Abstract
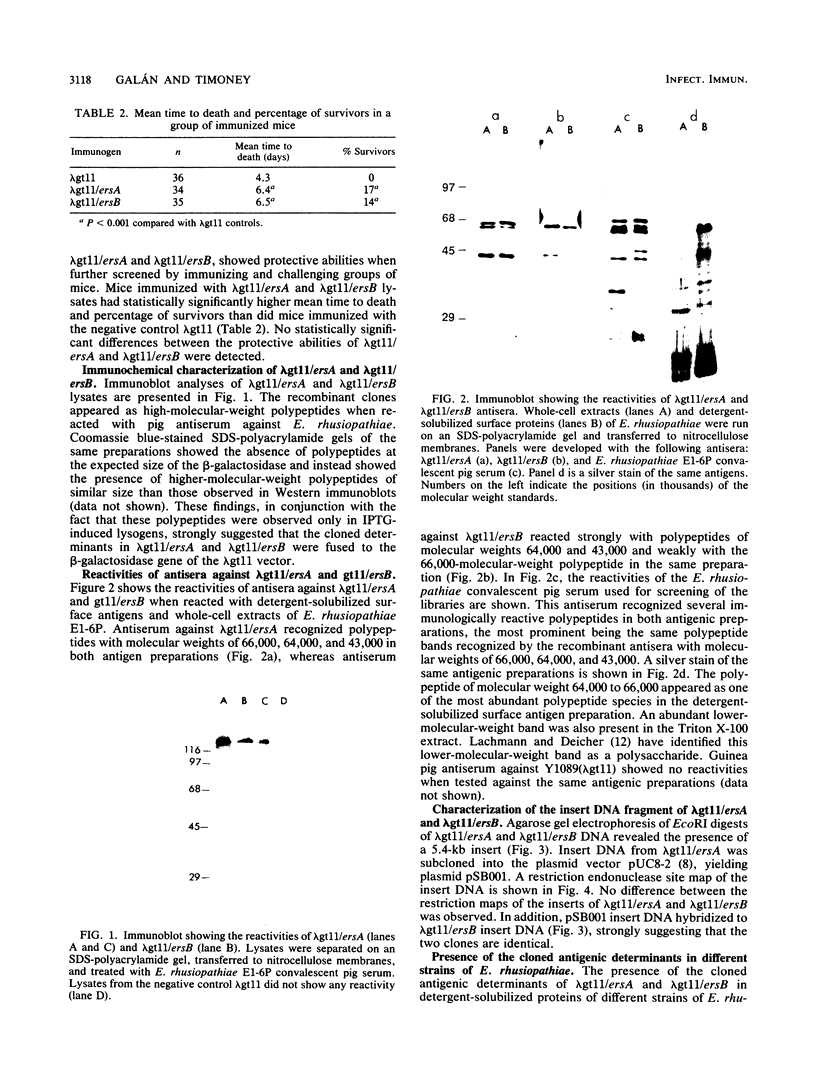
Erysipelothrix rhusiopathiae is a primary pathogen of swine and turkeys and sporadic cause of disease in a variety of other hosts, including humans. A genomic library of the highly virulent strain of E. rhusiopathiae E1-6P was constructed in the expression-cloning vector lambda gt11 and screened with serum from a pig convalescent from an E. rhusiopathiae experimental infection. Immunoreactive clones were screened for their ability to protectively immunized mice. Two clones, lambda gt11/ersA and lambda gt11/ersB, were obtained that protected mice against challenge with E. rhusiopathiae E1-6P. Antisera against the recombinant clones reacted with polypeptides of molecular weights 66,000, 64,000, and 43,000 in detergent-solubilized surface antigen preparations and whole-cell lysates of E. rhusiopathiae. These polypeptides were also the major antigens recognized by convalescent pig serum when reacted with the same preparations. Western immunoblot and Southern blot analysis revealed that the cloned genes and gene products were present in all of the E. rhusiopathiae strains tested.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bibler M. R. Erysipelothrix rhusiopathiae endocarditis. Rev Infect Dis. 1988 Sep-Oct;10(5):1062–1063. doi: 10.1093/clinids/10.5.1062. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Cases 16-1978. N Engl J Med. 1978 Apr 27;298(17):957–962. doi: 10.1056/NEJM197804272981708. [DOI] [PubMed] [Google Scholar]
- Galan J. E., Timoney J. F. Mucosal nasopharyngeal immune responses of horses to protein antigens of Streptococcus equi. Infect Immun. 1985 Mar;47(3):623–628. doi: 10.1128/iai.47.3.623-628.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galán J. E., Timoney J. F. Molecular analysis of the M protein of Streptococcus equi and cloning and expression of the M protein gene in Escherichia coli. Infect Immun. 1987 Dec;55(12):3181–3187. doi: 10.1128/iai.55.12.3181-3187.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorby G. L., Peacock J. E., Jr Erysipelothrix rhusiopathiae endocarditis: microbiologic, epidemiologic, and clinical features of an occupational disease. Rev Infect Dis. 1988 Mar-Apr;10(2):317–325. doi: 10.1093/clinids/10.2.317. [DOI] [PubMed] [Google Scholar]
- Hanna Z., Fregeau C., Préfontaine G., Brousseau R. Construction of a family of universal expression plasmid vectors. Gene. 1984 Oct;30(1-3):247–250. doi: 10.1016/0378-1119(84)90128-8. [DOI] [PubMed] [Google Scholar]
- Hohn B. In vitro packaging of lambda and cosmid DNA. Methods Enzymol. 1979;68:299–309. doi: 10.1016/0076-6879(79)68021-7. [DOI] [PubMed] [Google Scholar]
- Ivanov I., Gigova L. Isolation of lambda phage DNA by hydroxylapatite chromatography. Anal Biochem. 1985 May 1;146(2):389–392. doi: 10.1016/0003-2697(85)90556-1. [DOI] [PubMed] [Google Scholar]
