Abstract
Background
Poor nutritional status is often present among older adults who experience a fall. However, dietary intake and weight loss are often overlooked as potential factors. The objective of this study was to test the association between dietary protein intake and risk of subsequent falls in a population-based cohort of elderly men and women.
Methods
Dietary intake and clinic data from 807 men and women (ages 67–93 years) from the Framingham Original Cohort Study were analyzed. Protein intake (total, animal and plant) was assessed as a continuous variable and by tertile of intake. Falls were reported by participants using a validated questionnaire at two time points. Weight was ascertained at each examination to examine the effect of weight loss over follow-up.
Results
Higher dietary protein intakes were associated with a reduced odds of falling, although of borderline statistical significance (OR=0.80, 95% CI: 0.60–1.07) and were not associated with the rate of falls over follow-up (RR=0.93, 95%CI: 0.73–1.19). Tertile analyses tended towards a protective association, but most did not achieve statistical significance; there was no dose-response. For those who lost ≥ 5% of their baseline weight, higher intakes of total, animal and plant protein showed a significantly lower rate of subsequent falls.
Conclusion
This work highlights the importance of adequate protein intake as a potentially modifiable risk factor for fall prevention in older adults. Further exploration of the interaction of protein intake and weight loss as related to falls is needed.
Keywords: protein intake, falls, dietary protein, animal protein, elderly, cohort study
Introduction
Falls are a major health concern among older adults and a leading cause of morbidity and mortality. Annually, more than 30 percent of community-dwelling adults over the age of 65 years experience at least one fall [1–3]. After age 75, the rates are even higher [4]. While approximately half of all falls result in a minor injury [5], up to 10 percent result in a serious injury such as subdural hematoma, head trauma, or fracture [5–7]. Although only one percent of falls result in hip fracture [8], nearly all of hip fractures may be attributed to falls [9]. Falls can also lead to a decline in social and physical activities [10] and loss of independence [11], particularly amongst older adults.
Established risk factors for falls among community-dwelling older adults include advanced age, history of falls, balance or gait disorders, muscle weakness, cognitive or visual impairment, arthritis, stroke, and use of multiple prescription medications, [1, 3, 6, 12]. A comprehensive review ranked muscle weakness as the most common risk factor for falls in older persons[13]. Although poor nutritional status is often present among older adults who experience a fall [14–15], dietary intake and weight loss are often overlooked as potential factors in the etiology of falls.
Accumulating evidence indicates the importance of protein intake in the health of older adults, particularly as it relates to muscle [16–17] and bone [18–20], which are well known to decline with age. A number of small, short-term intervention studies have found that resistance training combined with protein intake resulted in increased muscle strength in older men and women [21–22]. Similarly, observational studies of older adults, including the Health ABC study, have shown that higher protein intake was associated with higher muscle mass and a reduced loss of lean mass over time [16–17]. When considering hip fracture as the ultimate fall outcome, our previous work, as well as that of others, demonstrates a lower risk of hip fracture in study participants consuming greater dietary protein [18–19].
Surprisingly, given these associations, there are few published studies that have directly examined the relationship between dietary protein intake and falls in older adults. In this study, we examine the association between protein intake and subsequent falls over a one-year period in older men and women in the population-based Framingham Study. We hypothesized that a higher intake of dietary protein would be associated with a lower risk of falls. We further hypothesized that this relationship would be influenced by weight loss over the follow-up.
Methods
Study participants
The Framingham Study was initiated in 1948 to examine risk factors for heart disease in a population-based sample consisting of two-thirds of the households in Framingham, MA, USA. The original cohort, comprised of 5,209 men and women between the ages of 28 and 62 years, has been examined biennially since the study began [23]. In 1988–1989, 1,402 surviving cohort members attended biennial exam 20 during which a food frequency questionnaire (FFQ) was administered. Of these participants, 426 resided in a nursing home, had incomplete FFQs (>12 food items left blank or extreme total energy intake <600 or >4,000 kilocalories/day) and 169 did not attend follow-up at biennial exam 21 in 1990–1991, when information on subsequent falls was collected. Thus, 807 participants were included in the current analyses.
This study was approved by the Institutional Review Boards for Human Research at Boston University and Hebrew Rehabilitation Center, and written informed consent was obtained for all study subjects.
Assessment of protein intake
Usual dietary intake was assessed by the 126-item Willett FFQ at the baseline exam in 1988–1989. The Willett FFQ has been validated for numerous nutrients against multiple food records and blood measures [24–26]. In addition to total protein intake (g/day), intakes of animal protein, and plant protein (g/day) were also estimated from the FFQ.
