and digested in a solution of 3 mg/mL type I collagenase and 4 mg/mL dispase for 1 h at 37°C. Single-cell suspensions were then obtained by passing these cells through a 70 μm strainer. A 200 μL aliquot of cells were then seeded onto tissue culture plates (96 well) at a density of 2 × 103 cells/well and grown with α-modified Eagle medium (α-MEM; Gibco, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, USA), 100 units/mL penicillin, and 100 μg/mL streptomycin (Gibco, USA). Culture medium was refreshed every 2-3 days. Cells maintained for 3-4 passages were used for further cell growth and differentiation study.
2.2. Stem-Cell Characterization
The stem-cell characteristics of hDPSCs were identified by means of immunofluorescence staining and fluorescence-activated cell sorting (FACS) analysis. For the immunostaining, hDPSCs were fixed with 4% paraformaldehyde for 20 minutes at room temperature followed by treatment with 0.2% Triton X-100 (Sigma) in PBS for 20 minutes. The cells were incubated with solutions of mouse monoclonal antibodies: anti-SSEA4 (1 : 100 dilution, MAB4303, Millipore, USA) and anti-STRO-1 (1 : 100 dilution, Developmental Studies Hybridoma Bank, Univ. Iowa, USA). After washing in PBS, cells were then incubated with secondary mouse and rabbit conjugated with a fluorescein isothiocyanate (FITC, Sigma, USA) for 1 h at room temperature. The SSEA4 and STRO-1 positive cells were revealed under confocal laser scanning microscopy (CLSM, LSM 510, Zeiss), and the fraction of cells was analyzed with the use of a flow cytometer (FACS Calibur, B.D. Co, USA). From our preliminary experiments, we observed that the odontogenic differentiation of the hDPSCs was pronounced approximately after 3 weeks; therefore, here, we monitored the cell proliferation mainly up to 2-3 weeks and the differentiation at the prolonged periods about 3-4 weeks.
2.3. 3D Granules for Assays
The porous granular form of the CaP scaffold was prepared by the polymer replication method as described previously, with slight modification [24]. Briefly, calcium phosphate nanopowders with a hydroxyapatite crystalline phase were made into a slurry that contained poly(vinyl butyral) and triethyl phosphate. Polyurethane foams (45 ppi, Customs foam system Ltd., Canada) were then dipped into the slurry and dried in an oven, after which this dipping-drying process was repeated to produce a thick layer of CaP. The dried sample was then thermally treated at 800°C for 5 h followed by treatment at 1250°C for 1 h. Next, the scaffolds were gently crushed into granules, and sizes in the range of 500 to 1000 μm were selected. Prior to the cellular tests, the granules were sterilized with 70% ethanol and dried. The characteristics of the CaP porous granules, such as crystalline phase and morphology were observed by X-ray diffraction (XRD; Rigaku, Japan) and scanning electron microscopy (SEM; Hitachi, Japan), respectively.
2.4. Cell Culture and Growth Kinetics
The 3D granules of 20 mg which was chosen to completely cover the surface of the well were placed in each well of a 96-well plate. The hDPSCs were then plated at a density of 4 × 104 cells per well. The samples were then cultured statically in α-MEM supplemented with 10% fetal bovine serum (FBS) containing 1% antibiotics/antimycotics (Gibco, USA). Odontoblastic differentiation medium was prepared by conditioning the culture medium with 50 μg/mL ascorbic acid, 10 mM β-glycerol phosphate, and 10−7 M dexamethasone. Cell growth kinetics was then examined by a MTS assay. After 1, 3, 7, and 14 days, CellTiter 96 Aqueous One Solution Reagent (Promega, Madison, WI) was added to each well according to the manufacturer's instructions. After 3 h in culture, the cell viability was detected quantitatively based on the optical density (OD) at 490 nm using a microplate ELISA reader.
2.5. Cell Morphology Observation
Scanning electron microscopy was used to observe the cell morphology (SEM, Hitach). The cell-cultured samples were rinsed with phosphate-buffered saline (PBS) and then fixed in 2% glutaraldehyde for 5 minutes, after which they were dehydrated in a graded series of ethanol and dried in hexamethyl disilane (HMDS). After sputter-coating with gold, the specimens were examined at an accelerating voltage of 10–15 kV.
The cells surrounding the granules were examined by staining with Hematoxylin & Eosin (H&E). Briefly, the samples were cultured for 21 days, after which the cell-constructed granules were fixed in 10% buffered formalin and embedded in paraffin. Thin sections (~4–6 μm) were then made using a microtome and subsequently stained with H&E. The images were examined by light microscopy.
2.6. Gene Expression Analysis by Real-Time Polymerase Chain Reaction
After culturing the cells for 7, 14, and 21 days, the total RNA was extracted from each sample using a Qiagen RNeasy Mini kit (Qiagen, South Korea), and then converted to complementary DNA (cDNA) with a first-strand cDNA synthesis kit (PrimeScript RT reagent Kit, Bioneer, Korea). Reverse transcriptase (RT) reaction was carried out using 2 μg aliquot of the total RNA. The differentiation of hDPSCs was monitored based on differences in the differentiation markers, including dentin sialophosphoprotein (DSPP), dentin matrix protein 1 (DMP1), collagen type I (Col I), and osteocalcin (OCN). The sense and antisense primers were designed according to published cDNA sequences available in GenBank, and Glyceraldehyde-phosphate-dehydrogenase (GAPDH) was used as a standard housekeeping gene for normalizing mRNA levels (as presented in Table 1). Accumulation of the real-time polymerase chain reaction (rt-PCR) products was monitored and quantified using a SYBR Green PCR kit (Quantace), which was carried out in a spectrofluorometric thermal cycler (Rotor-Gene 3000, Corbett Research, Korea). After the rt-PCR run, the amplification efficiency of different genes was analyzed using the comparative Ct method [25, 26]. Each measurement was assessed in triplicate.