- KALF G. F., WHITE T. G. The antigenic components of Erysipelothrix rhusiopathiae. II. Purification and chemical characterization of a type-specific antigen. Arch Biochem Biophys. 1963 Jul;102:39–47. doi: 10.1016/0003-9861(63)90317-5. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Lachmann P. G., Deicher H. Solubilization and characterization of surface antigenic components of Erysipelothrix rhusiopathiae T28. Infect Immun. 1986 Jun;52(3):818–822. doi: 10.1128/iai.52.3.818-822.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McCracken A. W., Mauney C. U., Huber T. W., McCloskey R. V. Endocarditis caused by Erysipelothrix insidiosa. Am J Clin Pathol. 1973 Feb;59(2):219–222. doi: 10.1093/ajcp/59.2.219. [DOI] [PubMed] [Google Scholar]
- Nørrung V. Two new serotypes of Erysipelothrix rhusiopathiae. Nord Vet Med. 1979 Nov;31(11):462–465. [PubMed] [Google Scholar]
- Simerkoff M. S., Rahal J. J., Jr Acute and subacute endocarditis due to Erysipelothrix rhusopathiae. Am J Med Sci. 1973 Jul;266(1):53–57. doi: 10.1097/00000441-197307000-00008. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Sawada T., Muramatsu M., Tamura Y., Fujisawa T., Benno Y., Mitsuoka T. Serotype, antimicrobial susceptibility, and pathogenicity of Erysipelothrix rhusiopathiae isolates from tonsils of apparently healthy slaughter pigs. J Clin Microbiol. 1987 Mar;25(3):536–539. doi: 10.1128/jcm.25.3.536-539.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Takagi M., Sawada T., Seto K. Cross protection in mice and swine immunized with live erysipelas vaccine to challenge exposure with strains of Erysipelothrix rhusiopathiae of various serotypes. Am J Vet Res. 1984 Oct;45(10):2115–2118. [PubMed] [Google Scholar]
- Timoney J. The inactivation of Erysipelothrix rhuopathiae in macrophages from normal and immune mice. Res Vet Sci. 1969 May;10(3):301–302. [PubMed] [Google Scholar]
- White R. R., Verwey W. F. Isolation and Characterization of a Protective Antigen-Containing Particle from Culture Supernatant Fluids of Erysipelothrix rhusiopathiae. Infect Immun. 1970 Apr;1(4):380–386. doi: 10.1128/iai.1.4.380-386.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. R., Verwey W. F. Solubilization and Characterization of a Protective Antigen of Erysipelothrix rhusiopathiae. Infect Immun. 1970 Apr;1(4):387–393. doi: 10.1128/iai.1.4.387-393.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T. G., Puls J. L., Hargrave P. Production of synovitis in rabbits by fractions of a cell-free extract of Erysipelothrix rhusiopathiae. Clin Immunol Immunopathol. 1975 Mar;3(4):531–540. doi: 10.1016/0090-1229(75)90078-1. [DOI] [PubMed] [Google Scholar]
- Wood R. L., Harrington R., Jr Serotypes of Erysipelothrix rhusiopathiae isolated from swine and from soil and manure of swine pens in the United States. Am J Vet Res. 1978 Nov;39(11):1833–1840. [PubMed] [Google Scholar]
- Wood R. L., Haubrich D. R., Harrington R., Jr Isolation of previously unreported serotypes of Erysipelothrix rhusiopathiae from swine. Am J Vet Res. 1978 Dec;39(12):1958–1961. [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]