Assessment of falls
Falls were reported by participants using a validated questionnaire [8] at both the baseline exam 1988–1989 and the follow-up exam in 1990–1991. Trained clinic staff asked participants, “In the past year have you accidentally fallen and hit the floor or ground?” and “If yes, how many times did you fall in the past year”. For this study, a fall was defined as any self-reported, unintended contact with the ground, regardless of whether the fall resulted in a fracture. The falls outcome was categorized as a yes/no response at the follow-up exam, and also evaluated as the rate of falls over follow-up (number of falls reported at follow-up/12 months).
Covariates and potential effect modifier
Information on covariates including age, sex, height, weight, total energy intake, baseline history of falls, dietary calcium intake, calcium supplement use, dietary vitamin D intake, vitamin D supplement use, alcohol intake, smoking status, and physical activity were collected at the baseline exam in 1988–1989. For subjects with missing information on height, weight, or physical activity at the baseline exam (n=34), we used these covariates as collected at the previous exam in 1986–1987.
Height without shoes was measured to the nearest quarter inch and weight was measured in light clothing and without shoes to the nearest pound using a standardized balance beam scale. Intake of total energy (kcal/day), dietary calcium (mg/day), dietary vitamin D (IU/day), and alcohol (g/day) were assessed using the food list section of the FFQ. Subjects were categorized for alcohol intake as non-drinker, moderate drinker (<13.2 g of alcohol/day for women and <26.4 g of alcohol/day for men), and heavy drinker (≥13.2 g of alcohol /day for women and ≥26.4 g of alcohol/day for men) using the cut-points recommended by the dietary guidelines for Americans [27]. Calcium and vitamin D supplement use were recorded in the supplement section of the FFQ and were categorized as a yes/no response. Smoking status was assessed via questionnaire and coded as current smoker (yes/no). Physical activity was estimated using the validated Framingham Physical Activity Index which scores the weighted sum of the number of hours spent doing heavy, moderate, light, or sedentary activities and sleeping during a typical day [28]. Baseline history of falls was assessed using the self-reported falls questionnaire for the number of falls in the year prior to the 1988–89 baseline exam and categorized as having had 0 falls, 1 fall, or ≥2 falls in the past year.
Relative weight change, specifically weight loss ≥ 5% of a participant’s baseline weight over follow-up, was considered as a potential effect modifier for the relation between dietary protein and falls. Weight loss was defined as a relative decrease in weight of ≥ 5%, based on the difference in weight measured at the baseline and follow-up exams. Weight change was missing for 17 study participants, thus they were excluded from these stratified analyses.
Statistical analysis
Baseline characteristics of the cohort members who attended both baseline and follow-up and those who only attended the baseline exam were compared using Student’s t-test for continuous variables and the chi-square test for categorical variables. Separate analyses were conducted for each protein intake variable (total protein, animal protein, and plant protein intakes). Protein intakes were analyzed as continuous variables (for 1 standard deviation (SD) difference in protein intake) and as tertiles. For the tertile analyses, protein intakes were adjusted for total energy intake using the residual method [29–30]. Using this method, each protein intake type was regressed on total energy intake and a constant was added to the resulting residuals. The constant is the predicted nutrient intake for the mean energy intake of the study population.
Odds ratios (OR) with 95% confidence intervals (95% CI) for the binary outcome of falling (yes/no) were estimated using multivariable logistic regression for a 1 SD in protein intake as well as for each of the upper 2 tertiles of protein intake relative to the lowest tertile (to examine possible threshold effects). Rate ratios (RR) for falls with 95% CI were estimated using multivariable negative binomial regression for continuous protein intake and for tertiles of protein intakes. Negative binomial regression was chosen for analyzing falls as it allows modeling when the count of falls may be over-dispersed [31], and allows full use of the numbers of reported falls. All of the regression models were adjusted for age, sex, height, weight, total energy intake, and history of falls in the previous year in the basic model. The full models were additionally adjusted for physical activity, dietary calcium, calcium supplement use, dietary vitamin D, vitamin D supplement use, alcohol use, and smoking status. Models for animal and plant protein intakes were adjusted for each other when examined.
Possible effect modification by weight change was evaluated by performing analyses stratified by weight loss (≥5% weight loss and no weight loss).
All analyses were conducted using SAS statistical analysis software (version 9.1, SAS Institute Inc., Cary, NC, USA). A two-sided p-value of <0.05 was considered statistically significant for all analyses.