Table 1.
Real-time PCR primer sequences of the genes coding collagen type I (Col I), osteocalcin (OCN), dentin sialophosphoprotein (DSPP), and dentin matrix protein 1 (DMP1).
| Sense primer | Antisense primer | |
|---|---|---|
| Col I. | 5′-aagtcttctgcaacatggag-3′ | 5′-tactcgaactggaatccatc-3′ |
| OCN | 5′-gtgcagagtccagcaaaggt-3′ | 5′-tcagccaactcgtcacagtc-3′ |
| DSPP | 5′-gaagatgctggcctggataa-3′ | 5′-tcttctttcccatggtcctg-3′ |
| DMP1 | 5′-cccttggagagcagtgagtc-3′ | 5′-ctccttttcctgtgctcctg-3′ |
| GAPDH | 5′-acatcaagaaggtggtgaag-3′ | 5′-aaatgagcttgacaaagtgg-3′ |
2.7. Alkaline Phosphatase (ALP) Activity Determination
Samples cultured for 7, 14, and 21 days were used for determination of the ALP. Cells from 3 wells were collected to ensure that a sufficient quantity of proteins was used for the assay. The cell pellets were gathered, added to cell lysis buffer (0.1% triton X-100, 1 M tris-HCl, 5 M NaCl, and 0.5 M ethylenediaminetetraacetic acid), and then disrupted by the freezing/thawing processes. Total protein content was assayed using a commercial DC protein assay kit (BioRad). For the enzymatic reaction, each sample was added to ALP reaction media using p-nitrophenyl phosphate as the substrate, according to the manufacturer's instructions (Sigma). The level of the enzymatic product, p-nitrophenol, was determined based on the change in the absorbance at 405 nm using a spectrophotometer. The ALP activity level was deduced from a protein standard curve made using bovine serum albumin (BSA). Three replicate samples were used for each condition (n = 3).
2.8. Western Blot Analysis for Dentin Sialoprotein (DSP)
After culturing for 21 days, the total cellular proteins were prepared using 0.1 mL RIPA lysis buffer. Equal amounts of each lysate were used for electrophoresis through a sodium dodecyl sulfate (SDS)-polyacrylamide gel in Tris-glycine-SDS running buffer. Proteins were transferred onto PVDF membrane, and the membranes were incubated in 5% BSA blocking solution for 2 h at room temperature. The membrane was incubated overnight at 4°C in the presence of the anti-DSP antibody (1 : 1000 dilutions, Santa Cruz Biotechnology, USA). After washing with TRIS-buffered saline (0.2% Tween-20) three times to remove anti-DSP antibody, the horseradish peroxidase-conjugated secondary antibody (1 : 10,000 dilution, Milipore Corporation, USA) was then added to the membrane and incubated for 2 h at 37°C. After washing in TRIS-buffered saline (0.2% Tween-20), the membrane was visualized with enhanced chemoluminescence reagents (Santa Cruz Biotechnology, Inc.) and exposed to Kodak X-ray film [27].
2.9. Mineralization Assays
The cellular mineralization was conducted on the cells which were indirectly affected by the CaP granules. For this, we first seeded cells on the culture dish and then placed CaP granules upon the cells. After culturing for 28 days, the CaP granules were removed from the culture wells, and the cells on the culture dish which were indirectly affected by the granules were assessed. Cells were fully washed with PBS and then fixed with phosphate-buffered formalin for 10 minutes. The fixed cells were washed vigorously with distilled water, after which they were stained with 1% Alizarin red S diluted in distilled water for 30 minutes. The remaining dye was washed out with distilled water, after which the cells were washed again. Finally, the cells were air dried, and images of the stained cells were captured.
For quantification of the cellular mineralization, the Ca content of the cellular products was assessed. Briefly, the cultured cells were washed with PBS and then dissolved in 1 mL of 1N HCL on a microplate shaker. After neutralizing the solution with 2 ml of 1 M NaOH, 5 μL aliquots of the resultant solution were placed in a 96-well plate. An equal amount (100 μL) of color reagent (0.01 wt% o-cresolphthalein complexone, 0.1 wt% 8-hydroxyquinoline) and buffer reagent (500 mM 2-amino-2-methyl-1-propanol, pH 10.7) was then added to each well, after which the plate was incubated for 15 minutes at room temperature. A standard curve was generated using serial dilutions of CaCO3 (0-0.1 mg/mL), and the absorbance of each well was then measured using a microplate reader at 570 nm. Finally, the amount of calcium deposited in each hydrogel was quantified as mg Ca2+ equivalents.
3. Results
3.1. DPSCs and Growth Behavior
The CaP porous granules used as a 3D matrix for the culturing of hDPSCs were characterized as shown in Figure 1. SEM morphology of the irregular shaped granules (sizes of 0.5–1 mm) showed a cylindrical stem and porous structure with pore sizes of ~200–300 μm (Figure 1(a)). The surface microstructure of the granules revealed granular morphology, being typical of sintered polycrystalline ceramics (inset in Figure 1(a)). The crystalline phase of the CaP granules was observed to be pure hydroxyapatite (Figure 1(b), detected from XRD).
Figure 1.
