Abstract
Background
The impact of body size, fat-free mass (FFM) and fat mass (FM) on cardiorespiratory fitness in pediatric renal transplant recipients (TX) has not been established. Study objectives were to assess maximal oxygen consumption (VO2max) in TX and controls, adjusted for body composition, and to identify risk factors for reduced fitness in TX.
Methods
Cycle ergometry and DXA were obtained in 50 TX and 70 controls, ages 8 to 21 yr. Control recruitment was targeted to include obese subjects with body mass index (BMI) Z-scores comparable to TX. Allometric regression models were utilized.
Results
TX had significantly lower height Z-scores (p < 0.001) and comparable BMI Z-scores. VO2max per body weight (ml/kg/min) and per FFM (ml/kgFFM/min) did not differ between groups. However, VO2max was 13% lower (95% CI 18, 8; p < 0.001) in TX, compared with controls, adjusted for FM, FFM, sex and race. Greater FFM, lower FM, non-black race, and male sex were independently associated with greater VO2max. Within TX, hemoglobin levels were positively associated with VO2max (p = 0.04) and sirolimus use was associated with lower VO2max (p < 0.01).
Conclusions
TX had significant VO2max deficits that were not captured by conventional measures (ml/kg/min). Greater FM was an independent risk factor for low VO2max. Lower fitness in TX may be related to sirolimus effects on skeletal muscle.
Keywords: Pediatric kidney transplant, exercise, fitness
Introduction
Young adults with a history of renal transplantation during childhood and adolescence have a greater than ten fold risk of cardiovascular death compared to the general population (1). Although research has focused on minimizing traditional cardiovascular risk factors such as hypertension and obesity, little attention has been paid to the role of physical inactivity and reduced cardiorespiratory fitness in this population. In healthy persons, it is well documented that improvements in aerobic fitness ameliorate many cardiovascular risk factors and decrease overall cardiovascular mortality (2-5). Therefore, further study of cardiorespiratory fitness in this high-risk population is of paramount importance.
Aerobic fitness is defined as the capacity to deliver oxygen to working skeletal muscles and the ability of muscles to utilize oxygen through the process of aerobic metabolism (6). The point at which the body reaches its limit for maximal utilization of oxygen is termed maximal oxygen consumption (VO2max). VO2max is the best single measure of aerobic capacity (6), and is measured in milliliters of oxygen consumed per minute (ml/min).
One of the greatest challenges in the interpretation of VO2max is normalizing the results for differences in body size and body composition (7-10). This is especially important in pediatric renal transplantation which is frequently complicated by poor growth and obesity (11). Traditionally, VO2max is divided by body mass (ml/kg/min) to facilitate comparisons across body sizes. The published normal reference data for VO2max in children are based on age or body size [body surface area (BSA) or weight](12-14). The use of such ratios to adjust VO2max for differences in body size assumes that VO2max and body size are isometrically related – that is, VO2max increases as a linear function of body size; this assumption is not however correct (8). In contrast, allometric scaling techniques, employing log-transformed regression models in children and adults, have consistently shown that VO2max increases as a function of body weight to the 0.60 to 0.75 power (e.g. ml/kg0.60/min) (8). Therefore, the expression of traditional measures of VO2max (ml/kg/min) will result in systematic underestimation of fitness in heavier individuals and overestimation in lighter subjects. Furthermore, scaling VO2max to fat-free mass (FFM) rather than total body weight provides better estimates of fitness since the metabolically active body cell mass is pertinent to the expression of oxygen consumption. A study in normal weight children demonstrated that log transformed regression models for VO2max relative to FFM eliminated the effect of body size (15).
The primary objective of this study was to assess aerobic fitness as measured by maximal oxygen consumption (VO2max) in renal transplant recipients relative to body size and body composition, compared with healthy controls. An additional objective was to identify risk factors for a lower level of aerobic fitness in renal transplant recipients.
Methods
Study Subjects
Renal transplant recipients and healthy control subjects were enrolled in this cross-sectional study from November 2005 through February 2008. Renal transplant recipients, ages 8-21 years, who were greater than 6 months after transplantation with stable renal function for three months were eligible. Children with cognitive impairment or orthopedic complications that limited their ability to perform the exercise protocol, as well as those with significant cardiac or pulmonary co-morbidity (such as restrictive lung disease secondary to obstructive uropathy) were excluded. Of a total of 160 transplant recipients, 110 were eligible to participate in the study. Of the eligible subjects, 50 subjects were enrolled and 11 transplant recipients declined to participate in the study. Healthy control subjects were recruited from general pediatric practices in Philadelphia and the surrounding community. Recruitment of healthy controls targeted overweight and obese children in order to achieve comparable distributions of body mass index (BMI, kg/m2) Z-scores in the transplant recipients and controls. Control subjects with chronic diseases or medications known to affect growth or body composition were excluded. All subjects had to be greater than 130 cm in height (minimum height required to perform cycle ergometry) and weigh less than 135 kg to meet dual-energy x-ray absorptiometry (DXA) scanner requirements. This study protocol was approved by the Institutional Review Board of The Children’s Hospital of Philadelphia. Informed consent was obtained directly from study participants older than 18 years, and assent along with parental consent from participants less than 18 years of age.
Subject Characteristics
Medical charts of the transplant recipients were reviewed for information regarding primary renal diagnosis, age at transplant, donor source, pre-transplant dialysis duration, interval since transplant, current medications and prior medications following transplantation. Laboratory data collected on the day of exercise testing included serum creatinine, phosphorus and hemoglobin levels. Glomerular filtration rate (eGFR) was estimated using the Schwartz formula (16).
