Abstract
Background
How phenylephrine and ephedrine treatments affect global and regional haemodynamics is of major clinical relevance. Cerebral tissue oxygen saturation ( )-guided management may improve postoperative outcome. The physiological variables responsible for
)-guided management may improve postoperative outcome. The physiological variables responsible for  changes induced by phenylephrine and ephedrine bolus treatment in anaesthetized patients need to be defined.
changes induced by phenylephrine and ephedrine bolus treatment in anaesthetized patients need to be defined.
Methods
A randomized two-treatment cross-over trial was conducted: one bolus dose of phenylephrine (100–200 µg) and one bolus dose of ephedrine (5–20 mg) were given to 29 ASA I–III patients anaesthetized with propofol and remifentanil.  , mean arterial pressure (MAP), cardiac output (CO), and other physiological variables were recorded before and after treatments. The associations of changes were analysed using linear-mixed models.
, mean arterial pressure (MAP), cardiac output (CO), and other physiological variables were recorded before and after treatments. The associations of changes were analysed using linear-mixed models.
Results
The CO decreased significantly after phenylephrine treatment [▵CO=−2.1 (1.4) litre min−1, P<0.001], but was preserved after ephedrine treatment [▵CO=0.5 (1.4) litre min−1, P>0.05]. The  was significantly decreased after phenylephrine treatment [▵
was significantly decreased after phenylephrine treatment [▵ =−3.2 (3.0)%, P<0.01] but preserved after ephedrine treatment [▵
=−3.2 (3.0)%, P<0.01] but preserved after ephedrine treatment [▵ =0.04 (1.9)%, P>0.05]. CO was identified to have the most significant association with
=0.04 (1.9)%, P>0.05]. CO was identified to have the most significant association with  (P<0.001). After taking CO into consideration, the other physiological variables, including MAP, were not significantly associated with
(P<0.001). After taking CO into consideration, the other physiological variables, including MAP, were not significantly associated with  (P>0.05).
(P>0.05).
Conclusions
Associated with changes in CO,  decreased after phenylephrine treatment, but remained unchanged after ephedrine treatment. The significant correlation between CO and
decreased after phenylephrine treatment, but remained unchanged after ephedrine treatment. The significant correlation between CO and  implies a cause–effect relationship between global and regional haemodynamics.
implies a cause–effect relationship between global and regional haemodynamics.
Keywords: cardiac output, cerebral tissue oxygen saturation, ephedrine, mean arterial pressure, phenylephrine
Editor's key points.
Effects of different vasopressor agents on cerebral oxygenation have been unclear.
Ephedrine and phenylephrine, used for intraoperative hypotension, were investigated in a cross-over design study.
Phenylephrine, but not ephedrine, decreased cardiac output (CO) and brain oxygenation.
This study highlights the importance of CO in preserving brain oxygenation during management of intraoperative hypotension.
Phenylephrine and ephedrine are routinely used in the perioperative setting to treat anaesthesia-related hypotension in order to maintain mean arterial pressure (MAP) and cerebral perfusion pressure.1 However, phenylephrine and ephedrine have very different pharmacological effects: phenylephrine is a pure α1-agonist, whereas ephedrine is a mixed-acting agent with positive inotropic and chronotropic effects.2 Indeed, the distinctive effects of phenylephrine and ephedrine on global haemodynamics (such as cardiac output, CO)3 and regional haemodynamics (such as cerebral tissue oxygen saturation,  )4 have been demonstrated.
)4 have been demonstrated.
Recently published studies show that near-infrared spectroscopy (NIRS)-guided brain protection protocols in cardiac surgery might lead to reduced neurocognitive complications and improved postoperative outcomes.5 Because the endpoint of haemodynamic optimization is to improve oxygen delivery, monitoring cerebral oxygenation may help to elucidate the effects of various clinical interventions on global and regional haemodynamics.6 Moreover, several studies have demonstrated that changes in  correlate with changes in cerebral blood flow (CBF) when cerebral metabolic rate of oxygen (CMRO2) and arterial blood oxygen content are kept constant.7 Understanding how the administration of phenylephrine and ephedrine affects cerebral perfusion and oxygenation is of major clinical relevance because both agents are routinely used to treat anaesthesia-related hypotension in surgical patients.
correlate with changes in cerebral blood flow (CBF) when cerebral metabolic rate of oxygen (CMRO2) and arterial blood oxygen content are kept constant.7 Understanding how the administration of phenylephrine and ephedrine affects cerebral perfusion and oxygenation is of major clinical relevance because both agents are routinely used to treat anaesthesia-related hypotension in surgical patients.
Consequently, the aims of our study were (i) to investigate the effect of phenylephrine and ephedrine bolus administration on cerebral oxygenation in anaesthetized patients and (ii) to identify the physiological variables [MAP, CO, heart rate (HR), stroke volume (SV), end-tidal CO2 ( ), oxygen saturation via pulse oximetry (
), oxygen saturation via pulse oximetry ( ), and bispectral index (BIS)] which are responsible for the changes in
), and bispectral index (BIS)] which are responsible for the changes in  induced by phenylephrine and ephedrine treatments.
induced by phenylephrine and ephedrine treatments.
Methods
Patients
After Institutional Research Board approval, a total of 33 patients undergoing elective surgery at University of California, Irvine Medical Center, were recruited for this study. Both verbal and written informed consents were obtained. Inclusion criteria were: age >18 yr, elective surgery, ASA physical status I–III, presenting with at least a 20% decrease in MAP or an MAP of <60 mm Hg after induction of general anaesthesia. Exclusion criteria were symptomatic cardiovascular disease, poorly controlled hypertension (systolic arterial pressure ≥160 mm Hg), cerebrovascular disease, and poorly controlled diabetes mellitus (blood glucose ≥200 mg dl−1).