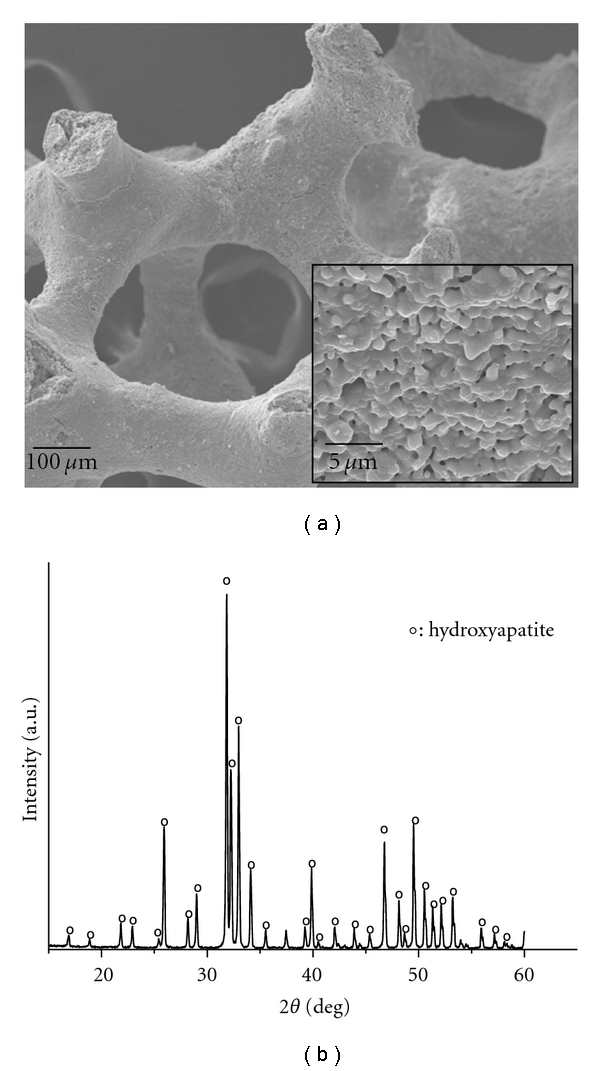
Characteristics of the CaP granules used as the 3D matrix for hDPSC culturing: (a) SEM morphology showing the porous structured granules with surface polycrystalline microstructure (inset) and (b) XRD pattern revealing a crystalline phase of pure hydroxyapatite (HA; hydroxyapatite).
The characteristics of the human dental pulp cells maintained for 3-4 passages were evaluated by immunofluorescence staining the cells with stem-cell markers (SSEA4 and STRO-1) and then analyzed with FACS, as presented in Figure 2. Results showed major population of dental pulp cells was positive to SSEA4 and STRO-1 staining, as revealed by CLSM (Figure 2(a)), which also amounts to approximately 80% of the total cells, as analyzed by FACS (Figure 2(b)).
Figure 2.
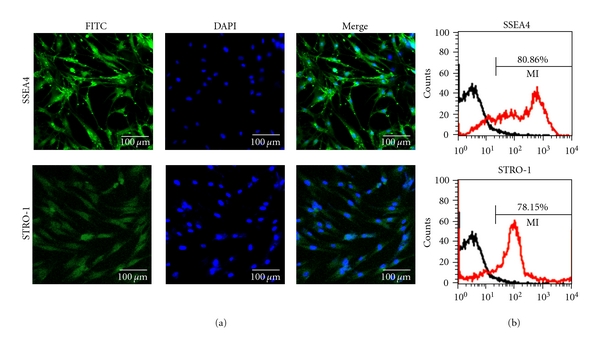
Stem-cell characterization of the human dental pulp cells. Cells cultured for passages of 3-4 were gathered and analyzed. Major population of cells maintained the characteristics of stem cells. (a) Fluorescence images of cells expressing SSEA4 and STRO-1 and (b) FACS analysis, presenting the percentages of cells that were immunoreactive with anti-SSEA4 and STRO-1.
The initial evaluation of the expanded hDPSCs was made by measuring the proliferative potential upon the 2D culture dish with or without using the odontogenic differentiation medium for periods of up to 21 days. The growth rate was measured by the MTS method. From a preliminary test, we observed when the cells were cultured on the 3D granules, about 90–95% of the total population remained on the granules while about 5–10% was on the underlying culture dish after culturing for 3 to 7 days. For convenience, we here considered the cells on both the granules and culture dish as those grown in the 3D condition. As shown in Figure 3(a), samples grown in the 2D culture dish without using differentiation medium showed an ongoing increase with culture time, reflecting good cell viability (dashed line). Interestingly, the cells grown in the 3D CaP granules continued to proliferate for up to 14 days, but this growth decreased at 21 days (line with unfilled circle).
Figure 3.