Results
Among the 807 study participants with both baseline and follow-up data, 63% were women. The mean age (±SD) of the men and women at baseline was 75±4.8 years with a range of 67 to 93 years (Table 1). Among participants at baseline, the mean total protein intake was 69±23.9 g/day. The majority of total protein intake came from animal food sources with mean animal and plant protein intakes of 46±19.1 g/day and 23±8.8 g/day, respectively. At the follow-up exam, 27% of participants reported at least one fall and 8% reported 2 or more falls. The number of falls experienced over the 1-year of follow-up ranged from 0 to 15, with a mean rate of 0.45 falls/year. Compared to the study participants, the nonparticipants, (who attended the baseline exam only), tended to be older men who were current smokers with lower BMIs. Although history of falls at the baseline exam did not differ between the participants and nonparticipants, the nonparticipants had significantly lower mean total, animal, and plant protein intakes (data not shown).
TABLE 1.
Characteristics of study participants of the Framingham Original Cohort at baseline (1988–1989)
| Descriptive variables | Total (n=807) |
Men (n=298) |
Women (n=509) |
|---|---|---|---|
| Age (years) | 75±4.8b | 75±4.8 | 75±4.8 |
| Height (in)c | 63.7±3.7 | 67.1±2.7 | 61.6±2.6 |
| Weight (lbs)c | 154±32.0 | 174±28.3 | 142±27.9 |
| BMI (kg/m2)c | 26.7±4.63 | 27.2±3.99 | 26.4±4.95 |
| Education (%) | |||
| No high school diploma | 29 | 30 | 28 |
| High school diploma | 37 | 34 | 38 |
| Higher education | 34 | 36 | 34 |
| Smoking status (%) | |||
| Current smokers | 10 | 7 | 11 |
| Former/Never smokers | 90 | 93 | 89 |
| Physical Activity Index (PAI)c | 33.5±5.51 | 33.9±6.19 | 33.3±5.05 |
| History of falls in past year (%) | 23 | 22 | 23 |
| Total energy (kcal/day) | 1750±584.9 | 1871±623.1 | 1680±549.8 |
| Total calcium intake (mg/day)d | 819±436.7 | 770±401.0 | 848±454.3 |
| Total vitamin D intake (IU/day)d | 330±265.8 | 322±279.1 | 335±257.9 |
| Total protein intake (g/day) | 69±23.9 | 70±23.8 | 69±24.0 |
| Animal protein | 46±19.1 | 46±18.5 | 46±19.5 |
| Plant protein | 23±8.8 | 24±9.6 | 22±8.3 |
Sample sizes varied from 290 to 298 for men and from 496 to 509 for women
Mean±SD
For subjects missing height, weight, BMI, and PAI values at baseline (1988–1989), values from previous exam (1986–1987) were used
Total nutrient intake = Dietary intake + Intake from supplements