Study protocol
After the patient's arrival in the operating theatre, a radial intra-arterial catheter, a BIS monitor, and two frequency-domain NIRS8 probes (left and right forehead) were placed in addition to the other routine monitors. After anaesthesia induction with fentanyl (1.5–2 µg kg−1) and propofol (2–3 mg kg−1), all patients were intubated and maintained with total i.v. anaesthesia (TIVA) using propofol 100–150 µg kg−1 min−1 and remifentanil 0.3–0.5 µg kg−1 min−1. The infusion rates of TIVA were based on the patient's age, ASA physical status, and BIS monitoring. The goal was to keep BIS between 25 and 35. An oesophageal Doppler probe was placed after tracheal intubation. Anaesthesia-related hypotension (at least a 20% decrease in MAP or MAP<60 mm Hg) was treated with either phenylephrine or ephedrine. This initial agent is referred to as the first treatment. The agent used for the first treatment was randomized based on a computer-generated randomization list (http://www.random.org). The first treatment was given at least 10 min after the start of TIVA in order to achieve relatively stable blood propofol and remifentanil concentrations. If hypotension persisted for more than 10 min after the first treatment, the alternative agent (the one not chosen for the first treatment) was then administered. This second agent is referred to as the second treatment. Each patient received one dose of phenylephrine and one dose of ephedrine as either the first or the second treatment. There were no additional doses given during the study period. Owing to interindividual differences in body weight, haemodynamic responses to pressor treatment, and severity of hypotension, varying doses of phenylephrine (100–200 µg) and ephedrine (5–20 mg) were used to increase MAP by at least 20% or above 60 mm Hg. The pressor treatments and physiological measurements were performed before the start of surgery in order to avoid the influence of surgical stimuli on systemic and cerebral haemodynamics.
Measurements
The cerebral oximeter used in this study was the Oxiplex TS (ISS, Inc., Champaign, IL, USA), a non-invasive, portable, and quantitative frequency-domain NIRS device.8 It emits and detects near-infrared light at two different wavelengths (690 and 830 nm). The emitted light is amplitude-modulated (i.e. turned on and off) at 110 MHz. The spacing between the source and detector fibres on the optical probe (1.96, 2.46, 2.92, and 3.45 cm) is sufficient for light to access the surface of the brain.9 The measured optical properties characterize cerebral tissues, primarily the haemoglobin in the capillary bed, and are not appreciably influenced by skin or surface contributions.10 The measured absolute concentrations of cerebral tissue oxyhaemoglobin and deoxyhaemoglobin are used to calculate  . The sampling frequency was set at 1.25 Hz.
. The sampling frequency was set at 1.25 Hz.  values from the right and left frontal lobes were averaged to represent regional cerebral oxygenation.
values from the right and left frontal lobes were averaged to represent regional cerebral oxygenation.
CO was monitored using an oesophageal Doppler (CardioQ, Deltex Medical, UK). The oesophageal Doppler measures blood flow velocity in the descending aorta and estimates SV via multiplying the cross-sectional area of the aorta by the blood flow distance (velocity multiplied by flow time). The aortic diameter is obtained from a built-in nomogram. The SV and CO values used for analysis were based on every 10 successive measurements by oesophageal Doppler. MAP was monitored at the external ear canal level via an intra-arterial catheter system (Vigileo-FloTrac, Edwards Lifesciences, Irvine, CA, USA).  was determined by the gas analyzer built in the anaesthesia machine (Aisys, GE Healthcare, Madison, WI, USA).
was determined by the gas analyzer built in the anaesthesia machine (Aisys, GE Healthcare, Madison, WI, USA).  was determined by pulse oximeter (LNOP Adt, Masimo Corp., Irvine, CA, USA). The depth of anaesthesia was monitored via the BIS monitor (Aspect Medical System, Norwood, MA, USA).
was determined by pulse oximeter (LNOP Adt, Masimo Corp., Irvine, CA, USA). The depth of anaesthesia was monitored via the BIS monitor (Aspect Medical System, Norwood, MA, USA).
All measurements were recorded before each treatment and repeated once MAP increased to the maximum level after each treatment. Owing to the fact that the maximal change in  lagged the maximal change in MAP (an observation in both this study and a previous study),11
lagged the maximal change in MAP (an observation in both this study and a previous study),11  measurements were recorded when corresponding changes reached the maximum level. The mean value of three successive recordings for each parameter was used for analysis. All measurements were performed before surgical incision. All patients were kept supine and still. The infusion rates of TIVA were kept constant. Volume-controlled ventilation was used with a tidal volume of 8–10 ml kg−1 and a ventilatory frequency of 8–12 bpm with a target
measurements were recorded when corresponding changes reached the maximum level. The mean value of three successive recordings for each parameter was used for analysis. All measurements were performed before surgical incision. All patients were kept supine and still. The infusion rates of TIVA were kept constant. Volume-controlled ventilation was used with a tidal volume of 8–10 ml kg−1 and a ventilatory frequency of 8–12 bpm with a target  between 4.7 and 5.3 kPa.
between 4.7 and 5.3 kPa.
Statistical analysis
Data are expressed as mean (sd). According to a previously published study, we calculated that 24 patients were required to detect a 10% decrease in  induced by phenylephrine administration with a two-tailed α risk of 5% and a β risk of 20%.4 Because this two-treatment cross-over study involved repeated measurements, we investigated the effect of drug treatment and physiological covariates (MAP, CO, HR, SV,
induced by phenylephrine administration with a two-tailed α risk of 5% and a β risk of 20%.4 Because this two-treatment cross-over study involved repeated measurements, we investigated the effect of drug treatment and physiological covariates (MAP, CO, HR, SV,  ,
,  , and BIS) on
, and BIS) on  using linear-mixed models. When testing the effect of drug treatment, we adjusted the potential effects of carry-over (the influence of the first treatment on the second treatment) by adding carry-over into our linear-mixed model. For a similar reason, when examining the effect of each physiological covariate, the effects of treatment and carry-over were also adjusted in our linear-mixed model. The differences in physiological values (
using linear-mixed models. When testing the effect of drug treatment, we adjusted the potential effects of carry-over (the influence of the first treatment on the second treatment) by adding carry-over into our linear-mixed model. For a similar reason, when examining the effect of each physiological covariate, the effects of treatment and carry-over were also adjusted in our linear-mixed model. The differences in physiological values ( , MAP, CO, HR, SV,
, MAP, CO, HR, SV,  ,
,  , and BIS) between pre- and post-treatments were analysed using paired Student's t-test. The differences in selected physiological values (
, and BIS) between pre- and post-treatments were analysed using paired Student's t-test. The differences in selected physiological values ( , MAP, and CO) between the first and the second treatments were analysed using unpaired Student's t-test. Relationships between variables were tested using Pearson's correlation.