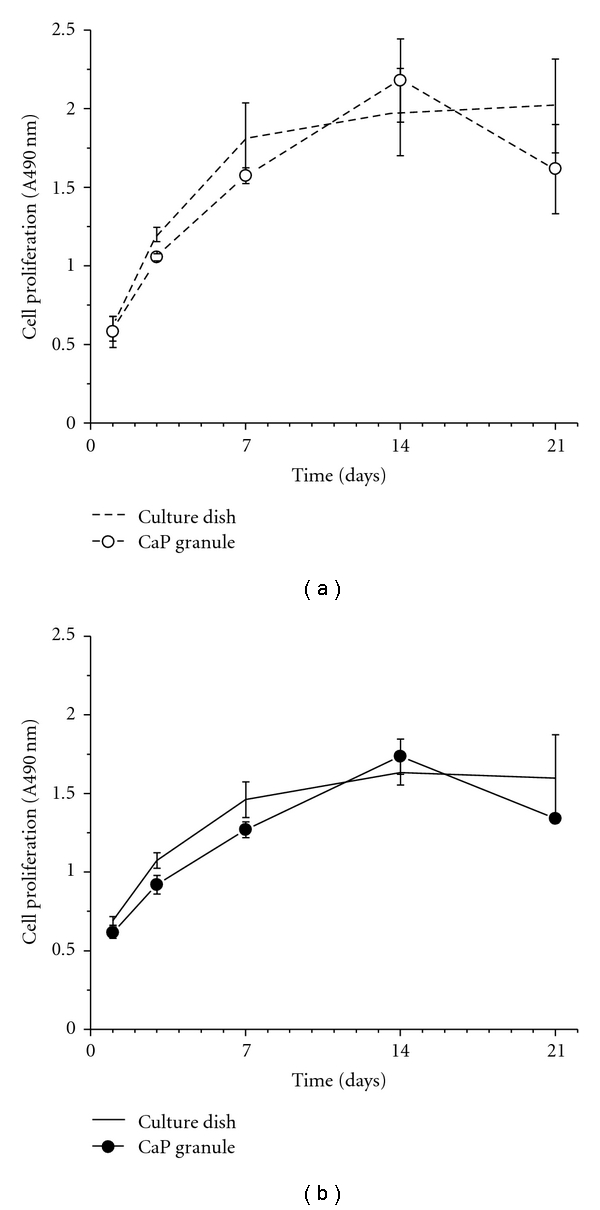
Viability of human dental pulp stem cells (hDPSCs) on the 2D culture dish and 3D CaP granules (a) without and (b) with the induction of odontogenic medium (50 μg/mL ascorbic acid, 10 mM β-glycerol phosphate, and 10−7 M dexamethasone). The cell viability in the culture dish was increased up to 14 days and maintained at 21 days, whereas that on the CaP granules was reduced at 21 days (statistical significant difference was noticed between 14 and 21 days in the CaP granules, **P < .01, ANOVA, n = 3). The odontogenic medium reduced the overall cell viability in both groups.
When treated with differentiation medium (Figure 3(b)), which containing 50 μg/mL ascorbic acid, 10 mM β-glycerol phosphate, and 10−7 M dexamethasone, the overall cell growth levels on both culture dishes (solid line) and CaP granules (line with filled circle) were reduced with respect to those grown without the use of differentiation medium. Furthermore, an abrupt decrease at day 21 was evident in the cells cultured on the 3D CaP granules.
The traits of hDPSCs cultured on a 2D culture dish conditioned with differentiation medium was confirmed by the expression of dentin-associated genes, including dentin sialophosphoprotein (DSPP) and dentin matrix protein 1 (DMP1) at day 7, as determined by RT-PCR (data not shown here).
3.2. ALP Activity
The ALP activity is thought to be one of the most important indicators of functional odontoblasts, because odontoblasts show much higher ALP activity than dental undifferentiated mesenchymal cells [28]. The ALP activity of cells cultured on 2D culture dishes and 3D CaP granules with the use of differentiation medium was assessed for up to 21 days, as shown in Figure 4. For both cases, a significant ALP increase was noticed at 14 days with respect to that observed at 7 days (##P < .01), and the incremental ALP was more noticeable in the CaP granules. At 14 and 21 days, the ALP activity was significantly higher on the CaP granules than on the culture dish (**P < .01).
Figure 4.
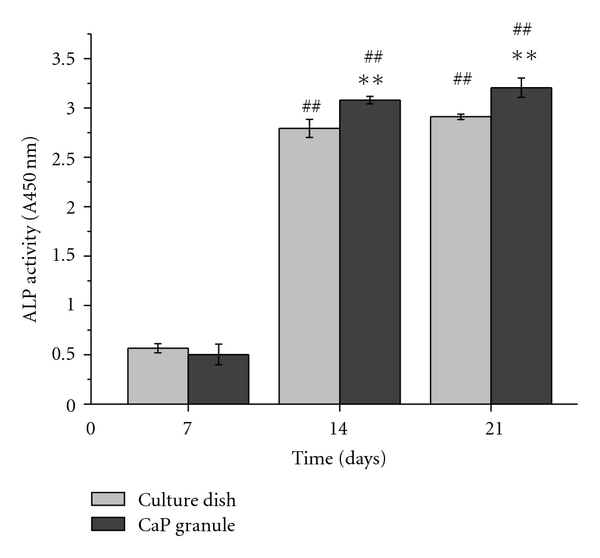
ALP activity of cells cultured for up to 21 days in the 2D culture dish and on the 3D CaP granules. For both 2D and 3D cases, significance increases were noticed at 14 and 21 with respect to day 7 (##P < 0.01, ANOVA, n = 3). In particular, significantly higher level was observed on the CaP granules than on the culture dish at both periods (**P < 0.01, ANOVA, n = 3).
3.3. Cell Morphology on Granules
The cell morphology grown on the CaP granules was observed by SEM during different culture periods (Figure 5). At day 7, most of the cells were spindle-shaped with active cytoskeletal extensions stretching out onto the rough granule surface (Figures 5(a) and 5(b)). The cells became confluent on the granules and formed a distinct layered structure at 14 days (Figures 5(c) and 5(d)). In addition, a network-like structure formed by the cell-secreted fibrous collagen was also detected (Figure 5(c)). At 21 days, some mineralized aggregates could be seen on the granules (arrows in Figures 5(e) and 5(f)).
Figure 5.