Anthropometry and Maturation
Height was measured to the nearest 0.1 cm with a stadiometer (Holtain, Crymych, UK) and weight to the nearest 0.1 kg with a digital scale (Scaletronix, White Plains, NY). The stage of pubertal development was determined using a validated self-assessment questionnaire (17) and classified according to the method of Tanner (18). Study participants and their parents were asked to categorize the participant’s race according to the National Institute of Health categories.
DXA Scans
DXA scans of the whole body were performed using a Delphi/Discovery (Hologic, Bedford, MA) densitometer with a fan beam in the array mode. All scans were analyzed using software version 12.3. The scans were analyzed to generate estimates of whole body FFM (kg) and fat mass (FM, kg).
Exercise Testing
Subjects were exercised to maximal volition using an electronically braked cycle ergometer (SensorMedics, Yorba Linda, CA). The protocol consisted of three minutes of pedaling in an unloaded state followed by a ramp increase in work rate (Watts) to maximal exercise. The steepness of the ramp protocol was determined by subject weight and designed to achieve predicted peak work rate in 10 to 12 minutes of cycling time (6). A 12-lead ECG (Marquette Case-8000, Milwaukee, WI) was obtained at rest in the supine, sitting, and standing position. Cardiac rhythm was monitored continuously throughout the study. Blood pressure was measured at rest and every three minutes during exercise and recovery by auscultation. Systemic arterial oxygen saturation (SaO2) was monitored continuously during exercise by pulse oximetry.
Metabolic data were obtained throughout the exercise study and for the first two minutes of recovery on a breath-by-breath basis using a metabolic cart (SensorMedics V29, Yorba Linda, CA). The primary outcome variable measured was VO2max. The respiratory exchange ratio (RER), defined as CO2 produced divided by O2 consumed, was calculated at peak exercise to assess effort. RER values greater than 1.10 were considered adequate for maximal effort (19).
Statistical Analysis
Descriptive analyses included means, standard deviation (SD), median and interquartile ranges of continuous variables and distributions of categorical variables. Differences in means were assessed using Student’s t-test, or the Wilcoxon Rank Sum test if the data were not normally distributed. Differences in proportions were assessed using the chi-square test. A p-value of < 0.05 was considered statistically significant, and two-sided tests of hypotheses were used throughout. The SPSS 15.0 (SPSS Inc., Chicago, IL) statistical package was used in the analysis.
Age- and sex-specific Z-scores (standard deviation scores) for height, weight and BMI were calculated using National Center for Health Statistics 2000 Center for Disease Control growth data (20). Obesity was defined as a BMI greater than the 95th percentile for age and sex (21). Blood pressure index was calculated by dividing the blood pressure of the subject by the age, sex and height specific 95th percentiles for systolic and diastolic blood pressures (22,23).
Correlations between VO2max and anthropometric measures were evaluated by Pearson correlation analysis, or Spearman rank correlation for non-normally distributed data. Variables with P values < 0.1 on univariate analysis were tested in log-transformed multivariate linear regression models to determine which measurement or combination of measurements explained the greatest proportion of the variability in VO2max in healthy control subjects. All models were adjusted for sex, race (black versus all others) and for Tanner stage of pubertal maturation (stage 1 through 3 as the referent group with an indicator variable for Tanner stages 4 and 5). Subsequently, a dummy variable was added to the model in order to compare VO2max in transplant recipients to that of controls, adjusted for the significant body composition and demographic factors. The independent effect of transplantation in the models is presented as the adjusted ratio of the outcome in transplant recipients divided by the outcome in controls. The adjusted ratios and 95% confidence intervals (C.I.) were calculated as exponentiated estimates of the regression coefficients for the transplant dummy variable. Tests for interactions between variables were performed using multiplicative interaction terms.
A secondary aim of the study was to determine potential transplant risk factors for lower level of fitness (VO2max). The risk factors were tested in the multivariable regression model in transplant recipients only. These included diagnosis (congenital vs. others), pre-emptive transplant, duration of end stage renal disease (ESRD), time since transplant, eGFR, hemoglobin, serum phosphorus, and medications.
Results
Subject Characteristics
Fifty pediatric renal transplant recipients and 70 healthy control subjects were enrolled in the study (Table 1). The male predominance in the renal transplant group is consistent with the demographics of ESRD in childhood (24). A greater proportion of the control subjects were black (p = 0.01), consistent with the demographics in the recruitment population. As compared with the controls, renal transplant recipients had significantly lower height Z-scores (p < 0.001) and a smaller proportion were pubertal (p = 0.001). Consistent with their shorter stature, body weight was significantly lower in the transplant recipients, compared with controls. However, BMI Z-scores and the proportion that were obese were comparable between groups. Transplant recipients had significantly lower whole body FFM compared to controls (p = 0.02). However, log transformed regression models demonstrated that whole body FFM was not significantly lower in transplant recipients compared with controls (ratio 0.99, 95% C.I. 0.93 to 1.05, p = 0.88), adjusted for height, race, pubertal stage and sex. Similarly, whole body fat mass was not significantly greater in the transplant recipients compared with controls (ratio 1.15, 95% C.I. 0.90 to 1.49, p = 0.25), adjusted for height, race, pubertal stage and sex.
Table 1.