, MAP, and CO) between the first and the second treatments were analysed using unpaired Student's t-test. Relationships between variables were tested using Pearson's correlation.
Results
Patient characteristics
Of the 33 patients recruited, we were able to administer both phenylephrine and ephedrine and finish all measurements before surgical incision in 29 patients [20 males, 9 females, age 59 (13) yr, height 173 (9) cm, weight 77 (13) kg]. Among the 29 patients, 10 were ASA I, 12 ASA II, and 7 ASA III. Detailed patients characteristics and planned surgeries are described in Supplementary Table S1. In 13 patients, phenylephrine was given as the first treatment and ephedrine as the second treatment. In 16 patients, ephedrine was given as the first treatment and phenylephrine as the second treatment. The interval between the first and the second treatments was 20 (14) min. In two patients, we did not administer phenylephrine or ephedrine because changes in MAP after anaesthesia induction did not meet the predefined criteria. In another two patients,  data were not analysable because of strong signal interference.
data were not analysable because of strong signal interference.
Responses to phenylephrine bolus treatment
An example of changes in MAP, CO, and  after a typical first phenylephrine treatment is illustrated in Figure 1a–c, respectively. MAP increased from the pretreatment level of ≈70 mm Hg to the highest level of ≈110 mm Hg within 1 min after phenylephrine administration. At the same time, CO decreased from the pretreatment level of ≈8 litre min−1 to the lowest level of ≈2 litre min−1, and
after a typical first phenylephrine treatment is illustrated in Figure 1a–c, respectively. MAP increased from the pretreatment level of ≈70 mm Hg to the highest level of ≈110 mm Hg within 1 min after phenylephrine administration. At the same time, CO decreased from the pretreatment level of ≈8 litre min−1 to the lowest level of ≈2 litre min−1, and  decreased from the pretreatment level of ≈65% to the lowest level of ≈58%. The measurements of MAP, CO, and
decreased from the pretreatment level of ≈65% to the lowest level of ≈58%. The measurements of MAP, CO, and  before and after phenylephrine treatment for every patient are presented in Figure 2a–c, respectively.
before and after phenylephrine treatment for every patient are presented in Figure 2a–c, respectively.
Fig 1.
Continuous MAP, CO, and 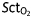 recordings from two selected patients. (a–c) Recordings during phenylephrine treatment. (d–f) Recordings during ephedrine treatment. Both agents were given during the first treatment. Vertical arrows indicate the drug administration time.
recordings from two selected patients. (a–c) Recordings during phenylephrine treatment. (d–f) Recordings during ephedrine treatment. Both agents were given during the first treatment. Vertical arrows indicate the drug administration time.
Fig 2.
Measurements of MAP, CO, and 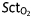 for every patient. (a–c) Measurements before (pre) and after (post) phenylephrine treatments. (d–f) Measurements before (pre) and after (post) ephedrine treatments.
for every patient. (a–c) Measurements before (pre) and after (post) phenylephrine treatments. (d–f) Measurements before (pre) and after (post) ephedrine treatments.
Grouped responses after the first and the second phenylephrine treatments are summarized in Table 1. MAP was consistently increased after the first [▵MAP=29.5 (9.3) mm Hg, P<0.001] and the second [▵MAP=42.6 (15.7) mm Hg, P<0.001] phenylephrine treatments. CO was significantly decreased after the first (▵CO=−1.7 (1.0) litre min−1, P<0.001) and the second (▵CO=−2.3 (1.7) litre min−1, P<0.001) phenylephrine treatments.  was also significantly decreased after the first (▵
was also significantly decreased after the first (▵ =−4.9 (2.8) %, P<0.001) and the second (▵
=−4.9 (2.8) %, P<0.001) and the second (▵ =−1.8 (2.4) %, P<0.01) phenylephrine treatments. However, the difference in
=−1.8 (2.4) %, P<0.01) phenylephrine treatments. However, the difference in  decreases between the first and the second phenylephrine treatments was significant (P<0.01; Fig. 3).
decreases between the first and the second phenylephrine treatments was significant (P<0.01; Fig. 3).
Table 1.
