Abstract
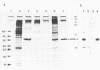
An outer membrane (OM) preparation from elementary bodies (EBs) of Chlamydia psittaci (ovine abortion strain) was used to vaccinate pregnant ewes in a single subcutaneous dose and was found to achieve protection after subcutaneous challenge with infectious organisms. Inactivated purified EBs used as a single-dose vaccine also gave protection. The ratio of live to dead lambs was significantly higher in the vaccinated groups (16:1 and 15:1, respectively) than in the placebo group (8:9). Polyacrylamide gel electrophoresis and immunoblotting showed that a 40-kilodalton protein was the main protein constituent of the OM preparation, and this was positively identified as the major outer membrane protein by protein microsequencing. Electron microscopy revealed that fine particulate structures on the outermost surface of the EB were also present in the OM preparation. The findings suggest that the major outer membrane protein is an important immunoprotective determinant in ovine abortion vaccines.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson I. E. Comparison of five ovine isolates of Chlamydia psittaci: an evaluation of three cell culture treatments. Med Lab Sci. 1986 Jul;43(3):241–248. [PubMed] [Google Scholar]
- Anderson I. E. Comparison of the virulence in mice of some ovine isolates of Chlamydia psittaci. Vet Microbiol. 1986 Sep;12(3):213–220. doi: 10.1016/0378-1135(86)90050-7. [DOI] [PubMed] [Google Scholar]
- Bavoil P., Ohlin A., Schachter J. Role of disulfide bonding in outer membrane structure and permeability in Chlamydia trachomatis. Infect Immun. 1984 May;44(2):479–485. doi: 10.1128/iai.44.2.479-485.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkelund S., Lundemose A. G., Christiansen G. Chemical cross-linking of Chlamydia trachomatis. Infect Immun. 1988 Mar;56(3):654–659. doi: 10.1128/iai.56.3.654-659.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown A., Hormaeche C. E., Demarco de Hormaeche R., Winther M., Dougan G., Maskell D. J., Stocker B. A. An attenuated aroA Salmonella typhimurium vaccine elicits humoral and cellular immunity to cloned beta-galactosidase in mice. J Infect Dis. 1987 Jan;155(1):86–92. doi: 10.1093/infdis/155.1.86. [DOI] [PubMed] [Google Scholar]
- Buxton D. Potential danger to pregnant women of Chlamydia psittaci from sheep. Vet Rec. 1986 May 3;118(18):510–511. doi: 10.1136/vr.118.18.510. [DOI] [PubMed] [Google Scholar]
- Buzoni-Gatel D., Rodolakis A., Plommet M. T cell mediated and humoral immunity in a mouse Chlamydia psittaci systemic infection. Res Vet Sci. 1987 Jul;43(1):59–63. [PubMed] [Google Scholar]
- Caldwell H. D., Kromhout J., Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981 Mar;31(3):1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell H. D., Perry L. J. Neutralization of Chlamydia trachomatis infectivity with antibodies to the major outer membrane protein. Infect Immun. 1982 Nov;38(2):745–754. doi: 10.1128/iai.38.2.745-754.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell H. D., Schachter J. Antigenic analysis of the major outer membrane protein of Chlamydia spp. Infect Immun. 1982 Mar;35(3):1024–1031. doi: 10.1128/iai.35.3.1024-1031.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J. J., Leonard K., Arad T., Pitt T., Zhang Y. X., Zhang L. H. Structural studies of the outer envelope of Chlamydia trachomatis by electron microscopy. J Mol Biol. 1982 Nov 15;161(4):579–590. doi: 10.1016/0022-2836(82)90409-0. [DOI] [PubMed] [Google Scholar]
- Cui Z. D., LaScolea L. J., Jr, Fisher J., Ogra P. L. Immunoprophylaxis of Chlamydia trachomatis lymphogranuloma venereum pneumonitis in mice by oral immunization. Infect Immun. 1989 Mar;57(3):739–744. doi: 10.1128/iai.57.3.739-744.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson M., Zaghloul A., Wilsmore A. J. Ovine enzootic abortion: experimental studies of immune responses. Res Vet Sci. 1986 Jan;40(1):59–64. [PubMed] [Google Scholar]
- Eckerskorn C., Mewes W., Goretzki H., Lottspeich F. A new siliconized-glass fiber as support for protein-chemical analysis of electroblotted proteins. Eur J Biochem. 1988 Oct 1;176(3):509–519. doi: 10.1111/j.1432-1033.1988.tb14308.x. [DOI] [PubMed] [Google Scholar]
- Grayston J. T., Wang S. P., Yeh L. J., Kuo C. C. Importance of reinfection in the pathogenesis of trachoma. Rev Infect Dis. 1985 Nov-Dec;7(6):717–725. doi: 10.1093/clinids/7.6.717. [DOI] [PubMed] [Google Scholar]
- Herring A. J., Anderson I. E., McClenaghan M., Inglis N. F., Williams H., Matheson B. A., West C. P., Rodger M., Brettle P. P. Restriction endonuclease analysis of DNA from two isolates of Chlamydia psittaci obtained from human abortions. Br Med J (Clin Res Ed) 1987 Nov 14;295(6608):1239–1239. doi: 10.1136/bmj.295.6608.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring A. J., Tan T. W., Baxter S., Inglis N. F., Dunbar S. Sequence analysis of the major outer membrane protein gene of an ovine abortion strain of Chlamydia psittaci. FEMS Microbiol Lett. 1989 Nov;53(1-2):153–158. doi: 10.1016/0378-1097(89)90383-2. [DOI] [PubMed] [Google Scholar]