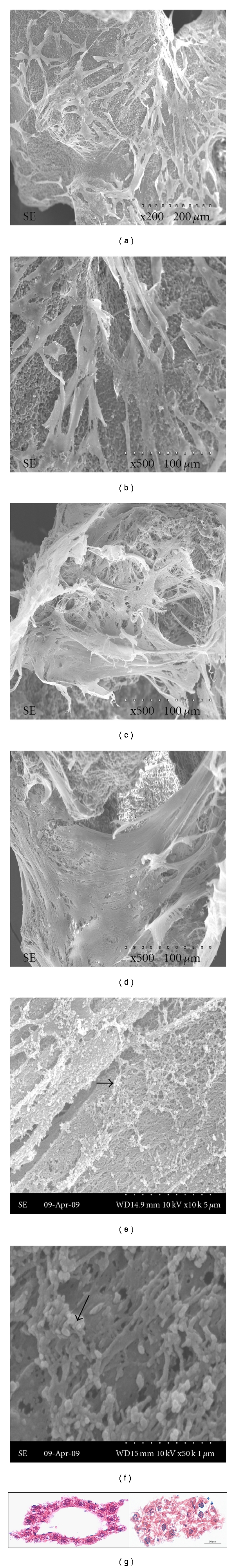
Cell growth morphology following culture on CaP granules for (a, b) 7, (c, d) 14, and (e, f) 21 days, as observed by SEM. Cells with active lamellipodia extensions at day 7 formed a layered structure covering the entire surface at day 14. At 21 days of culture, a number of mineral-like aggregates were produced on the cellular matrix. (g) Histological view of H&E stained cells populated on the granules. Cell nuclei stained with the dark-blue and surrounding pink-colored cytosols were well-developed to show viable cell formation.
As shown in Figure 5(g), the H&E stained image of cells that covered the surface of 3D granules during 21 days of culture clearly demonstrated the formation of a thick cellular construct over the granule that was decalcified during the sample preparation. Dark-stained nuclei and collagenous matrix components were also shown to be well developed.
3.4. Gene Expressions by Real-Time PCR
The expression levels of mRNA for DSPP, DMP1, Col I, and OCN were compared on the 2D culture dish and 3D CaP granules after 7, 14, and 21 days of culture (Figure 6). The mRNA level of the culture dish at 7 days was set to baseline (=1.0). Col I in the culture dish was expressed actively during the early stages of growth and reached a peak at day 21. Col I on the CaP granules was relatively low at day 7, but it was expressed in greater levels with time up to 21 days. Other genes coding noncollagenous proteins related to the dentin matrix were quite inactive during the first 14 days. However, they were highly upregulated at 21 days and this upregulation was more conspicuous on the 3D CaP granules than on the 2D culture dish.
Figure 6.
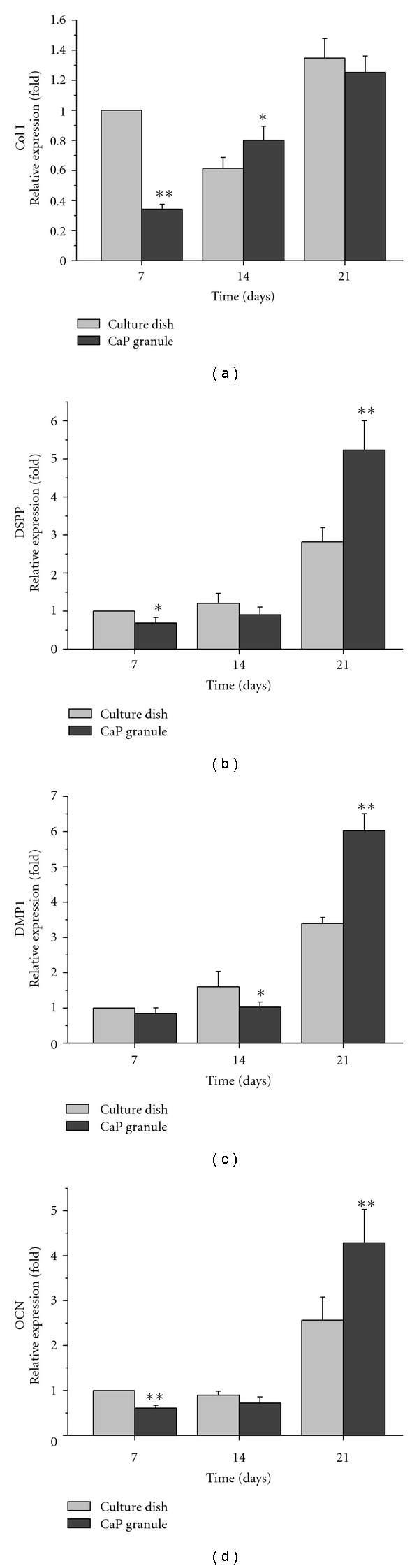
Expressions of genes associated with dentin, including collagen type I (col I), dentin sialophosphoprotein (DSPP), dentin matrix protein 1 (DMP1), and osteocalcin (OCN), as assessed by real time PCR. Great upregulations were noticed on the CaP granules, particularly during the later stage of culture (21 days). Significantly different levels were observed between groups (*P < .05 and **P < .01, ANOVA, n = 3).
3.5. Dentin Sialoprotein Synthesis by Western Blotting
To detect the synthesis of DSP, which is a major protein of the dentin matrix, Western blot analysis was conducted at 21 days (Figure 7). The results clearly revealed the expression of DSP when cultured on the 3D CaP granules for 21 days, but DSP was not detected in samples that were grown in the 2D culture dish.
Figure 7.
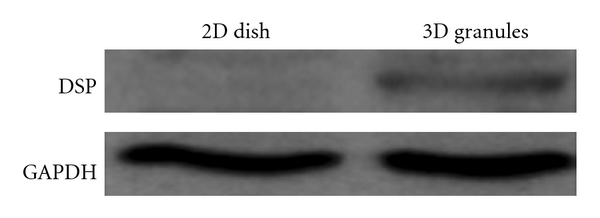
Dentin sialoprotein (DSP) synthesis by the cells on the 3D CaP granules after 21 days of culture. Western blot analysis of the protein extracts with anti-DSP revealed a clear band for samples from the 3D cultures, but no clear band formation was observed for samples from the 2D cultures.