Characteristics of renal transplant recipients and healthy controls
| Characteristic | Transplant N = 50 | Controls N = 70 | p value |
|---|---|---|---|
| N (%) or Mean ± SD (Range) | |||
| Age (years) | 14.5 ± 3.0 | 14.9 ± 3.7 | 0.61 |
| Range | (8.5 to 20) | (8.1 to 21.9) | |
| Male | 35 (70%) | 28 (40%) | 0.001 |
| Black race | 15 (30%) | 40 (57%) | 0.01 |
| Tanner stage 4-5 | 25 (50%) | 55 (79%) | 0.001 |
| Body weight (kg) | 58.0 ± 21.5 | 66.2 ± 20.4 | 0.049 |
| Range | (31.0 to 126.7) | (25.4 to 126.7) | |
| Weight Z-score | 0.5 ± 1.7 | 1.1 ± 1.2 | 0.02 |
| Height (cm) | 153.2 ± 14.5 | 160.4 ± 13.0 | <0.01 |
| Range | (126.2 to 182.3) | (129.1 to 182.4) | |
| Height Z-score | -1.0 ± 1.2 | 0.2 ± 1.0 | <0.001 |
| BMI (kg/m2) | 24.1 ± 6.0 | 25.4 ± 6.2 | 0.34 |
| Range | (14.7 to 43.2) | (14.9 to 44.4) | |
| BMI Z-score | 0.9 ± 1.2 | 1.0 ± 1.1 | 0.54 |
| Fat-Free Mass (kg) | 37.0 ± 13.0 | 42.5 ± 12.8 | 0.02 |
| Range | (20.5 to 76.1) | (17.2 to 72.4) | |
| Fat mass (kg) | 16.1 ± 10.2 | 17.8 ± 10.5 | 0.40 |
| Range | (3.6 to 57.5) | (3.6 to 51.5) | |
| Obese | 17 (34%) | 26 (37%) | 0.72 |
BMI, body mass index
The medical histories of the renal transplant recipients are summarized in Table 2. eGFR ranged from 24.7 to 183.2 ml/min/1.73 m2. Only 4 subjects had eGFR less than 50 ml/min/1.73 m2. Of the 50 transplant recipients, 10% had hemoglobin levels below the normal range for age and four were on treatment with erythropoietin at the time of the study. All were on prednisone; dosages ranged from 2.5 mg to 10 mg daily or every other day. Ten renal transplant recipients had a history of treatment with growth hormone prior to transplantation; none were treated with growth hormone following transplantation. The percentages of concurrent medications are summarized in the Table.
Table 2.
Disease characteristics of renal transplant recipients
| Characteristic | N= 50 |
|---|---|
| N (%) or Median and inter-quartile range (25%-75%) | |
|
| |
| Diagnosis: | |
| FSGS | 9 (18%) |
| Congenital renal disease | 25 (50%) |
| Other glomerular disease | 6 (12%) |
| Other | 10 (20%) |
| Duration of ESRD (months) | 39.2 (24.0-92.4) |
| Age at transplant (years) | 11.1 (6.7-15.1) |
| Duration since transplant (months) | 26.3 (16.2-72.5) |
| Living related donor | 24 (48%) |
| History of dialysis | 29 (58%) |
| Duration of dialysis (months) | 9.8 (3.5-21.8) |
| eGFR (ml/min/1.73 m2) | 87.2 (73.4-96.3) |
| eGFR ≥90 ml/min/1.73 m2 | 20 (40%) |
| eGFR 60 -89 ml/min/1.73 m2 | 18 (36%) |
| eGFR 30-59 ml/min/1.73 m2 | 3 (6%) |
| eGFR 15 -29 ml/min/1.73 m2 | 1 (2%) |
| Hemoglobin (g/dl) | 12.6 (11.9-13.4) |
| Serum phosphorus (g/dl) | 4.3 (3.8-4.8) |
| Current Medications | |
| Antihypertensive | 21 (42%) |
| Calcineurin inhibitor | 43 (86%) |
| Sirolimus | 18 (36%) |
| Mycophenolate mofetil | 20 (40%) |
| Cyclosporine | 2 (4%) |
| Azathioprine | 7 (14) |
| Prednisone, mean daily dose | 50 (100%), 0.069 mg/kg/day |
FSGS, focal segmental glomerulosclerosis; ESRD, end stage renal disease; eGFR, estimated glomerular filtration rate
Exercise Outcomes
The results of the exercise testing are summarized in Table 3. Both groups achieved maximal effort on exercise testing, average RER > 1.10. The control group reached a higher peak heart rate than the transplant recipients, 96% and 93% of predicted, respectively. Transplant recipients had a higher diastolic blood pressure index compared to controls during rest (p = 0.01) and peak exercise (p = 0.01). Absolute VO2max (L/min) was significantly lower in the transplant group as compared to the control group (p < 0.01). However, no significant differences were detected between transplant recipients and controls when VO2max was divided by body weight (ml/kg/min) or fat free mass (ml/kgFFM/min). There was no significant difference in VO2max in transplant recipients based on chronic kidney disease stage.
Table 3.