When we examined the OR of falling per 1 SD difference in protein intakes, a slight reduction in the odds was observed for each type of protein intake; yet these findings were not statistically significant [OR (95%CI)for total protein: 0.84 (0.60, 1.16), animal protein: 0.89 (0.69, 1.16), and plant protein 0.75 (0.55, 1.02); P range: 0.06–0.20]. As seen in Table 2, there was no association found with the rate of falls over follow-up (P range: 0.33–0.65, Table 2). Analyses were conducted for both the basic and full models; however, as the additional adjustments in the full model did not substantially change the results, we present the basic model only, with adjustments for sex, age, height, weight, total energy intake, and history of falls.
TABLE 2.
Adjusteda rate ratiosb at follow-up (1990–1991) by standard deviation difference in protein intake at baseline (1988–1989) in the Framingham Original Cohort
| Protein intake (n=772) |
Rate ratio (95% CI) |
|---|---|
| Total protein | 0.93 (0.73, 1.19) |
| Animal protein | 0.96 (0.79, 1.16) |
| Plant protein | 0.88 (0.68, 1.14) |
Adjusted for sex, age, height, weight, total energy, and history of falls. Animal protein and plant protein intake were adjusted for each other when examined
Rate ratios were estimated using negative binomial regression models. The rate ratio is the average number of falls per person per year of follow-up for 1 standard deviation difference in protein intake
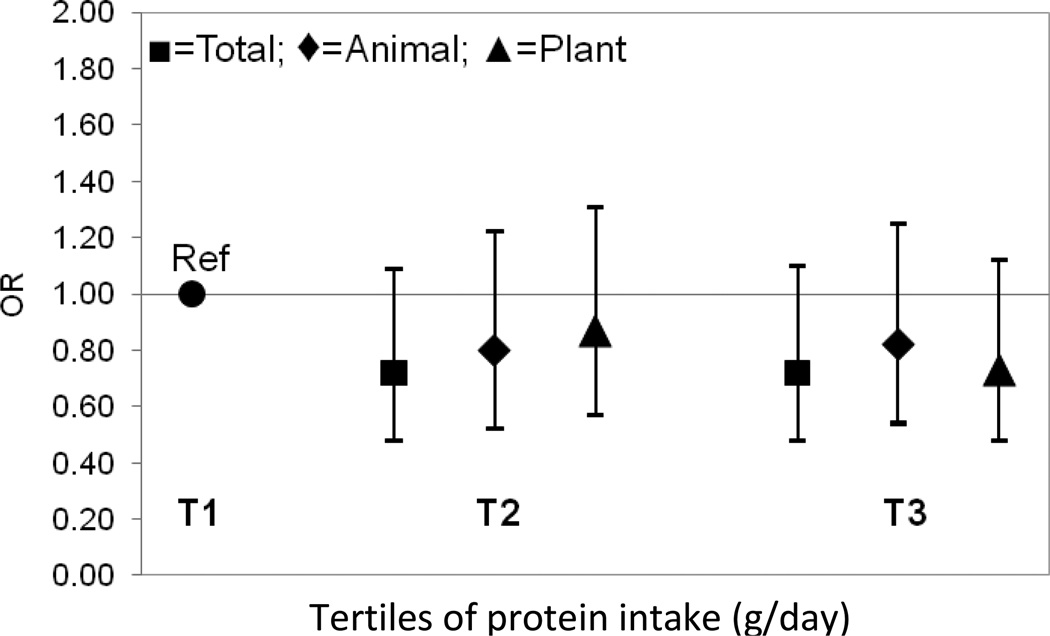
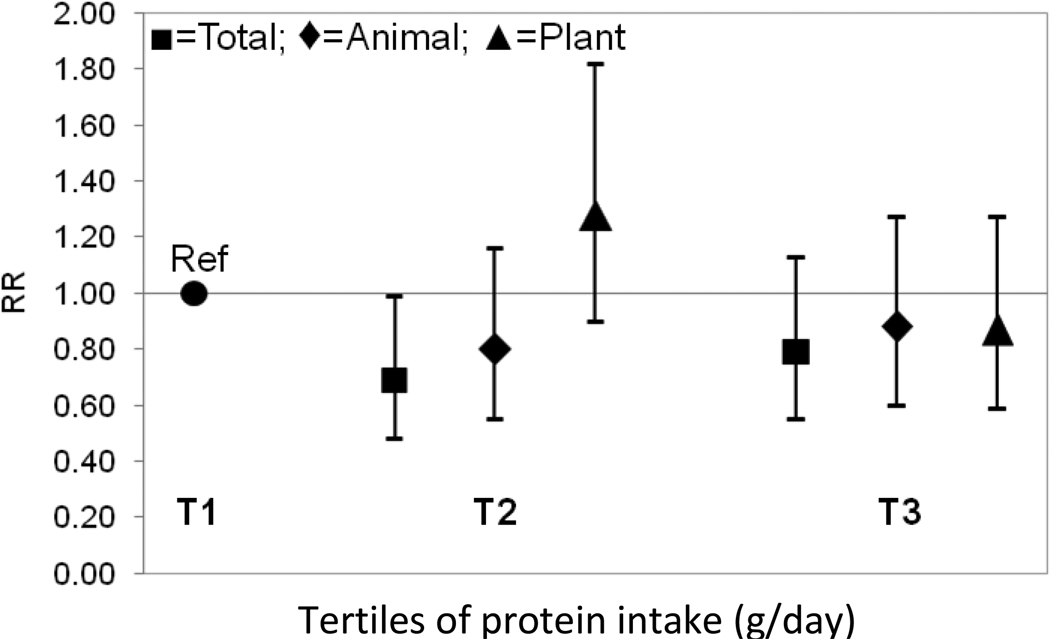
In the tertile analysis shown in Figure 1, protein intakes (total, animal, and plant) were protective against the odds of falling; however these results were not statistically significant (P range: 0.12–0.50, Figure 1). As seen in Table 2, for the rate of falls, total protein intake was found to be protective falls, but was only of borderline statistical significance for tertile 2 relative to tertile 1 (the reference group) (RR T3: 0.79, 95% CI: 0.55–1.13, RR T2: 0.69, 95% CI: 0.48–0.99, Figure 2). A similar pattern was observed for animal protein intake (RR T3: 0.88, 95% CI: 0.60–1.27, RR T2: 0.80, 95% CI 0.55–1.16). No significant associations were observed for plant protein intake.
FIGURE 1.
Adjusteda odds ratio of falls at follow-up (1990–1991) by tertile of protein intakeb at baseline (1988–1989) in the Framingham Original Cohort
aAdjusted for sex, age, height, weight, total energy, and history of falls. Animal protein and plant protein intake were adjusted for each other when examined (n=773)
bProtein intakes were energy-adjusted residuals added to a constant, where the constant equals the protein intake for the mean energy intake of the study population
FIGURE 2.