3.6. Cellular Mineralization
Cellular mineralization during the culture period was first observed by staining the Ca mineral with Alizarin red S (Figure 8(a)). Strong positive staining in red was observed throughout the cellular construct which was affected by the addition of 3D CaP granule for 28 days, whereas only some parts of the cellular construct were weakly stained in the culture dish without the CaP granules. Quantitative analysis of the Ca content was also performed (Figure 8(b)). The Ca present in the cellular matrix reacts with the reagent used and results in color change (blue). The change was more noticeable in the cells affected by the 3D CaP granules than those without the granules (**P < .01).
Figure 8.
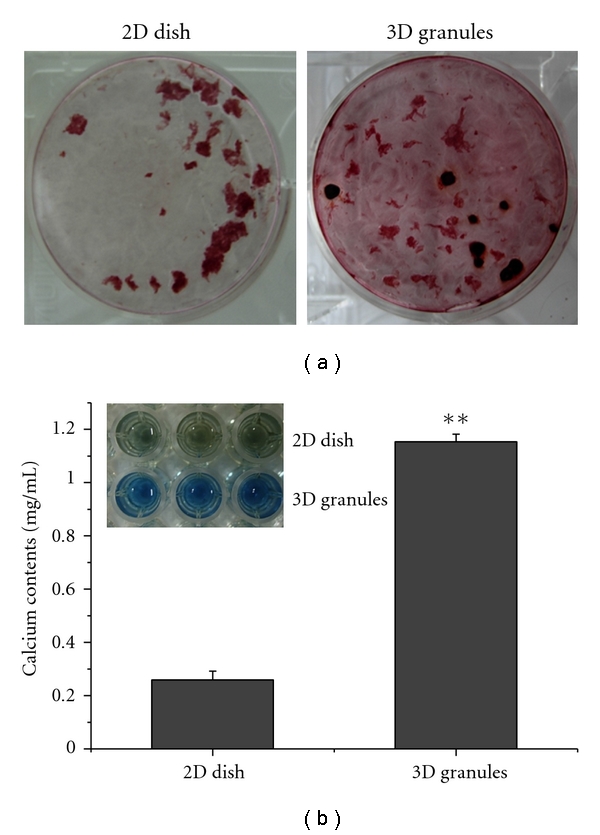
Cellular mineralization assays of the cell constructs grown on 2D culture dishes and 3D CaP granules at 28 days of culture: (a) Alizarin red S treatment to stain the calcium deposits dark red to qualitatively reveal a better staining in the granule group. (b) The calcium content was found to be significantly higher on cells grown on the CaP granules than on the culture dishes (**P < 0.01, ANOVA, n = 3).
4. Discussion
Regenerative dentistry is now considered a facile therapy to efficiently restore tooth function. After a tooth injury, dental pulp is involved in reparative dentinogenesis, where the cells elaborate microenvironments and deposit a new dentin matrix to repair the injured site [29]. This is possible because of the presence of precursor/stem cells in the adult dental pulp, which are able to form odontoblasts under appropriate microenvironments [30]. Conventional endodontic surgery depends largely on the therapeutic treatment of disease and injured sites and/or the removal of the tissue and subsequent restoration with inert filling materials [28]. However, elucidation of the existence of stem/precursor cells in adult dental pulp, and their successful cultivation, has allowed stem cell-based therapy to enable the regeneration of dentin-pulp complex tissues [31].
For the successful use of stem cells in defective sites, the introduction of scaffolds/matrices is of crucial importance. A variety of inorganic biomaterials, such as calcium hydroxide mineral trioxide aggregates, have been used during the repair of dental tissues targeting pulp capping and dentin remineralization [32]. In this study, we used a porous granular type of calcium phosphate (hydroxyapatite composition) and observed the effects of the granules on the growth of DPSCs and their differentiation into odontoblasts. Some recent studies have shown the potential of 3-dimensional (3D) matrices for use in the odontogenic regulation of DPSCs [23, 25–27]. However, most studies have mainly employed 2D culture of DPSCs to identify regulatory factors and to control proliferative and differentiation behavior [6–9]. When compared to the 2D culture condition, the 3D matrix may significantly alter stem-cell behavior such as initial growth and matrix synthesis of the target tissues. Moreover, the specific use of CaP bioactive materials for the regeneration of dentin-pulp complex is considered to be useful as an alternative therapy in DPSCs-based dental tissue engineering.
The hDPSCs cultured on the 2D culture dish showed excellent proliferative potential, with confluence occurring rapidly (approximately 2 weeks, Figure 3), but this was retarded by the input of odontogenic medium. The cell proliferation on the 3D granules was also favorable during the initial period. However, it was significantly reduced with prolonged culture (at 21 days), even in the absence of odontogenic factors, which reflects the dominant occurrence of odontoblastic differentiation. These findings suggest that 3D CaP granules play some roles in stimulating hDPSCs to switch to an odontoblastic pathway, which was further demonstrated by other evidences. The onset of in vitro odontogenesis of the hDPSCs is believed to become dominant after culture for approximately 21 days, which is supported by the significant increase in ALP activity that was observed at 21 days (Figure 4). The highly proliferative cell morphology observed for up to 14 days during the formation of abundant fibrillar collagenous protein also appeared to change during the later stage of culture (week 3), as indicated by the presence of mineral aggregates on the 3D CaP surface (Figure 5).