Exercise outcomes in renal transplant recipients and control subjects
| Characteristic | Transplant N = 50 | Controls N = 70 | p value |
|---|---|---|---|
| Peak heart rate | 186 ± 16 | 191 ± 12 | 0.04 |
| RER | 1.18 ± 0.08 | 1.20 ± 0.09 | 0.35 |
| Resting SBP index | 0.92 ± 0.10 | 0.89 ± 0.10 | 0.12 |
| Peak SBP index | 1.28 ± 0.18 | 1.29 ± 0.19 | 0.73 |
| Resting DBP index | 0.96 ± 0.15 | 0.90 ± 0.09 | 0.01 |
| Peak DBP index | 0.88 ± 0.12 | 0.83 ± 0.09 | 0.01 |
| Change in BP | 44 ± 17 | 51 ± 19 | 0.06 |
| VO2max (L/min) | 1.7 ± 0.6 | 2.0 ± 0.6 | < 0.01 |
| VO2max (ml/kg/min) | 29.6 ± 7.7 | 31.5 ± 7.8 | 0.20 |
| VO2max (ml/kgFFM/min) | 45.5 ± 8.8 | 47.7 ± 8.0 | 0.14 |
RER, respiratory exchange ratio; SBP, systolic blood pressure; DBP, diastolic blood pressure; BP, blood pressure; VO2max, maximal oxygen consumption; FFM, fat free mass.
The relationships between VO2max and anthropometric measures were examined. The significant univariate relationship between log VO2max (ml/min) and log body weight (β = 0.72, 95% CI: 0.59 to 0.85; R = 0.71, p < 0.001) is illustrated in Figure 1; the slope is significantly less than 1.0, illustrating the need for allometric scaling techniques. The stronger correlation between log VO2max and log FFM (β = 0.87, 95% CI: 0.78 to 0.97; R = 0.86, p < 0.001) is illustrated in Figure 2. Other potential confounders of VO2max with p < 0.1 in univariate analyses were entered in a log transformed multivariate linear regression model of VO2max within the control subjects only. In the final model (R2 = 0.87), FFM was independently associated with greater VO2max, while FM, female sex, and black race were independently associated with lower VO2max. Pubertal stage was not associated with VO2max.
Figure 1. Association between log transformed maximal oxygen consumption (VO2max) and log transformed body weight in renal transplant recipients and control subjects.

R = 0.71, β = 0.72, p < 0.001
Figure 2. Association between log transformed maximal oxygen consumption (VO2max) and log transformed fat free mass in renal transplant recipients and control subjects.
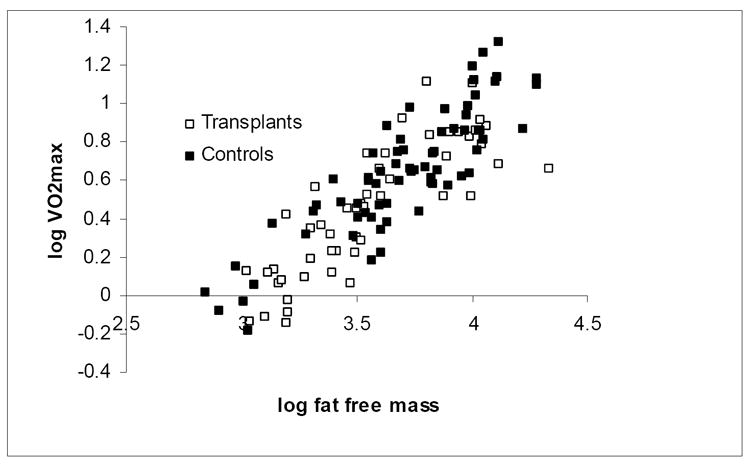
R = 0.86, β = 0.87 p < 0.001
Transplant recipients were subsequently added to the above model (Table 4). Ratios for each covariate were calculated as exponentiated estimates of the regression coefficients, adjusted for other covariates in the model. VO2max, adjusted for FFM, FM, gender and race, was significantly lower in transplant recipients, compared with healthy controls. The adjusted ratio for VO2max in the transplant recipients compared with that in the controls was 0.87 (95% C.I. 0.83 to 0.92, p < 0.001), indicating that VO2max was, on average, 13% lower in the transplant recipients. Although there were insufficient numbers of control subjects to generate age and sex specific Z-scores (standard deviation scores) for the VO2 measures in the renal transplant recipients, the effect size was estimated by comparing the standardized residuals from the regression models in the renal transplant recipients and the controls. The standardized residuals differed by 0.72 (95% C.I. 0.38, 1.07; p < 0.0001) suggesting that the VO2 levels were 0.72 SD lower in transplant recipients, compared with controls, adjusted for body composition, race and gender.
Table 4.
Multiple linear regression analysis of predictors of maximal oxygen consumption (log VO2max)
| β | 95% Confidence Interval | p value | |
|---|---|---|---|
| Renal transplant (vs. controls) | -0.136 | -0.192 to -0.079 | p < 0.001 |
| Black race (vs. non-black) | -0.128 | -0.179 to -0.077 | p < 0.001 |
| Female sex (vs. male sex) | -0.109 | -0.170 to -0.048 | p = 0.001 |
| Fat free mass | 0.923 | 0.822 to 1.024 | p < 0.001 |
| Fat mass | -0.093 | -0.144 to -.042 | p < 0.001 |
| Constant | -2.144 |
R2 = 0.84. Model is described by VO2max = exp [-2.144 + (-0.128 * Race) + (-0.109 * Sex) + (-0.136 * Renal Transplant)] * Fat Free Mass0.923 * Fat Mass-0.093.
VO2max, fat free mass and fat mass were log transformed.
VO2max in black subjects (transplants and controls) was 12% lower compared with all other subjects (ratio 0.88, 95% C.I. 0.84 to 0.93, p < 0.001). VO2max in female subjects (transplants and controls) was 10% lower compared with male subjects (ratio 0.90, 95% C.I. 0.84 to 0.95, p = 0.001). The values for fat free mass and fat mass were log transformed. Therefore, VO2max increases as a function of fat free mass to the power of 0.923, and VO2max decreases as a function of fat mass to the power of 0.093. Tests for interaction between transplant status and race, gender or FFM were not significant. Specifically, the VO2max deficits in the transplant recipients compared with controls were of comparable magnitude within the black and non-black subjects (ratios of 0.86 and 0.84, respectively).