Summarized physiological measurements before (pre) and after (post) treatments (Tx). Data are presented as means (sd). ▵=post–pre; 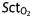 , cerebral tissue oxygen saturation; MAP, mean arterial pressure; CO, cardiac output; HR, heart rate (beats min−1); SV, stroke volume;
, cerebral tissue oxygen saturation; MAP, mean arterial pressure; CO, cardiac output; HR, heart rate (beats min−1); SV, stroke volume;  , end-tidal CO2;
, end-tidal CO2;  , oxygen saturation per pulse oximetry; BIS, bispectral index. *P<0.001, †P<0.01, and ‡P<0.05 (post vs pre, paired Student's t-test)
, oxygen saturation per pulse oximetry; BIS, bispectral index. *P<0.001, †P<0.01, and ‡P<0.05 (post vs pre, paired Student's t-test)
| Phenylephrine first Tx (n=13) |
Phenylephrine second Tx (n=16) |
Ephedrine first Tx (n=16) |
Ephedrine second Tx (n=13) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Δ | Pre | Post | Δ | Pre | Post | Δ | Pre | Post | Δ | |
 (%) (%) |
68.8 (8.8) | 63.9 (10.4) | −4.9 (2.8)* | 66.4 (6.7) | 64.5 (6.7) | −1.8 (2.4)† | 67.4 (6.3) | 67.1 (6.0) | −0.4 (2.3) | 65.7 (8.5) | 66.2 (8.9) | 0.5 (1.1) |
| MAP (mm Hg) | 60.8 (12.1) | 90.3 (14.5) | 29.5 (9.3)* | 58.8 (9.3) | 101.3 (14.7) | 42.6 (15.7)* | 48.1 (8.9) | 72.3 (10.7) | 24.1 (13.5)* | 62.4 (5.3) | 90.7 (13.7) | 28.3 (13.3)* |
| CO (litre min−1) | 5.3 (1.1) | 3.6 (0.7) | −1.7 (1.0)* | 6.8 (1.7) | 4.5 (2.2) | −2.3 (1.7)* | 6.0 (1.8) | 6.5 (1.8) | 0.5 (1.7) | 5.0 (0.9) | 5.4 (1.0) | 0.4 (0.9) |
| HR (beats min−1) | 71.2 (15.2) | 53.9 (8.6) | −17.3 (11.4)* | 64.7 (12.1) | 48.0 (6.6) | −16.7 (12.1)* | 65.3 (14.1) | 67.6 (12.6) | 2.3 (5.8)† | 59.5 (9.6) | 67.0 (11.3) | 7.5 (7.8) |
| SV (ml) | 77.2 (16.7) | 68.7 (14.4) | −8.5 (9.2)† | 105.8 (31.0) | 90.7 (39.5) | −15.2 (22.3)‡ | 92.2 (30.4) | 97.4 (29.7) | 5.3 (21.6) | 85.5 (17.9) | 83.1 (18.0) | −2.4 (11.9) |
 (kPa) (kPa) |
5.1 (0.7) | 4.9 (0.6) | −0.2 (0.3)‡ | 4.7 (0.4) | 4.6 (0.5) | −0.1 (0.3) | 4.7 (0.4) | 4.7 (0.4) | 0.04 (0.3) | 4.9 (0.7) | 5.0 (0.6) | 0.2 (0.3) |
 (%) (%) |
99.5 (0.7) | 99.9 (0.3) | 0.4 (0.7) | 99.1 (1.4) | 99.5 (1.2) | 0.4 (0.7) | 99.3 (1.4) | 99 (2.7) | −0.3 (1.8) | 99.1 (1.9) | 99.4 (0.9) | 0.3 (1.2) |
| BIS | 25.5 (11.9) | 27.8 (11.4) | 2.3 (5.2) | 34.9 (7.8) | 30.5 (11.0) | −4.3 (8.4) | 25.7 (12.5) | 26.5 (8.5) | 0.7 (6.0) | 29.8 (12.9) | 29.0 (11.2) | −0.9 (3.3) |
Fig 3.
Grouped MAP (a), CO (b), and 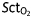 (c) changes after the first and second phenylephrine (Phe) and the first and second ephedrine (Eph) treatments. The blue bars represent pretreatment values; the green striped bars represent post-treatment values. *P<0.01 (first treatment vs second treatment, unpaired Student's t-test).
(c) changes after the first and second phenylephrine (Phe) and the first and second ephedrine (Eph) treatments. The blue bars represent pretreatment values; the green striped bars represent post-treatment values. *P<0.01 (first treatment vs second treatment, unpaired Student's t-test).
Changes in  correlated well with changes in CO after the first (r=0.74, P=0.004) and the second (r=0.67, P=0.005) phenylephrine treatments (Fig. 4b), but only weakly correlated with changes in MAP after the first (r=0.40, P=0.17) and the second (r=0.48, P=0.06) phenylephrine treatments (Fig. 4a).
correlated well with changes in CO after the first (r=0.74, P=0.004) and the second (r=0.67, P=0.005) phenylephrine treatments (Fig. 4b), but only weakly correlated with changes in MAP after the first (r=0.40, P=0.17) and the second (r=0.48, P=0.06) phenylephrine treatments (Fig. 4a).
Fig 4.
Pearson's correlations between 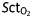 and global haemodynamics (MAP and CO) during the first and second phenylephrine (a and b) and the first and second ephedrine treatments (Tx) (c and d). ▵=post–pre.
and global haemodynamics (MAP and CO) during the first and second phenylephrine (a and b) and the first and second ephedrine treatments (Tx) (c and d). ▵=post–pre.
Responses to ephedrine bolus treatment
An example of changes in MAP, CO, and  after one of the first ephedrine treatments is illustrated in Figure 1d–f, respectively. MAP increased from the pretreatment level of ≈50 mm Hg to the highest level of ≈80 mm Hg within 2 min after ephedrine administration. However, CO remained unchanged at ≈5 litre min−1 and
after one of the first ephedrine treatments is illustrated in Figure 1d–f, respectively. MAP increased from the pretreatment level of ≈50 mm Hg to the highest level of ≈80 mm Hg within 2 min after ephedrine administration. However, CO remained unchanged at ≈5 litre min−1 and  remained unchanged at ≈62%. The measurements of MAP, CO, and
remained unchanged at ≈62%. The measurements of MAP, CO, and  before and after ephedrine treatment for every patient are presented in Figure 2d–f, respectively.
before and after ephedrine treatment for every patient are presented in Figure 2d–f, respectively.
Grouped responses after the first and second ephedrine treatments are summarized in Table 1. MAP was consistently increased after the first [▵MAP=24.1 (13.5) mm Hg, P<0.001] and the second [▵MAP=28.3 (13.3) mm Hg, P<0.001] ephedrine treatments. CO was slightly, but insignificantly, increased after the first [▵CO=0.5 (1.7) litre min−1, P=0.15] and the second [▵CO=0.4 (0.9) litre min−1, P=0.28] ephedrine treatments. The changes in  were also insignificant after the first [▵
were also insignificant after the first [▵ =−0.4 (2.3)%, P=0.11] and the second [▵
=−0.4 (2.3)%, P=0.11] and the second [▵ =0.5 (1.1)%, P=0.54] ephedrine treatments. The difference in
=0.5 (1.1)%, P=0.54] ephedrine treatments. The difference in  changes between the first and second ephedrine treatments was not significant (P=0.19) (Fig. 3).
changes between the first and second ephedrine treatments was not significant (P=0.19) (Fig. 3).