The odontogenic differentiation behavior of the hDPSCs was also investigated in terms of gene expression and protein synthesis, which are specific to dentin (Figure 6). A series of dentin-associated genes including dentin sialophosphoprotein (DSPP), dentin matrix protein-1 (DMP-1), and osteocalcin (OCN) were expressed in cells grown on the 3D CaP matrix in levels that were significantly higher than those observed in the 2D culture dish, particularly during the later stage when the hDPSCs underwent dominant odontogenic differentiation. The upregulation of the dentin-specific genes DSPP and DMP-I in addition to collagen type I and OCN demonstrates the potential for CaP granules to be used in the odontogenesis of hDPSCs, which would facilitate the use of CaP granules during dentin regeneration and tooth engineering in conjunction with hDPSCs. In addition to the evidence observed at the gene level, the synthesis of dentin sialoprotein (DSP), the key protein present in dentin, was confirmed by the Western blot analysis (Figure 7). In contrast to the culture plate, the CaP granules led to a significant increase in the secretion of DSP.
Subsequent mineralization of the odontogenic cellular construct that was affected by the addition of 3D CaP granules was also demonstrated during the prolonged culture of hDPSCs (Figure 8). Although the treatment with differentiation medium, particularly β-glycerophosphate, induced a level of cell mineralization, the involvement of CaP was shown to increase calcium deposition within the cellular construct. Based on the CaP structure (hydroxyapatite crystalline phase), the calcium and phosphate ions can be released and involved in the calcification of dentin extracellular matrices developed in vitro including collagenous and noncollagenous proteins. Not only the high expression level of genes such as DSPP, DMP1 and OCN, particularly during the later stages of culture, but also the clear synthesis profile of DSP supports that the cellular mineralization may be affected by those proteins, because the major nanocollagenous dentin matrix proteins are known to play crucial roles in calcium binding and mineralization [29].
Along with the compositional effect of the CaP, it is also envisaged that the 3-dimensional substrate condition should trigger the hDPSCs to better develop into odontogenic cells and further secrete dentin extracellular matrices. This is because the substrate is similar, although not identical, to the native tissue condition [33]. Taken together, these findings indicate that the CaP porous granules may be useful as a scaffold for tissue engineering of dentin-pulp complex tissues in conjugation with hDPSCs. Further studies to facilitate the use of these granules in tissue engineering will be conducted in the near future.
5. Conclusions
The potential for the use of calcium phosphate (CaP) porous granules in the cultivation of human dental pulp stem cells (hDPSCs) and their odontoblastic differentiation was investigated. hDPSCs were shown to actively populate upon the 3D granules with a proliferative potential similar to that observed in the 2D culture dish. In particular, odontogenic development was supported by the CaP granules, as determined by the expression of dentin-associated genes, matrix synthesis, and mineralization. The use of CaP granules in conjunction with hDPSCs may be employed in future applications to induce the regeneration of dentin.
Acknowledgment
This work was supported by Priority Research Centers Program (Grant no. 2009-0093829) and WCU (World Class University) program (Grant no. R31-10069) through the National Research Foundation (NRF) funded by the Ministry of Education, Science and Technology.
References
- 1.Zhang W, Walboomers XF, Van Osch GJVM, Van Den Dolder J, Jansen JA. Hard tissue formation in a porous HA/TCP ceramic scaffold loaded with stromal cells derived from dental pulp and bone marrow. Tissue Engineering—Part A. 2008;14(2):285–294. doi: 10.1089/tea.2007.0146. [DOI] [PubMed] [Google Scholar]
- 2.Yang X, Walboomers XF, Van Den Dolder J, et al. Non-viral bone morphogenetic protein 2 transfection of rat dental pulp stem cells using calcium phosphate nanoparticles as carriers. Tissue Engineering—Part A. 2008;14(1):71–81. doi: 10.1089/ten.a.2007.0102. [DOI] [PubMed] [Google Scholar]
- 3.Miura M, Gronthos S, Zhao M, et al. SHED: stem cells from human exfoliated deciduous teeth. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(10):5807–5812. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(25):13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hargreaves KM, Giesler T, Henry M, Wang Y. Regeneration potential of the young permanent tooth: what does the future hold? Journal of Endodontics. 2008;34(7):S51–S56. doi: 10.1016/j.joen.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 6.Gronthos S, Brahim J, Li W, et al. Stem cell properties of human dental pulp stem cells. Journal of Dental Research. 2002;81(8):531–535. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- 7.Laino G, D’Aquino R, Graziano A, et al. A new population of human adult dental pulp stem cells: a useful source of living autologous fibrous bone tissue (LAB) Journal of Bone and Mineral Research. 2005;20(8):1394–1402. doi: 10.1359/JBMR.050325. [DOI] [PubMed] [Google Scholar]
- 8.Laino G, Graziano A, D’Aquino R, et al. An approachable human adult stem cell source for hard-tissue engineering. Journal of Cellular Physiology. 2006;206(3):693–701. doi: 10.1002/jcp.20526. [DOI] [PubMed] [Google Scholar]