Adjusteda rate ratiosb at follow-up (1990–1991) by tertile of protein intakec at baseline (1988–1989) in the Framingham Original Cohort
aAdjusted for sex, age, height, weight, total energy, and history of falls. Animal protein and plant protein intake were adjusted for each other when examined (n=772)
bRate ratios were estimated using negative binomial regression models.
cProtein intakes were energy-adjusted residuals added to a constant, where the constant equals the protein intake for the mean energy intake of the study population
When stratifying the results by weight loss, greater intakes of each type of protein intake was associated with lower rates of falls among participants who had ≥5% decrease in weight (Table 3), although these results were only statistically significant for total and animal protein intakes (RR: 0.46, 95% CI: 0.22–0.93, P=0.03, RR: 0.53, 95% CI: 0.30–0.93, P=0.03, respectively, Table 3). Among those participants who did not lose weight, protein intake was not associated with the rate of falls (P-value range=0.55–0.80)
TABLE 3.
Adjusteda rate ratiosb at follow-up (1990–1991) by standard deviation difference in protein intake at baseline (1988–1989) in the Framingham Original Cohort stratified by percent weight change
| Weight loss (≥ −5% change) (n=116) |
No weight loss (n=639) |
|
|---|---|---|
| Protein intake | Rate ratio (95% CI) | Rate ratio (95% CI) |
| Total protein | 0.46 (0.22, 0.93) | 1.04 (0.79, 1.36) |
| Animal protein | 0.53 (0.30, 0.93) | 1.04 (0.84, 1.29) |
| Plant protein | 0.48 (0.21, 1.08) | 0.92 (0.69, 1.22) |
Adjusted for sex, age, height, weight, total energy, and history of falls. Animal protein and plant protein intake were adjusted for each other when examined
Rate ratios were estimated using negative binomial regression models.
Discussion
In this cohort study of older men and women with protein intakes that correspond with the U.S. Recommended Daily Allowance for dietary protein [32], moderate total dietary protein intake was associated with a reduced rate of subsequent falls over a one-year period. However, statistical significance was borderline and no dose-response was observed. There were similar patterns of results when type of protein intake (animal vs. plant) was examined separately, However, when stratifying by weight loss, our study showed that increased total, animal, and plant protein intakes were associated with a decreased rate of falls among participants with a weight loss of ≥5% of their baseline weight (RR of 0.4–0.5), while no associations were observed for the participants with no weight loss.
There are very few studies that have examined the effect of protein intake on the risk of falls. In a large population-based study of Danish adults of ages over 65 years, Larsen et al. [33], reported that not eating fish in the past month was associated with a two-fold increased risk of fall in a 24-hour period in women, but not in men. However, they did not examine isolated nutrients from fish, such as protein intakes, in relation to the odds of falling. A cross-sectional study by Shahar et al. [34], reported no difference in protein intake between 100 fallers and non-fallers including both men and women 65 years of age and older. Similar to this study, we did not detect significant protection or increased risks between higher protein intakes and the risk of falls. However, we did observe protective effects for each of the types of protein in the men and women who experienced a weight loss of ≥5 percent.
Weight loss, and particularly unintentional weight loss, is of concern among older adults [35] and is associated with an increased risk of fractures and falling [36]. To our knowledge, the association between increased dietary protein intake and a decreased rate of falls observed in our study among men and women who experienced weight loss has not been previously reported in other studies. Studies that have examined intentional weight loss among middle-aged men and women [37–39] and older women [40–41] have found that caloric restriction diets with higher proportions of protein intake, both with and without exercise, are associated with weight loss and preservation of lean mass. Interestingly, among Health ABC study participants with weight change (gain and loss), increased protein intake was associated with less muscle loss, while those with a stable weight showed no association. These findings support our suspected pathway between protein intake, muscle mass and perhaps falls, although falls were not reported in their analysis of muscle mass.
While the source of dietary protein intake has not been fully examined as it relates to falls, prior studies have examined protein source as it relates to lean muscle mass, which is the likely pathway between protein intake and falls. However, studies of resistance training in those consuming omnivorous compared to lactoovovegetarian diets reported mixed results on the association between protein source and muscle mass and strength [22, 42]. Houston et al [16] found significant positive associations between animal protein and lean mass, but not with plant protein in the Health ABC study of older adults ages 70–79, although the range of plant protein intake was limited in this study [17]. In our study, we found similar associations for each type of protein, albeit of borderline statistical significance.