Transplant Risk Factors
Each one-unit increase in hemoglobin was associated with a five percent greater VO2max in the transplant recipients (ratio = 1.05, 95% C.I. 1.01 to 1.09, p = 0.04), adjusted for sex, race, FM and FFM. Sirolimus use was associated with a lower VO2max (ratio = 0.87, 95% C.I. 0.80 to 0.95, p < 0.01), adjusted for sex, race, FM, and FFM. The effect of sirolimus persisted after adjustment for hemoglobin in the model. There was no difference in prednisone dose between those on sirolimus (0.0787 mg/kg/day) vs. those not on sirolimus (0.0626 mg/kg/day, p = 0.105). None of the other transplant covariates described in Table 2 were significant, independent determinants of VO2max.
Discussion
Using allometric scaling techniques, we found that cardiorespiratory fitness in children and adolescents with renal transplants was reduced by an average of 13% when compared to healthy controls, after adjusting for race, sex, FM, and FFM. Assuming that VO2 is at the 50th percentile in the controls (standard deviation score = 0) on average, these data would indicate that the transplant recipients are at the 24th percentile (standard deviation score = -0.72) on average. This reduction likely represents a clinically significant increase in the number of renal transplant recipients below the normal range relative to body composition, race and gender. This VO2max deficit in transplant recipients was not captured by conventional ratios, dividing VO2max by weight or FFM (Table 3). This was likely due to overestimation of cardiorespiratory fitness in the transplant recipients using the ratio method because they were smaller (lower weight and FFM) than the controls. As such, the use of ratios to express the nonlinear relationship between body weight and VO2max leads to several types of statistical error and misinterpretations. Log-linear regression (allometric) analyses in adults have demonstrated that scaled expressions of VO2max are more appropriate. VO2max in adults increases as body mass raised to the 0.6 - 0.75 power (e.g. ml/kg0.75/min) (8). Our results illustrate similar relations in children and adolescents.
To our knowledge, this is the first study in pediatric transplant recipients to include measures of body composition in an allometric analysis of VO2max relative to body size in comparison to control subjects. Earlier studies comparing VO2max in pediatric renal transplant recipients to controls (25, 26) or reference data (27-29) have yielded a broad range of VO2max deficits in renal transplant recipients. Different approaches to calculate VO2max utilizing ratios were carried out in these reports. Our data demonstrated a slope of 0.72 for log VO2 max relative to log body weight, consistent with adult studies (8). Therefore, we submit that our analysis of VO2max using allometric regression models instead of conventional ratios more accurately adjusts VO2max for differences in body size and composition between transplant recipients and controls.
Furthermore, no prior studies of pediatric renal transplant recipients utilized measures of FFM in their assessments of cardiorespiratory fitness. FFM has been found to be the best single predictor of VO2max (10, 30, 31). Three previous studies obtained DXA scans in transplant recipients (27, 29, 32), however, body composition measures were not analyzed with respect to VO2max. Weaver et al. included estimates of FFM that were derived from predictor equations for FFM based on height and weight that may not be appropriate in children with ESRD (26). Our analyses that incorporate DXA measures of whole body FM and FFM in transplant recipients and controls confirmed the strong, positive association between FFM and VO2max, and also demonstrated an additional, independent adverse effect of greater adiposity (FM). Given that transplant recipients have a high incidence of obesity following transplant (33), their cardiorespiratory fitness may be compromised further by increasing adiposity.
Our findings highlight the importance of adjusting for race in the renal transplant population. No other pediatric transplant studies reported associations between race and VO2max. Our results confirm prior reports in healthy children of lower VO2 max in black children versus white children (34-37). Factors such as physical activity level, body composition and hematologic profile may account for the racial differences in VO2 max (34). However, our study demonstrated racial differences independent of body composition. Furthermore, the high proportion of black subjects in the control group may have contributed to the lack of difference in VO2max between the transplant group and controls when using conventional ratios.
The recruitment of controls targeted overweight and obese children in order to achieve sufficiently overlapping distributions of body weight, FM and FFM compared with the transplant recipients. Given that conventional ratio measures of VO2max (ml/kg/min) result in an underestimation of fitness in larger individuals (greater absolute body weight), these measures may result in an underestimation of VO2max in obese transplant recipients compared with non-obese controls. In contrast, if predominantly overweight and obese transplant recipients weigh less than the non-obese controls due to shorter stature, the ratio measures may result in an overestimation of VO2max. Given that transplant recipients have lower height Z-scores and greater BMI Z-score than the general population, it is important to use a control population that allows for adjustment for these variable effects of body size and composition.
The mechanism of decreased fitness in the pediatric renal transplant population is likely multi-factorial. Studies in adults have implicated skeletal muscle weakness due to residual effects of renal failure on neuromuscular structure and function (38), prednisone effects on skeletal muscle structure and function (39-41), decreased oxygen transport capacity in anemia (42), ventricular dysfunction (43), and sedentary lifestyle (43). Evidence documenting the risk factors for low fitness in pediatric renal transplant recipients is sparse. Weaver et al. found that diastolic dysfunction was an independent predictor of lower VO2max in a group of children with chronic kidney disease and renal transplant recipients. In agreement with Krull et al., our study showed that hemoglobin was positively associated with VO2max although published results are conflicting (28, 29). Our results confirm previous studies demonstrating no significant relationship between VO2max and duration of dialysis or time since transplant (28, 29). We also found no relationship between VO2max and underlying renal disease, pre-emptive transplant, treatment with blood pressure medication, eGFR or serum phosphorus level.