- 9.Papaccio G, Graziano A, D’Aquino R, et al. Long-term cryopreservation of dental pulp stem cells (SBP-DPSCs) and their differentiated osteoblasts: a cell source for tissue repair. Journal of Cellular Physiology. 2006;208(2):319–325. doi: 10.1002/jcp.20667. [DOI] [PubMed] [Google Scholar]
- 10.Hubbell JA. Biomaterials in tissue engineering. Nature Biotechnology. 1995;13(6):565–576. doi: 10.1038/nbt0695-565. [DOI] [PubMed] [Google Scholar]
- 11.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260(5110):920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 12.Peppas NA, Langer R. New challenges in biomaterials. Science. 1994;263(5154):1715–1720. doi: 10.1126/science.8134835. [DOI] [PubMed] [Google Scholar]
- 13.Roche S, Provansal M, Tiers L, Jorgensen C, Lehmann S. Proteomics of primary mesenchymal stem cells. Regenerative medicine. 2006;1(4):511–517. doi: 10.2217/17460751.1.4.511. [DOI] [PubMed] [Google Scholar]
- 14.Wei G, Ma PX. Nanostructured biomaterials for regeneration. Advanced Functional Materials. 2008;18(22):3568–3582. doi: 10.1002/adfm.200800662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jarcho M. Calcium phosphate ceramics as hard tissue prosthetics. Clinical Orthopaedics and Related Research. 1981;157:259–279. [PubMed] [Google Scholar]
- 16.Hench LL. Bioceramics: from concept to clinic. Journal of the American Ceramic Society. 1991;74(7):1487–1510. [Google Scholar]
- 17.Wilson T, Stark C, Holmbom J, et al. Fate of bone marrow-derived stromal cells after intraperitoneal infusion or implantation into femoral bone defects in the host animal. Journal of Tissue Engineering. 2010;2010:8 pages. doi: 10.4061/2010/345806. Article ID 345806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gatti AM, Zaffe D. Short-term behaviour of two similar active glasses used as granules in the repair of bone defects. Biomaterials. 1991;12(5):497–504. doi: 10.1016/0142-9612(91)90149-5. [DOI] [PubMed] [Google Scholar]
- 19.Gatti AM, Zaffe D. Long-term behaviour of active glasses in sheep mandibular bone. Biomaterials. 1991;12(3):345–350. doi: 10.1016/0142-9612(91)90044-b. [DOI] [PubMed] [Google Scholar]
- 20.Schepers EJ, Ducheyne P, Barbier L, Schepers S. Bioactive glass particles of narrow size range: a new material for the repair of bone defects. Implant dentistry. 1993;2(3):151–156. doi: 10.1097/00008505-199309000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Schepers E, de Clercq M, Ducheyne P, Kempeneers R. Bioactive glass particulate material as a filler for bone lesions. Journal of Oral Rehabilitation. 1991;18(5):439–452. doi: 10.1111/j.1365-2842.1991.tb01689.x. [DOI] [PubMed] [Google Scholar]
- 22.Aho AJ, Tirri T, Kukkonen J, et al. Injectable bioactive glass/biodegradable polymer composite for bone and cartilage reconstruction: concept and experimental outcome with thermoplastic composites of poly(ε-caprolactone-co-D,L-lactide) and bioactive glass S53P4. Journal of Materials Science: Materials in Medicine. 2004;15(10):1165–1173. doi: 10.1023/B:JMSM.0000046401.50406.9b. [DOI] [PubMed] [Google Scholar]
- 23.Holtgrave EA, Donath K. Response of odontoblast-like cells to hydroxyapatite ceramic granules. Biomaterials. 1995;16(2):155–159. doi: 10.1016/0142-9612(95)98280-r. [DOI] [PubMed] [Google Scholar]
- 24.Kim HW, Lee SY, Bae CJ, et al. Porous ZrO bone scaffold coated with hydroxyapatite with fluorapatite intermediate layer. Biomaterials. 2003;24(19):3277–3284. doi: 10.1016/s0142-9612(03)00162-5. [DOI] [PubMed] [Google Scholar]
- 25.Yang X, Walboomers XF, Van Den Beucken JJJP, Bian Z, Fan M, Jansen JA. Hard tissue formation of STRO-1-selected rat dental pulp stem cells in vitro. Tissue Engineering—Part A. 2009;15(2):367–375. doi: 10.1089/ten.tea.2008.0133. [DOI] [PubMed] [Google Scholar]
- 26.Oh SA, Kim SH, Kim JE, Kim JJ, Shin US, Kim HW. Effects on growth and osteogenic differentiation of mesenchymal stem cells by the zinc-added sol-gel bioactive glass granules. Journal of Tissue Engineering. 2010;2010:10 pages. doi: 10.4061/2010/475260. Article ID 475260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu J, Deng Z, Shi J, et al. Differentiation of dental pulp stem cells into regular-shaped dentin-pulp complex induced by tooth germ cell conditioned medium. Tissue Engineering. 2006;12(11):3097–3105. doi: 10.1089/ten.2006.12.3097. [DOI] [PubMed] [Google Scholar]
- 28.Bluteau G, Luder HU, De Bari C, Mitsiadis TA. Stem cells for tooth engineering. European Cells and Materials. 2008;16:1–9. doi: 10.22203/ecm.v016a01. [DOI] [PubMed] [Google Scholar]
- 29.Bergenholtz G, Spangberg L. Controversies in endodontics. Critical Reviews in Oral Biology & Medicine. 2004;15(2):99–114. doi: 10.1177/154411130401500204. [DOI] [PubMed] [Google Scholar]
- 30.Hao J, Zou B, Narayanan K, George A. Differential expression patterns of the dentin matrix proteins during mineralized tissue formation. Bone. 2004;34(6):921–932. doi: 10.1016/j.bone.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 31.Rotstein I, Simon JH. The endo-perio lesion: a critical appraisal of the disease condition. Endodontic Topic. 2006;13(1):34–56. [Google Scholar]
- 32.Murray PE, Garcia-Godoy F. Stem cell responses in tooth regeneration. Stem Cells and Development. 2004;13(3):255–262. doi: 10.1089/154732804323099181. [DOI] [PubMed] [Google Scholar]
- 33.Grayson WL, Ma T, Bunnell B. Human mesenchymal stem cells tissue development in 3D PET matrices. Biotechnology Progress. 2004;20(3):905–912. doi: 10.1021/bp034296z. [DOI] [PubMed] [Google Scholar]