Our study had several strengths and weaknesses. Rather than examining a selected group of patients, we studied a population-based cohort that included both men and women across a wide range of older ages. Unlike most studies which were often limited to the examination of total protein only, we were able to examine total, animal and plant protein intakes in our analyses. Detailed information was also available on confounders and covariates, which may have helped in minimizing residual confounding. This study also has limitations that, if present, would have mostly biased our results toward the null. We had no falls outcome information for the nonparticipants who had lower protein intake and other risk factors associated with poor health outcomes. Additionally, protein intake was assessed by a semi-quantitative FFQ, which cannot determine the absolute nutrient intakes; however, it is a valid measure for ranking individuals and average nutrient intakes and has been shown to be stable and reproducible over time [25–26]. Non-differential misclassification of the falls outcome is also possible, as falls were assessed based on one-year recall with potentially limited accuracy [43–44], which could lead to results that were biased towards the null. Lastly, the Framingham Study cohort is predominantly Caucasian, which limits the generalizability of our results.
In conclusion, this large, population-based study of older men and women showed that increased protein intake was associated with reduced rates of falls, especially in those who had lost weight. We believe this study may be the first to directly examine the association with types of protein intake and subsequent falls in a population-based cohort of older men and women. This work highlights the importance of adequate protein intake as a potentially modifiable risk factor for fall prevention in older persons. More work is needed to focus upon the possible pathway and links between types of protein intakes and their possible influences upon muscle mass and subsequent falls. Further exploration of the interaction of protein intake and weight change as they relate to falls is needed before prevention programs can be targeted to older persons experiencing significant weight loss.
Acknowledgements
We would like to thank the Framingham Osteoporosis Study research team and the study participants for their time, effort, and dedication. We would also like to thank the Institute for Aging Research Musculoskeletal Program including Elizabeth Newton, Ph.D. for her statistical advice.
Funding Support: Supported by the National Institutes of Health BIDMC/Harvard T32 Translational Research in Aging Pre-Doctoral Training Program, the National Institute for Arthritis, Musculoskeletal & Skin Diseases (R01-AR053205), and the National Heart, Lung, and Blood Institute’s Framingham Heart Study (Contract No. N01-HC-25195).
References
- 1.Nevitt MC, Cummings SR, Kidd S, Black D. Risk factors for recurrent nonsyncopal falls. A prospective study. JAMA. 1989 May 12;261(18):2663–2668. [PubMed] [Google Scholar]
- 2.Tinetti ME. Clinical practice. Preventing falls in elderly persons. N Engl J Med. 2003 Jan 2;348(1):42–49. doi: 10.1056/NEJMcp020719. [DOI] [PubMed] [Google Scholar]
- 3.Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988 Dec 29;319(26):1701–1707. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- 4.Rubenstein LZ, Josephson KR. The epidemiology of falls and syncope. In: Kenny RA, O'Shea D, editors. Falls and Syncope in Elderly Patients. Clinics in Geriatric Medicine. Philadelphia: W.B. Saunders Co.; 2002. [Google Scholar]
- 5.Tinetti ME, Doucette J, Claus E, Marottoli R. Risk factors for serious injury during falls by older persons in the community. J Am Geriatr Soc. 1995 Nov;43(11):1214–1221. doi: 10.1111/j.1532-5415.1995.tb07396.x. [DOI] [PubMed] [Google Scholar]
- 6.Sattin RW, Lambert Huber DA, DeVito CA, et al. The incidence of fall injury events among the elderly in a defined population. Am J Epidemiol. 1990 Jun;131(6):1028–1037. doi: 10.1093/oxfordjournals.aje.a115594. [DOI] [PubMed] [Google Scholar]
- 7.Nevitt MC, Cummings SR, Hudes ES. Risk factors for injurious falls: a prospective study. J Gerontol. 1991 Sep;46(5):M164–M170. doi: 10.1093/geronj/46.5.m164. [DOI] [PubMed] [Google Scholar]