A novel finding of this study is that sirolimus use was associated with lower fitness in renal transplant recipients. Animal studies have shown that activation of the mammalian target of rapamycin (mTOR) signaling pathway is vital in the regulation of skeletal muscle size through an increase in protein synthesis (44). Sirolimus inhibits the ability of mTOR to phosphorylate its downstream targets, resulting in decreased cell growth and disregulation of cell cycle proliferation and cell cycle progression. Treatment with sirolimus in two week old rat pups resulted in a 40% reduction in hind leg muscle mass. Treatment at four weeks of age resulted in a 20-25% reduction and, interestingly, sirolimus had no effect on muscle mass of young adult rats (10-12 weeks) (44). Another murine study demonstrated that muscle hypertrophy in response to altered mechanical loading is blocked by sirolimus (45). These data suggest that sirolimus inhibits skeletal muscle growth and hypertrophy; however, in our study, we saw an association between sirolimus and VO2max even after adjustment for FFM. Currently, there are no published reports on the effects of sirolimus on muscle mass or metabolism in humans or the relationship of sirolimus with VO2max.
The present study is limited by the cross-sectional design and heterogeneity of the transplant population. The difficulty in measuring residual confounding factors such as the complexity of ESRD course, past medical histories and fluctuating values of covariates (e.g. recent rejection episode) is also a limitation. Additionally, transplant recipients who agreed to participate in the study may be more fit or more interested in physical activity than those who declined to participate. However, since lower fitness was found, such a bias would lead to an underestimation of the difference. Given the small control group, we could not fully adjust for differences in maturation and body composition, but our study has more concurrent controls than any prior study. Our study also lacked a measure of physical activity in cases and controls, such as accelerometry, which has been shown to have a positive association with VO2max in healthy children (46). Despite the aforementioned limitations, we believe that our conclusions remain valid. And, our study is strengthened by the use of allometric scaling techniques, DXA measures of body composition and concurrent controls with specific attention to race and obesity.
CONCLUSION
Our findings illustrate the importance of the consideration of race and body size and composition in the assessment of cardiorespiratory fitness in pediatric transplant recipients. Our results also highlight the independent relation between greater adiposity and lower cardiorespiratory fitness. Future prospective longitudinal studies are needed that include accelerometric measures of physical activity, assessment of cardiovascular disease, including diastolic dysfunction, and measures of concurrent medication exposures. Decreased cardiorespiratory fitness is a cardiovascular risk factor and post-transplant care should include Interventions to improve physical functioning of pediatric renal transplant recipients.
Acknowledgments
Funding Sources: This project was supported by Grants UL1-RR-024134 and R01-HD040714 and by NIH Clinical Research Training in Kidney Disease Grant T32-DK07785. The study was supported by the Clinical Translational Research Center under the grant MO1-RR00240. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Author Contributions
C.B. Sethna participated in performance of the research, data analysis, and writing of the paper.
A.E. Salerno participated in research design and writing of the paper.
M.G. McBride participated in performance of the research and writing of the paper.
J. Shults participated in data analysis.
S.M. Paridon participated in research design.
N. Sharma participated in performance of the research.
K.E.C Meyers participated in data analysis and writing of the paper.
M.B. Leonard participated in research design, data analysis, and writing of the paper.
Conflict of Interest: None
References
- 1.Foley RN, Parfrey PS, Sarnak MJ. Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol. 1998;9(12 Suppl):S16–23. [PubMed] [Google Scholar]
- 2.Blair SN, Kohl HW, 3rd, Paffenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA. 1989;262(17):2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 3.Carnethon MR, Gidding SS, Nehgme R, Sidney S, Jacobs DR, Jr, Liu K. Cardiorespiratory fitness in young adulthood and the development of cardiovascular disease risk factors. JAMA. 2003;290(23):3092–3100. doi: 10.1001/jama.290.23.3092. [DOI] [PubMed] [Google Scholar]
- 4.Ekelund LG, Haskell WL, Johnson JL, Whaley FS, Criqui MH, Sheps DS. Physical fitness as a predictor of cardiovascular mortality in asymptomatic North American men. The Lipid Research Clinics Mortality Follow-up Study. N Engl J Med. 1988;319(21):1379–1384. doi: 10.1056/NEJM198811243192104. [DOI] [PubMed] [Google Scholar]