Changes in  correlated with changes in CO after the first (r=0.84, P<0.001) and the second (r=0.68, P=0.01) ephedrine treatments (Fig. 4d), but very weakly correlated with changes in MAP after the first (r=0.24, P=0.38) and the second (r=0.39, P=0.18) ephedrine treatments (Fig. 4c).
correlated with changes in CO after the first (r=0.84, P<0.001) and the second (r=0.68, P=0.01) ephedrine treatments (Fig. 4d), but very weakly correlated with changes in MAP after the first (r=0.24, P=0.38) and the second (r=0.39, P=0.18) ephedrine treatments (Fig. 4c).
Associations between  and physiological covariates (pooled data)
and physiological covariates (pooled data)
We first fitted a linear-mixed model to examine the effects of treatment and carryover on  . Our results showed that the treatment effect on
. Our results showed that the treatment effect on  was significant (P<0.001) and that the carry-over effect on
was significant (P<0.001) and that the carry-over effect on  was not significant (P=0.11). After adjusting the effects of treatment and carryover on
was not significant (P=0.11). After adjusting the effects of treatment and carryover on  , linear-mixed models showed that there were significant associations (in the order of significance from high to low, data available upon request) between
, linear-mixed models showed that there were significant associations (in the order of significance from high to low, data available upon request) between  and CO (P<0.001), between
and CO (P<0.001), between  and SV (P<0.001), between
and SV (P<0.001), between  and HR (P<0.001), between
and HR (P<0.001), between  and MAP (P<0.001), and between
and MAP (P<0.001), and between  and
and  (P<0.01); however, there were no significant associations between
(P<0.01); however, there were no significant associations between  and
and  (P=0.60) and between
(P=0.60) and between  and BIS (P=1.0). After taking CO into consideration, SV (P=0.85), HR (P=0.95), MAP (P=0.48), and
and BIS (P=1.0). After taking CO into consideration, SV (P=0.85), HR (P=0.95), MAP (P=0.48), and  (P=0.64) were no longer significantly associated with
(P=0.64) were no longer significantly associated with  . Further analysis showed that the associations between CO and SV (P<0.001), between CO and HR (P<0.001), between CO and MAP (P<0.001), and between CO and
. Further analysis showed that the associations between CO and SV (P<0.001), between CO and HR (P<0.001), between CO and MAP (P<0.001), and between CO and  (P<0.001) were all significant.
(P<0.001) were all significant.
Discussion
This study demonstrates that concordant with changes in CO, cerebral oxygenation ( ) significantly decreased after phenylephrine bolus treatment and remained unchanged after ephedrine bolus treatment, even though MAP was significantly increased by both agents. Among all physiological variables being considered (MAP, CO, HR, SV,
) significantly decreased after phenylephrine bolus treatment and remained unchanged after ephedrine bolus treatment, even though MAP was significantly increased by both agents. Among all physiological variables being considered (MAP, CO, HR, SV,  ,
,  , and BIS), CO was identified as the variable associated most significantly with
, and BIS), CO was identified as the variable associated most significantly with  . The other variables (MAP, HR, SV, and
. The other variables (MAP, HR, SV, and  ) which associated significantly with
) which associated significantly with  became insignificant after taking CO into consideration.
became insignificant after taking CO into consideration.
Cerebral oxygenation is determined by oxygen delivery to the brain and oxygen consumption by the brain (CMRO2). Oxygen delivery to the brain depends on cerebral perfusion (CBF) and arterial blood oxygen content. Studies have shown that changes in  correlate with changes in CBF if CMRO2 and arterial blood oxygen content are kept constant.7 In the present study, we considered CMRO2 to be constant because our patients were under general Anaesthesia and the infusion rates of propofol and remifentanil were kept constant. Moreover, in order to achieve stable blood propofol and remifentanil concentrations, we waited for at least 10 min between starting TIVA and giving the first pressor treatment. We also considered arterial blood oxygen content to be constant because there was no surgical haemorrhage and no sign of desaturation. Therefore, we submit that the observed changes of
correlate with changes in CBF if CMRO2 and arterial blood oxygen content are kept constant.7 In the present study, we considered CMRO2 to be constant because our patients were under general Anaesthesia and the infusion rates of propofol and remifentanil were kept constant. Moreover, in order to achieve stable blood propofol and remifentanil concentrations, we waited for at least 10 min between starting TIVA and giving the first pressor treatment. We also considered arterial blood oxygen content to be constant because there was no surgical haemorrhage and no sign of desaturation. Therefore, we submit that the observed changes of  in this study were mainly caused by changes in CBF.
in this study were mainly caused by changes in CBF.
The importance of arterial pressure management in patients undergoing anaesthesia has been substantiated by the significant relationship between intraoperative hypotension and postoperative neurocognitive impairment.12 Despite the fact that arterial pressure monitoring is a standard practice, consensus in terms of when and how to treat intraoperative hypotension is still lacking. Among all options, phenylephrine and ephedrine belong to the set of typical sympathomimetic agents routinely chosen to increase arterial pressure.2 However, little is known about the impacts of these agents on cerebral oxygenation and the relationship between global and regional haemodynamics. If treating hypotension is an attempt to avoid organ ischaemia and hypoxia, we are actually achieving the opposite result (decreased cerebral oxygenation) by administering phenylephrine, as demonstrated in this study using a quantitative NIRS device and in previous studies using a trend NIRS device.4,11,13 Another study also demonstrated the negative impact of norepinephrine infusion on cerebral oxygenation.14 Thus, the routine and indiscriminate use of vasopressors might be less beneficial than previously thought. Nonetheless, prospective and randomized studies are needed to address whether the negative impact of vasopressor treatment on  relates to adverse patient outcomes, especially because recent studies have suggested that low
relates to adverse patient outcomes, especially because recent studies have suggested that low  values are related to poor postoperative outcome.5 In contrast, ephedrine's
values are related to poor postoperative outcome.5 In contrast, ephedrine's  -preserving ability is reassuring. However, studies are again needed to demonstrate whether or not the administration of ephedrine rather than phenylephrine relates to a better outcome.