- 8.Onambele-Pearson GL, Breen L, Stewart CE. Influences of carbohydrate plus amino acid supplementation on differing exercise intensity adaptations in older persons: skeletal muscle and endocrine responses. Age (Dordr) 2010 Jun;32(2):125–138. doi: 10.1007/s11357-009-9129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parkkari J, Kannus P, Palvanen M. Majority of hip fractures occur as a result of a fall and impact on the greater trochanter of the femur: a prospective controlled hip fracture study with 206 consecutive patients. Calcif Tissue Int. 1999 Sep;65(3):183–187. doi: 10.1007/s002239900679. [DOI] [PubMed] [Google Scholar]
- 10.Tinetti ME, Williams CS. The effect of falls and fall injuries on functioning in community-dwelling older persons. J Gerontol A Biol Sci Med Sci. 1998 Mar;53(2):M112–M119. doi: 10.1093/gerona/53a.2.m112. [DOI] [PubMed] [Google Scholar]
- 11.Kiel DP, O'Sullivan P, Teno JM, Mor V. Health care utilization and functional status in the aged following a fall. Med Care. 1991 Mar;29(3):221–228. doi: 10.1097/00005650-199103000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Campbell AJ, Borrie MJ, Spears GF. Risk factors for falls in a community-based prospective study of people 70 years and older. J Gerontol. 1989 Jul;44(4):M112–M117. doi: 10.1093/geronj/44.4.m112. [DOI] [PubMed] [Google Scholar]
- 13.Guideline for the prevention of falls in older persons. American Geriatrics Society, British Geriatrics Society, and American Academy of Orthopaedic Surgeons Panel on Falls Prevention. J Am Geriatr Soc. 2001 May;49(5):664–672. [PubMed] [Google Scholar]
- 14.Vivanti AP, McDonald CK, Palmer MA, Sinnott M. Malnutrition associated with increased risk of frail mechanical falls among older people presenting to an emergency department. Emerg Med Australas. 2009 Oct;21(5):386–394. doi: 10.1111/j.1742-6723.2009.01223.x. [DOI] [PubMed] [Google Scholar]
- 15.Bauer JD, Isenring E, Torma J, Horsley P, Martineau J. Nutritional status of patients who have fallen in an acute care setting. J Hum Nutr Diet. 2007 Dec;20(6):558–564. doi: 10.1111/j.1365-277X.2007.00832.x. [DOI] [PubMed] [Google Scholar]
- 16.Meng X, Zhu K, Devine A, Kerr DA, Binns CW, Prince RL. A 5-year cohort study of the effects of high protein intake on lean mass and BMC in elderly postmenopausal women. J Bone Miner Res. 2009 Nov;24(11):1827–1834. doi: 10.1359/jbmr.090513. [DOI] [PubMed] [Google Scholar]
- 17.Houston DK, Nicklas BJ, Ding J, et al. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the Health, Aging, and Body Composition (Health ABC) Study. Am J Clin Nutr. 2008 Jan;87(1):150–155. doi: 10.1093/ajcn/87.1.150. [DOI] [PubMed] [Google Scholar]
- 18.Misra D, Berry SD, Broe KE, et al. Does dietary protein reduce hip fracture risk in elders? The Framingham osteoporosis study. Osteoporos Int. 2010 May 5; doi: 10.1007/s00198-010-1179-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munger RG, Cerhan JR, Chiu BC. Prospective study of dietary protein intake and risk of hip fracture in postmenopausal women. Am J Clin Nutr. 1999 Jan;69(1):147–152. doi: 10.1093/ajcn/69.1.147. [DOI] [PubMed] [Google Scholar]
- 20.Hannan MT, Tucker KL, Dawson-Hughes B, Cupples LA, Felson DT, Kiel DP. Effect of dietary protein on bone loss in elderly men and women: the Framingham Osteoporosis Study. J Bone Miner Res. 2000 Dec;15(12):2504–2512. doi: 10.1359/jbmr.2000.15.12.2504. [DOI] [PubMed] [Google Scholar]
- 21.Holm L, Olesen JL, Matsumoto K, et al. Protein-containing nutrient supplementation following strength training enhances the effect on muscle mass, strength, and bone formation in postmenopausal women. J Appl Physiol. 2008 Jul;105(1):274–281. doi: 10.1152/japplphysiol.00935.2007. [DOI] [PubMed] [Google Scholar]
- 22.Haub MD, Wells AM, Tarnopolsky MA, Campbell WW. Effect of protein source on resistive-training-induced changes in body composition and muscle size in older men. Am J Clin Nutr. 2002 Sep;76(3):511–517. doi: 10.1093/ajcn/76.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dawber TR, Meadors GF, Moore FE., Jr. Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951 Mar;41(3):279–281. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacques PF, Sulsky SI, Sadowski JA, Phillips JC, Rush D, Willett WC. Comparison of micronutrient intake measured by a dietary questionnaire and biochemical indicators of micronutrient status. Am J Clin Nutr. 1993 Feb;57(2):182–189. doi: 10.1093/ajcn/57.2.182. [DOI] [PubMed] [Google Scholar]