- 5.Mora S, Redberg RF, Cui Y, Whiteman MK, Flaws JA, Sharrett AR, et al. Ability of exercise testing to predict cardiovascular and all-cause death in asymptomatic women: a 20-year follow-up of the lipid research clinics prevalence study. JAMA. 2003;290(12):1600–1607. doi: 10.1001/jama.290.12.1600. [DOI] [PubMed] [Google Scholar]
- 6.Rowland TW. Pediatric laboratory exercise testing : clinical guidelines. Champaign, IL: Human Kinetics Publishers; 1992. [Google Scholar]
- 7.Beunen GP, Rogers DM, Woynarowska B, Malina RM. Longitudinal study of ontogenetic allometry of oxygen uptake in boys and girls grouped by maturity status. Ann Hum Biol. 1997;24(1):33–43. doi: 10.1080/03014469700004752. [DOI] [PubMed] [Google Scholar]
- 8.Buresh R, Berg K. Scaling oxygen uptake to body size and several practical applications. J Strength Cond Res. 2002;16(3):461–465. [PubMed] [Google Scholar]
- 9.Rogers DM, Olson BL, Wilmore JH. Scaling for the VO2-to-body size relationship among children and adults. J Appl Physiol. 1995;79(3):958–967. doi: 10.1152/jappl.1995.79.3.958. [DOI] [PubMed] [Google Scholar]
- 10.Toth MJ, Goran MI, Ades PA, Howard DB, Poehlman ET. Examination of data normalization procedures for expressing peak VO2 data. J Appl Physiol. 1993;75(5):2288–2292. doi: 10.1152/jappl.1993.75.5.2288. [DOI] [PubMed] [Google Scholar]
- 11.Hanevold CD, Ho PL, Talley L, Mitsnefes MM. Obesity and renal transplant outcome: a report of the North American Pediatric Renal Transplant Cooperative Study. Pediatrics. 2005;115(2):352–356. doi: 10.1542/peds.2004-0289. [DOI] [PubMed] [Google Scholar]
- 12.Cooper DM, Weiler-Ravell D, Whipp BJ, Wasserman K. Aerobic parameters of exercise as a function of body size during growth in children. J Appl Physiol. 1984;56(3):628–634. doi: 10.1152/jappl.1984.56.3.628. [DOI] [PubMed] [Google Scholar]
- 13.Nagle FJ, Hagberg J, Kamei S. Maximal O2 uptake of boys and girls -- ages 14--17. Eur J Appl Physiol Occup Physiol. 1977;36(2):75–80. doi: 10.1007/BF00423114. [DOI] [PubMed] [Google Scholar]
- 14.Washington RL, van Gundy JC, Cohen C, Sondheimer HM, Wolfe RR. Normal aerobic and anaerobic exercise data for North American school-age children. J Pediatr. 1988;112(2):223–233. doi: 10.1016/s0022-3476(88)80059-3. [DOI] [PubMed] [Google Scholar]
- 15.Janz KF, Burns TL, Witt JD, Mahoney LT. Longitudinal analysis of scaling VO2 for differences in body size during puberty: the Muscatine Study. Med Sci Sports Exerc. 1998;30(9):1436–1444. doi: 10.1097/00005768-199809000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz GJ, Feld LG, Langford DJ. A simple estimate of glomerular filtration rate in full-term infants during the first year of life. J Pediatr. 1984;104(6):849–854. doi: 10.1016/s0022-3476(84)80479-5. [DOI] [PubMed] [Google Scholar]
- 17.Morris N, Udry J. Validation of a self-administered instrument to assess stage of adolescent development. Journal of Youth and Adolescence. 1980;9(3):271–280. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- 18.Tanner JM. Assessment of skeletal maturity and prediction of adult height (TW2 method) London ; New York: Academic Press; 1975. [Google Scholar]
- 19.Paridon SM, Alpert BS, Boas SR, Cabrera ME, Caldarera LL, Daniels SR, et al. Clinical stress testing in the pediatric age group: a statement from the American Heart Association Council on Cardiovascular Disease in the Young, Committee on Atherosclerosis, Hypertension, and Obesity in Youth. Circulation. 2006;113(15):1905–1920. doi: 10.1161/CIRCULATIONAHA.106.174375. [DOI] [PubMed] [Google Scholar]
- 20.Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109(1):45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 21.Krebs NF, Jacobson MS. Prevention of pediatric overweight and obesity. Pediatrics. 2003;112(2):424–430. doi: 10.1542/peds.112.2.424. [DOI] [PubMed] [Google Scholar]
- 22.The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 Suppl):555–576. 4th Report. [PubMed] [Google Scholar]
- 23.Sorof JM, Cardwell G, Franco K, Portman RJ. Ambulatory blood pressure and left ventricular mass index in hypertensive children. Hypertension. 2002;39:903–908. doi: 10.1161/01.hyp.0000013266.40320.3b. [DOI] [PubMed] [Google Scholar]
- 24.Smith JM, Stablein DM, Munoz R, Hebert D, McDonald RA. Contributions of the Transplant Registry: The 2006 Annual Report of the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) Pediatr Transplant. 2007;11(4):366–373. doi: 10.1111/j.1399-3046.2007.00704.x. [DOI] [PubMed] [Google Scholar]
- 25.Bonzel KE, Wildi B, Weiss M, Scharer K. Spiroergometric performance of children and adolescents with chronic renal failure. Pediatr Nephrol. 1991;5(1):22–28. doi: 10.1007/BF00852834. [DOI] [PubMed] [Google Scholar]