-preserving ability is reassuring. However, studies are again needed to demonstrate whether or not the administration of ephedrine rather than phenylephrine relates to a better outcome.
The mechanism of how phenylephrine administration leads to a decreased cerebral oxygenation is intriguing. It has been found that sympathetic nerve activity (SNA) originating from the superior cervical ganglion increases promptly after pharmacologically (including phenylephrine) induced rapid increase in arterial pressure.15 Considering that the cerebral vasculature is largely innervated by the superior cervical ganglion,16 we speculate that phenylephrine bolus treatment may constrict cerebral resistance vessels indirectly via reflexively increased SNA to the brain. This assertion is supported by the findings that cerebral arteries are abundantly innervated by sympathetic nerve fibres17 and that both α- and β-adrenoceptors are demonstrated in the vascular walls in the brain.18 It is also supported by the finding that stellate ganglion block leads to a decreased cerebral vascular tone.19 Nonetheless, the discussion over whether or not SNA affects cerebral perfusion and oxygenation has lasted for more than 100 yr, witnessed by the recently well-organized point20–counterpoint21 debate. It should be noted that direct action of either phenylephrine or ephedrine on cerebral resistance vessels is practically nil since we know that vasoactive amines do not cross the blood–brain barrier.22
To the best of our knowledge, this study is the first one to demonstrate a significant relationship between global haemodynamics (CO) and regional haemodynamics ( ) in situations where CO changes are induced by sympathomimetic agents in anaesthetized patients. The distinctive effects of phenylephrine and ephedrine on
) in situations where CO changes are induced by sympathomimetic agents in anaesthetized patients. The distinctive effects of phenylephrine and ephedrine on  are thus explained by their distinctive impacts on CO. Our data concur with previous reports that a reduced CO correlates with decreased cerebral haemodynamics, despite maintained MAP in situations where CO changes are induced by preload swing in healthy non-anaesthetized volunteers.23,24 The mechanism behind the modulation of cerebral haemodynamics by CO is believed to be sympathetically mediated vasoconstriction consequent to a reduced CO.20 This assertion is supported by the finding that dynamic inputs from CO and SV are important in the regulation of baroreflex control of muscle SNA in healthy, normotensive humans.25 Alternatively, the influence of CO on cerebral haemodynamics may depend on circulating blood volume distribution rather than autonomic control.20 Consequently, our study emphasizes the relationship between global and regional haemodynamics and supports the importance of studies focusing on this relationship as well as studies evaluating the impact of regional haemodynamics on patients' outcome.26,27 Our finding also supports the emerging practice of goal-directed haemodynamic optimization because optimized global haemodynamics is related to a minimized risk of regional ischaemia and hypoxia.28
are thus explained by their distinctive impacts on CO. Our data concur with previous reports that a reduced CO correlates with decreased cerebral haemodynamics, despite maintained MAP in situations where CO changes are induced by preload swing in healthy non-anaesthetized volunteers.23,24 The mechanism behind the modulation of cerebral haemodynamics by CO is believed to be sympathetically mediated vasoconstriction consequent to a reduced CO.20 This assertion is supported by the finding that dynamic inputs from CO and SV are important in the regulation of baroreflex control of muscle SNA in healthy, normotensive humans.25 Alternatively, the influence of CO on cerebral haemodynamics may depend on circulating blood volume distribution rather than autonomic control.20 Consequently, our study emphasizes the relationship between global and regional haemodynamics and supports the importance of studies focusing on this relationship as well as studies evaluating the impact of regional haemodynamics on patients' outcome.26,27 Our finding also supports the emerging practice of goal-directed haemodynamic optimization because optimized global haemodynamics is related to a minimized risk of regional ischaemia and hypoxia.28
Interestingly, our data showed that the difference in  changes was significant between the first and second phenylephrine treatments (P<0.01) and not significant between the first and second ephedrine treatments (P=0.19) based on unpaired Student's t-test. These results suggest that the effect of phenylephrine treatment on cerebral haemodynamics is negated by the previous ephedrine treatment. In contrast, the effect of ephedrine treatment is less affected by phenylephrine. This finding may be caused by the longer clinical half-life of ephedrine than phenylephrine (clinical observation). Our analysis of the carry-over effect based on linear-mixed models showed that this is not significant (P=0.11) at the 0.05 level. This might be due to the fact that testing carry-over effects usually requires a larger sample size than that of our study.
changes was significant between the first and second phenylephrine treatments (P<0.01) and not significant between the first and second ephedrine treatments (P=0.19) based on unpaired Student's t-test. These results suggest that the effect of phenylephrine treatment on cerebral haemodynamics is negated by the previous ephedrine treatment. In contrast, the effect of ephedrine treatment is less affected by phenylephrine. This finding may be caused by the longer clinical half-life of ephedrine than phenylephrine (clinical observation). Our analysis of the carry-over effect based on linear-mixed models showed that this is not significant (P=0.11) at the 0.05 level. This might be due to the fact that testing carry-over effects usually requires a larger sample size than that of our study.