- Huang H. S., Tan T. W., Buxton D., Anderson I. E., Herring A. J. Antibody response of the ovine lymph node to experimental infection with an ovine abortion strain of Chlamydia psittaci. Vet Microbiol. 1990 Feb;21(4):345–351. doi: 10.1016/0378-1135(90)90006-h. [DOI] [PubMed] [Google Scholar]
- Linklater K. A., Dyson D. A. Field studies on enzootic abortion of ewes in south east Scotland. Vet Rec. 1979 Oct 27;105(17):387–389. doi: 10.1136/vr.105.17.387. [DOI] [PubMed] [Google Scholar]
- Matsumoto A. Fine structures of cell envelopes of Chlamydia organisms as revealed by freeze-etching and negative staining techniques. J Bacteriol. 1973 Dec;116(3):1355–1363. doi: 10.1128/jb.116.3.1355-1363.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClenaghan M., Herring A. J., Aitken I. D. Comparison of Chlamydia psittaci isolates by DNA restriction endonuclease analysis. Infect Immun. 1984 Aug;45(2):384–389. doi: 10.1128/iai.45.2.384-389.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEWEN A. D., STAMP J. T., LITTLEJOHN A. I. Enzootic abortion in ewes. II. Immunization and infection experiments. Vet Rec. 1951 Mar 17;63(11):197–201. doi: 10.1136/vr.63.11.197. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Nano F. E., Barstad P. A., Mayer L. W., Coligan J. E., Caldwell H. D. Partial amino acid sequence and molecular cloning of the encoding gene for the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1985 May;48(2):372–377. doi: 10.1128/iai.48.2.372-377.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhall W. J., Jones R. B. Disulfide-linked oligomers of the major outer membrane protein of chlamydiae. J Bacteriol. 1983 May;154(2):998–1001. doi: 10.1128/jb.154.2.998-1001.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurminen M., Leinonen M., Saikku P., Mäkelä P. H. The genus-specific antigen of Chlamydia: resemblance to the lipopolysaccharide of enteric bacteria. Science. 1983 Jun 17;220(4603):1279–1281. doi: 10.1126/science.6344216. [DOI] [PubMed] [Google Scholar]
- Peeling R., Maclean I. W., Brunham R. C. In vitro neutralization of Chlamydia trachomatis with monoclonal antibody to an epitope on the major outer membrane protein. Infect Immun. 1984 Nov;46(2):484–488. doi: 10.1128/iai.46.2.484-488.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAMP J. T., McEWEN A. D., WATT J. A. A., NISBET D. I. Enzootic abortion in ewes; transmission of the disease. Vet Rec. 1950 Apr 29;62(17):251–254. doi: 10.1136/vr.62.17.251. [DOI] [PubMed] [Google Scholar]
- STAMP J. T., WATT J. A. A., COCKBURN R. B. Enzootic abortion in ewes; complement fixation test. J Comp Pathol. 1952 Apr;62(2):93–101. doi: 10.1016/s0368-1742(52)80009-8. [DOI] [PubMed] [Google Scholar]
- Stephens R. S., Sanchez-Pescador R., Wagar E. A., Inouye C., Urdea M. S. Diversity of Chlamydia trachomatis major outer membrane protein genes. J Bacteriol. 1987 Sep;169(9):3879–3885. doi: 10.1128/jb.169.9.3879-3885.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H., Zhang Y. X., Barrera O., Watkins N. G., Caldwell H. D. Differential effect of trypsin on infectivity of Chlamydia trachomatis: loss of infectivity requires cleavage of major outer membrane protein variable domains II and IV. Infect Immun. 1988 Aug;56(8):2094–2100. doi: 10.1128/iai.56.8.2094-2100.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor H. R., Prendergast R. A. Attempted oral immunization with chlamydial lipopolysaccharide subunit vaccine. Invest Ophthalmol Vis Sci. 1987 Oct;28(10):1722–1726. [PubMed] [Google Scholar]
- Taylor H. R., Whittum-Hudson J., Schachter J., Caldwell H. D., Prendergast R. A. Oral immunization with chlamydial major outer membrane protein (MOMP). Invest Ophthalmol Vis Sci. 1988 Dec;29(12):1847–1853. [PubMed] [Google Scholar]
- Taylor H. R., Young E., MacDonald A. B., Schachter J., Prendergast R. A. Oral immunization against chlamydial eye infection. Invest Ophthalmol Vis Sci. 1987 Feb;28(2):249–258. [PubMed] [Google Scholar]
- Waldhalm D. G., DeLong W. J., Hall R. F. Efficacy of a bacterin prepared from Chlamydia psittaci grown in cell culture for experimental immunization of ewes. Vet Microbiol. 1982 Nov;7(5):493–498. doi: 10.1016/0378-1135(82)90066-9. [DOI] [PubMed] [Google Scholar]
- Wills J. M., Gruffydd-Jones T. J., Richmond S. J., Gaskell R. M., Bourne F. J. Effect of vaccination on feline Chlamydia psittaci infection. Infect Immun. 1987 Nov;55(11):2653–2657. doi: 10.1128/iai.55.11.2653-2657.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. X., Stewart S., Joseph T., Taylor H. R., Caldwell H. D. Protective monoclonal antibodies recognize epitopes located on the major outer membrane protein of Chlamydia trachomatis. J Immunol. 1987 Jan 15;138(2):575–581. [PubMed] [Google Scholar]