- 26.Weaver DJ, Jr, Kimball TR, Knilans T, Mays W, Knecht SK, Gerdes YM, et al. Decreased maximal aerobic capacity in pediatric chronic kidney disease. J Am Soc Nephrol. 2008;19(3):624–630. doi: 10.1681/ASN.2007070773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krasnoff JB, Mathias R, Rosenthal P, Painter PL. The comprehensive assessment of physical fitness in children following kidney and liver transplantation. Transplantation. 2006;82(2):211–217. doi: 10.1097/01.tp.0000226160.40527.5f. [DOI] [PubMed] [Google Scholar]
- 28.Krull F, Schulze-Neick I, Hatopp A, Offner G, Brodehl J. Exercise capacity and blood pressure response in children and adolescents after renal transplantation. Acta Paediatr. 1994;83(12):1296–1302. doi: 10.1111/j.1651-2227.1994.tb13020.x. [DOI] [PubMed] [Google Scholar]
- 29.Painter P, Krasnoff J, Mathias R. Exercise capacity and physical fitness in pediatric dialysis and kidney transplant patients. Pediatr Nephrol. 2007;22(7):1030–1039. doi: 10.1007/s00467-007-0458-6. [DOI] [PubMed] [Google Scholar]
- 30.Batterham AM, Vanderburgh PM, Mahar MT, Jackson AS. Modeling the influence of body size on V(O2) peak: effects of model choice and body composition. J Appl Physiol. 1999;87(4):1317–1325. doi: 10.1152/jappl.1999.87.4.1317. [DOI] [PubMed] [Google Scholar]
- 31.Vanderburgh PM, Katch FI. Ratio scaling of VO2max penalizes women with larger percent body fat, not lean body mass. Med Sci Sports Exerc. 1996;28(9):1204–1208. doi: 10.1097/00005768-199609000-00019. [DOI] [PubMed] [Google Scholar]
- 32.Feber J, Dupuis JM, Chapuis F, Braillon P, Jocteur-Monrozier D, Daudet G, et al. Body composition and physical performance in children after renal transplantation. Nephron. 1997;75(1):13–19. doi: 10.1159/000189493. [DOI] [PubMed] [Google Scholar]
- 33.Mitsnefes MM, Khoury P, McEnery PT. Body mass index and allograft function in pediatric renal transplantation. Pediatr Nephrol. 2002;17(7):535–539. doi: 10.1007/s00467-002-0863-9. [DOI] [PubMed] [Google Scholar]
- 34.Andreacci JL, Robertson RJ, Dube JJ, Aaron DJ, Balasekaran G, Arslanian SA. Comparison of maximal oxygen consumption between black and white prepubertal and pubertal children. Pediatr Res. 2004;56(5):706–713. doi: 10.1203/01.PDR.0000141521.77229.8D. [DOI] [PubMed] [Google Scholar]
- 35.Gutin B, Islam S, Manos T, Cucuzzo N, Smith C, Stachura ME. Relation of percentage of body fat and maximal aerobic capacity to risk factors for atherosclerosis and diabetes in black and white seven- to eleven-year-old children. J Pediatr. 1994;125(6 Pt 1):847–852. doi: 10.1016/s0022-3476(05)81997-3. [DOI] [PubMed] [Google Scholar]
- 36.Pivarnik JM, Fulton JE, Taylor WC, Snider SA. Aerobic capacity in black adolescent girls. Res Q Exerc Sport. 1993;64(2):202–207. doi: 10.1080/02701367.1993.10608797. [DOI] [PubMed] [Google Scholar]
- 37.Trowbridge CA, Gower BA, Nagy TR, Hunter GR, Treuth MS, Goran MI. Maximal aerobic capacity in African-American and Caucasian prepubertal children. Am J Physiol. 1997;273(4 Pt 1):E809–814. doi: 10.1152/ajpendo.1997.273.4.E809. [DOI] [PubMed] [Google Scholar]
- 38.Diesel W, Emms M, Knight BK, Noakes TD, Swanepoel CR, van Zyl Smit R, et al. Morphologic features of the myopathy associated with chronic renal failure. Am J Kidney Dis. 1993;22(5):677–684. doi: 10.1016/s0272-6386(12)80430-6. [DOI] [PubMed] [Google Scholar]
- 39.Horber FF, Hoppeler H, Herren D, Claassen H, Howald H, Gerber C, et al. Altered skeletal muscle ultrastructure in renal transplant patients on prednisone. Kidney Int. 1986;30(3):411–416. doi: 10.1038/ki.1986.199. [DOI] [PubMed] [Google Scholar]
- 40.Horber FF, Scheidegger JR, Grunig BE, Frey FJ. Thigh muscle mass and function in patients treated with glucocorticoids. Eur J Clin Invest. 1985;15(6):302–307. doi: 10.1111/j.1365-2362.1985.tb00276.x. [DOI] [PubMed] [Google Scholar]
- 41.Topp KS, Painter PL, Walcott S, Krasnoff JB, Adey D, Sakkas GK, et al. Alterations in skeletal muscle structure are minimized with steroid withdrawal after renal transplantation. Transplantation. 2003;76(4):667–673. doi: 10.1097/01.TP.0000076096.45542.1B. [DOI] [PubMed] [Google Scholar]
- 42.Painter P, Messer-Rehak D, Hanson P, Zimmerman SW, Glass NR. Exercise capacity in hemodialysis, CAPD, and renal transplant patients. Nephron. 1986;42(1):47–51. doi: 10.1159/000183632. [DOI] [PubMed] [Google Scholar]
- 43.Painter P. Exercise after renal transplantation. Adv Ren Replace Ther. 1999;6(2):159–164. doi: 10.1016/s1073-4449(99)70034-8. [DOI] [PubMed] [Google Scholar]
- 44.Bodine SC. mTOR signaling and the molecular adaptation to resistance exercise. Med Sci Sports Exerc. 2006;38(11):1950–1957. doi: 10.1249/01.mss.0000233797.24035.35. [DOI] [PubMed] [Google Scholar]
- 45.Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3(11):1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- 46.LeMura LM, Andreacci J, Carlonas R, Klebez JM, Chelland S. Evaluation of physical activity measured via accelerometry in rural fourth-grade children. Percept Mot Skills. 2000;90(1):329–337. doi: 10.2466/pms.2000.90.1.329. [DOI] [PubMed] [Google Scholar]