The main methodological considerations are as follows. The method of phenylephrine and ephedrine administration in this study was bolus, not infusion. The effects of bolus and infusion administrations on systemic and cerebral haemodynamics may be different. For example, the gradual increase in MAP caused by infusion might not be able to elicit the same increase in SNA in the superior cervical ganglion as that seen with bolus. A comparison study between bolus and infusion would be informative. Secondly, we used  based on NIRS measurements to assess cerebral haemodynamics. Middle cerebral artery flow velocity (MCAV) based on transcranial Doppler (TCD) measurement, which is also non-invasive and portable, is another technology being used for the same purpose. However, MCAV may not provide a valid CBF estimation should vessel calibre or flow profile change.29 Indeed, in studies where both technologies were adopted, it was found that MCAV increased, whereas
based on NIRS measurements to assess cerebral haemodynamics. Middle cerebral artery flow velocity (MCAV) based on transcranial Doppler (TCD) measurement, which is also non-invasive and portable, is another technology being used for the same purpose. However, MCAV may not provide a valid CBF estimation should vessel calibre or flow profile change.29 Indeed, in studies where both technologies were adopted, it was found that MCAV increased, whereas  decreased after phenylephrine bolus and infusion administration in healthy non-anaesthetized volunteers.11,13 One of the possible explanations for this discrepancy lies in the fact that TCD measures flow velocity in large cerebral arteries, whereas NIRS measures oxygen saturation mainly at the capillary bed.
decreased after phenylephrine bolus and infusion administration in healthy non-anaesthetized volunteers.11,13 One of the possible explanations for this discrepancy lies in the fact that TCD measures flow velocity in large cerebral arteries, whereas NIRS measures oxygen saturation mainly at the capillary bed.
In summary, associated with the changes in CO, cerebral oxygenation decreases after phenylephrine but remains unchanged after ephedrine bolus treatment in anaesthetized patients, even though both agents consistently increase MAP. The significant correlation between CO and  implies a cause–effect relationship between global haemodynamics (CO) and regional haemodynamics (
implies a cause–effect relationship between global haemodynamics (CO) and regional haemodynamics ( ).
).
Supplementary material
Supplementary material is available at British Journal of Anaesthesia online.
Conflict of interest
The authors (A.E.C., B.J.T., and W.W.M.) consult for ISS, Inc.
Funding
This study is supported by the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) through the following programmes: the Institute for Clinical and Translational Science (ICTS) grant UL1 RR031985 (B.J.T.); the Laser Microbeam and Medical Program [LAMMP, an NIH BRTP resource (P41-RR01192)] (B.J.T.); and the Laboratory for Fluorescence Dynamics (LFD) grant P41 RR03155 (W.W.M.). It is also supported by the Department of Anesthesiology and Perioperative Care, University of California, Irvine, CA, USA.
Supplementary Material
Acknowledgements
We acknowledge the generous loan of the Oxiplex TX oximeter from ISS, Inc. Special thanks go to Debra E. Morrison, MD, for her insightful discussions.
References
- 1.Sookplung P, Siriussawakul A, Malakouti A, et al. Vasopressor use and effect on blood pressure after severe adult traumatic brain injury. Neurocrit Care. doi: 10.1007/s12028-010-9448-9. Online FirstTM 2010, September 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moss J, Glick D. The autonomic nervous system. In: Miller RD, editor. Miller's Anesthesia. 6th Edn. Philadelphia: Elsevier Churchill Livingstone; 2005. pp. 617–77. [Google Scholar]
- 3.Dyer RA, Reed AR, van Dyk D, et al. Hemodynamic effects of ephedrine, phenylephrine, and the coadministration of phenylephrine with oxytocin during spinal anesthesia for elective cesarean delivery. Anesthesiology. 2009;111:753–65. doi: 10.1097/ALN.0b013e3181b437e0. doi:10.1097/ALN.0b013e3181b437e0. [DOI] [PubMed] [Google Scholar]
- 4.Nissen P, Brassard P, Jørgensen TB, et al. Phenylephrine but not ephedrine reduces frontal lobe oxygenation following anesthesia-induced hypotension. Neurocrit Care. 2010;12:17–23. doi: 10.1007/s12028-009-9313-x. doi:10.1007/s12028-009-9313-x. [DOI] [PubMed] [Google Scholar]
- 5.Vohra HA, Modi A, Ohri SK. Does use of intra-operative cerebral regional oxygen saturation monitoring during cardiac surgery lead to improved clinical outcomes? Interact Cardiovasc Thorac Surg. 2009;9:318–22. doi: 10.1510/icvts.2009.206367. doi:10.1510/icvts.2009.206367. [DOI] [PubMed] [Google Scholar]
- 6.Murkin JM, Arango M. Near-infrared spectroscopy as an index of brain and tissue oxygenation. Br J Anaesth. 2009;103:i3–13. doi: 10.1093/bja/aep299. doi:10.1093/bja/aep299. [DOI] [PubMed] [Google Scholar]
- 7.Wong FY, Nakamura M, Alexiou T, et al. Tissue oxygenation index measured using spatially resolved spectroscopy correlates with changes in cerebral blood flow in newborn lambs. Intensive Care Med. 2009;35:1464–70. doi: 10.1007/s00134-009-1486-4. doi:10.1007/s00134-009-1486-4. [DOI] [PubMed] [Google Scholar]
- 8.Fantini S, Franceschini-Fantini MA, Maier JS, et al. Frequency-domain multichannel optical detector for non-invasive tissue spectroscopy and oximetry. Opt Eng. 1995;34:32–42. doi:10.1117/12.183988. [Google Scholar]
- 9.Choi J, Wolf M, Toronov V, et al. Noninvasive determination of the optical properties of adult brain: near-infrared spectroscopy approach. J Biomed Opt. 2004;9:221–9. doi: 10.1117/1.1628242. doi:10.1117/1.1628242. [DOI] [PubMed] [Google Scholar]