- 25.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992 May 15;135(10):1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. discussion 1127–1136. [DOI] [PubMed] [Google Scholar]
- 26.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985 Jul;122(1):51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 27.Dietary Guidelines for Americans. Washington, DC: U.S. Government Printing Office; 2005. U.S. Department of Health and Human Services and U.S. Department of Agriculture. [Google Scholar]
- 28.Kannel WB, Sorlie P. Some health benefits of physical activity. The Framingham Study. Arch Intern Med. 1979 Aug;139(8):857–861. [PubMed] [Google Scholar]
- 29.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997 Apr;65(4 Suppl):1220S–1228S. doi: 10.1093/ajcn/65.4.1220S. discussion 1229S–1231S. [DOI] [PubMed] [Google Scholar]
- 30.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986 Jul;124(1):17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- 31.Glynn RJ, Stukel TA, Sharp SM, Bubolz TA, Freeman JL, Fisher ES. Estimating the variance of standardized rates of recurrent events, with application to hospitalizations among the elderly in New England. Am J Epidemiol. 1993 Apr 1;137(7):776–786. doi: 10.1093/oxfordjournals.aje.a116738. [DOI] [PubMed] [Google Scholar]
- 32.Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients) Washington, D.C.: he National Academies Press; 2005. [DOI] [PubMed] [Google Scholar]
- 33.Larsen ER, Mosekilde L, Foldspang A. Correlates of falling during 24 h among elderly Danish community residents. Prev Med. 2004 Aug;39(2):389–398. doi: 10.1016/j.ypmed.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Shahar D, Levi M, Kurtz I, et al. Nutritional status in relation to balance and falls in the elderly: a preliminary look at serum folate. Ann Nutr Metab. 2009;54(1):59–66. doi: 10.1159/000207356. [DOI] [PubMed] [Google Scholar]
- 35.Bales CW, Ritchie CS. Sarcopenia, weight loss, and nutritional frailty in the elderly. Annu Rev Nutr. 2002;22:309–323. doi: 10.1146/annurev.nutr.22.010402.102715. [DOI] [PubMed] [Google Scholar]
- 36.Ensrud KE, Cauley J, Lipschutz R, Cummings SR. Weight change and fractures in older women. Study of Osteoporotic Fractures Research Group. Arch Intern Med. 1997 Apr 28;157(8):857–863. [PubMed] [Google Scholar]
- 37.Leidy HJ, Carnell NS, Mattes RD, Campbell WW. Higher protein intake preserves lean mass and satiety with weight loss in pre-obese and obese women. Obesity (Silver Spring) 2007 Feb;15(2):421–429. doi: 10.1038/oby.2007.531. [DOI] [PubMed] [Google Scholar]
- 38.Layman DK, Evans E, Baum JI, Seyler J, Erickson DJ, Boileau R. Dietary protein and exercise have additive effects on body composition during weight loss in adult women. J Nutr. 2005 Aug;135(8):1903–1910. doi: 10.1093/jn/135.8.1903. [DOI] [PubMed] [Google Scholar]
- 39.Layman DK, Boileau RA, Erickson DJ, et al. A reduced ratio of dietary carbohydrate to protein improves body composition and blood lipid profiles during weight loss in adult women. J Nutr. 2003 Feb;133(2):411–417. doi: 10.1093/jn/133.2.411. [DOI] [PubMed] [Google Scholar]
- 40.Bopp MJ, Houston DK, Lenchik L, Easter L, Kritchevsky SB, Nicklas BJ. Lean mass loss is associated with low protein intake during dietary-induced weight loss in postmenopausal women. J Am Diet Assoc. 2008 Jul;108(7):1216–1220. doi: 10.1016/j.jada.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gordon MM, Bopp MJ, Easter L, et al. Effects of dietary protein on the composition of weight loss in post-menopausal women. J Nutr Health Aging. 2008 Oct;12(8):505–509. doi: 10.1007/BF02983202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Campbell WW, Barton ML, Jr., Cyr-Campbell D, et al. Effects of an omnivorous diet compared with a lactoovovegetarian diet on resistance-training-induced changes in body composition and skeletal muscle in older men. Am J Clin Nutr. 1999 Dec;70(6):1032–1039. doi: 10.1093/ajcn/70.6.1032. [DOI] [PubMed] [Google Scholar]
- 43.Hannan MT, Gagnon MM, Aneja J, et al. Optimizing the tracking of falls in studies of older participants: comparison of quarterly telephone recall with monthly falls calendars in the MOBILIZE Boston Study. Am J Epidemiol. 2010 May 1;71(9):1031–1036. doi: 10.1093/aje/kwq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cummings SR, Nevitt MC, Kidd S. Forgetting falls. The limited accuracy of recall of falls in the elderly. J Am Geriatr Soc. 1988 Jul;36(7):613–616. doi: 10.1111/j.1532-5415.1988.tb06155.x. [DOI] [PubMed] [Google Scholar]