- 10.Liu H, Chance B, Hielscher AH, et al. Influence of blood vessels on the measurement of hemoglobin oxygenation as determined by time-resolved reflectance spectroscopy. Med Phys. 1995;22:1209–17. doi: 10.1118/1.597520. doi:10.1118/1.597520. [DOI] [PubMed] [Google Scholar]
- 11.Brassard P, Seifert T, Wissenberg M, et al. Phenylephrine decreases frontal lobe oxygenation at rest but not during moderately intense exercise. J Appl Physiol. 2010;108:1472–8. doi: 10.1152/japplphysiol.01206.2009. doi:10.1152/japplphysiol.01206.2009. [DOI] [PubMed] [Google Scholar]
- 12.Yocum GT, Gaudet JG, Teverbaugh LA, et al. Neurocognitive performance in hypertensive patients after spine surgery. Anesthesiology. 2009;110:254–61. doi: 10.1097/ALN.0b013e3181942c7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lucas SJ, Tzeng YC, Galvin SD, et al. Influence of changes in blood pressure on cerebral perfusion and oxygenation. Hypertension. 2010;55:698–705. doi: 10.1161/HYPERTENSIONAHA.109.146290. doi:10.1161/HYPERTENSIONAHA.109.146290. [DOI] [PubMed] [Google Scholar]
- 14.Brassard P, Seifert T, Secher NH. Is cerebral oxygenation negatively affected by infusion of norepinephrine in healthy subjects? Br J Anaesth. 2009;102:800–5. doi: 10.1093/bja/aep065. doi:10.1093/bja/aep065. [DOI] [PubMed] [Google Scholar]
- 15.Cassaglia PA, Griffiths RI, Walker AM. Sympathetic nerve activity in the superior cervical ganglia increases in response to imposed increases in arterial pressure. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1255–61. doi: 10.1152/ajpregu.00332.2007. doi:10.1152/ajpregu.00332.2007. [DOI] [PubMed] [Google Scholar]
- 16.Arbab MA, Wiklund L, Svendgaard NA. Origin and distribution of cerebral vascular innervation from superior cervical, trigeminal and spinal ganglia investigated with retrograde and anterograde WGA-HRP tracing in the rat. Neuroscience. 1986;19:695–708. doi: 10.1016/0306-4522(86)90293-9. doi:10.1016/0306-4522(86)90293-9. [DOI] [PubMed] [Google Scholar]
- 17.Hamel E. Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol. 2006;100:1059–64. doi: 10.1152/japplphysiol.00954.2005. doi:10.1152/japplphysiol.00954.2005. [DOI] [PubMed] [Google Scholar]
- 18.Edvinsson L, Owman C. Pharmacological characterization of adrenergic alpha and beta receptors mediating the vasomotor responses of cerebral arteries in vitro. Circ Res. 1974;35:835–49. doi: 10.1161/01.res.35.6.835. [DOI] [PubMed] [Google Scholar]
- 19.Gupta MM, Bithal PK, Dash HH, et al. Effects of stellate ganglion block on cerebral haemodynamics as assessed by transcranial Doppler ultrasonography. Br J Anaesth. 2005;95:669–73. doi: 10.1093/bja/aei230. doi:10.1093/bja/aei230. [DOI] [PubMed] [Google Scholar]
- 20.van Lieshout JJ, Secher NH. Point:counterpoint: sympathetic activity does/does not influence cerebral blood flow. Point: sympathetic activity does influence cerebral blood flow. J Appl Physiol. 2008;105:1364–6. doi: 10.1152/japplphysiol.90597.2008. doi:10.1152/japplphysiol.90597.2008. [DOI] [PubMed] [Google Scholar]
- 21.Strandgaard S, Sigurdsson ST. Point:counterpoint: Sympathetic activity does/does not influence cerebral blood flow. Counterpoint: Sympathetic nerve activity does not influence cerebral blood flow. J Appl Physiol. 2008;105:1366–7. doi: 10.1152/japplphysiol.90597.2008a. doi:10.1152/japplphysiol.90597.2008a. [DOI] [PubMed] [Google Scholar]
- 22.Olesen J. The effect of intracarotid epinephrine, norepinephrine, and angiotensin on the regional cerebral blood flow in man. Neurology. 1972;22:978–87. doi: 10.1212/wnl.22.9.978. [DOI] [PubMed] [Google Scholar]
- 23.van Lieshout JJ, Pott F, Madsen PL, et al. Muscle tensing during standing: effects on cerebral tissue oxygenation and cerebral artery blood velocity. Stroke. 2001;32:1546–51. doi: 10.1161/01.str.32.7.1546. [DOI] [PubMed] [Google Scholar]
- 24.Ogoh S, Brothers RM, Barnes Q, et al. The effect of changes in cardiac output on middle cerebral artery mean blood velocity at rest and during exercise. J Physiol. 2005;569:697–704. doi: 10.1113/jphysiol.2005.095836. doi:10.1113/jphysiol.2005.095836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charkoudian N, Joyner MJ, Johnson CP, et al. Balance between cardiac output and sympathetic nerve activity in resting humans: role in arterial pressure regulation. J Physiol. 2005;568:315–21. doi: 10.1113/jphysiol.2005.090076. doi:10.1113/jphysiol.2005.090076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jhanji S, Lee C, Watson D, et al. Microvascular flow and tissue oxygenation after major abdominal surgery: association with post-operative complications. Intensive Care Med. 2009;35:671–7. doi: 10.1007/s00134-008-1325-z. doi:10.1007/s00134-008-1325-z. [DOI] [PubMed] [Google Scholar]
- 27.Jhanji S, Vivian-Smith A, Lucena-Amaro S, et al. Haemodynamic optimisation improves tissue microvascular flow and oxygenation after major surgery: a randomised controlled trial. Crit Care. 2010;14:R151. doi: 10.1186/cc9220. doi:10.1186/cc9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giglio MT, Marucci M, Testini M, et al. Goal-directed haemodynamic therapy and gastrointestinal complications in major surgery: a meta-analysis of randomized controlled trials. Br J Anaesth. 2009;103:637–46. doi: 10.1093/bja/aep279. doi:10.1093/bja/aep279. [DOI] [PubMed] [Google Scholar]
- 29.Taylor GA, Short BL, Walker LK, et al. Intracranial blood flow: quantification with duplex Doppler and color Doppler flow US. Radiology. 1990;176:231–6. doi: 10.1148/radiology.176.1.2112768. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






