In this issue of Molecular Cell, Skourti-Stathaki et al. report that human Senataxin, like its yeast homolog Sen1, promotes termination by RNA polymerase II and resolves RNA/DNA duplexes formed during transcription. Their results may help uncover a cause of motor neuron degeneration.
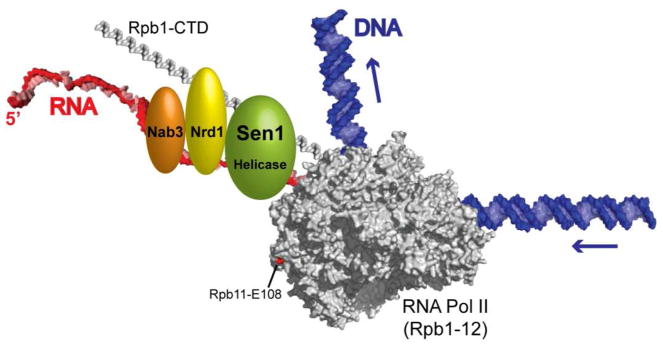
Transcription termination by eukaryotic RNA polymerase II (Pol II) must be tightly controlled. How else could the same core enzyme synthesize both a 200-nucleotide long small nuclear (sn) RNA and the >2 million-nucleotide long dystrophin pre-mRNA? The stop sign for Pol II is recognized in the nascent transcript rather than the DNA, and differs for different classes of transcripts. The termination signal for most mRNAs is the poly(A) signal, so cleavage and polyadenylation factors also act as termination factors (Kuehner et al., 2011). The termination factors for short, non-coding Pol II transcripts, such as snRNAs and small nucleolar (sno) RNAs, were first identified in yeast, and include the Sen1 helicase and two RNA-binding proteins, Nrd1 and Nab3 (Steinmetz et al., 2001). These three proteins associate directly or indirectly with Pol II by binding to each other and to the C-terminal domain (CTD) of the largest Pol II subunit, Rpb1 (Fig. 1). Binding of Nrd1 and/or Nab3 to specific sequences in the nascent transcript somehow results in Sen1-dependent termination of Pol II transcription. Humans have a Sen1 homolog, but it was unknown if it also functions in Pol II termination. In this issue of Molecular Cell, Skourti-Stathaki et al. (2011) provide direct evidence for the function of human Sen1 in transcription termination.
Figure 1.
Schematic of the yeast Sen1-dependent termination machinery scanning a nascent transcript. Elongating yeast Pol II is shown in gray (PDB #1Y1W), with the Rpb1-C-terminal domain (PDB #1SZA), DNA and RNA modeled in. The red residue in the Rpb11 subunit of Pol II causes Sen1-dependent terminator read-through when mutated (Steinmetz et al., 2006a). The order of the Sen1, Nrd1 and Nab3 proteins along the nascent transcript and Rpb1-CTD is unknown.
Interest in human Sen1 is heightened by the discovery that mutations in its gene (SETX) result in two diseases caused by motor neuron degeneration: ataxia ocular apraxia type 2 (AOA2) in the case of recessive mutations and amyotrophic lateral sclerosis type 4 (ALS4) in the case of dominant mutations (Lemmens et al., 2010). Thus, human Sen1 is called Senataxin, short for Sen1 homolog associated with ataxia (lack of muscle coordination). It is intriguing that AOA2 is strongly correlated with elevated alpha-fetoprotein levels in the serum, consistent with misregulation of gene expression (Anheim et al., 2009).
In the current study, Skourti-Stathaki and coworkers depleted Senataxin from HeLa cells by RNAi and observed read-through of the Pol II terminators of two transiently transfected reporter constructs, as well as the endogenous beta-actin gene. Chromatin immunoprecipitation across the beta-actin gene showed that Senataxin is enriched between the poly(A) site and a downstream transcription pause site that enhances termination, consistent with a direct role of Senataxin in the process. Why does depletion of Senataxin affect termination on protein-coding genes? While genes for short, non-coding transcripts are a primary target of yeast Sen1, some protein-coding yeast genes also require Sen1 for efficient termination (Steinmetz et al., 2006b; Rondon et al. 2009). Thus, the human and yeast results are congruent.
The mechanism of Sen1-dependent termination is still a mystery. Sen1 may be functionally analogous to the bacterial Rho termination factor (Peters et al., 2009). Like Rho, Sen1 is a 5′-to-3′ RNA translocase in vitro (Kim et al., 1999), and so could track down the nascent transcript until it collides with Pol II. As proposed previously for Rho, Sen1 could then either yank the nascent transcript out of the Pol II active site or alter Pol II’s conformation to induce termination. Of possible relevance to the latter model is the fact that a substitution in the Rpb11 subunit at the back end of yeast Pol II induces read-through of some Sen1-dependent terminators (Fig. 1).
However, the ability of Sen1 to unwind RNA/DNA duplexes in vitro (Kim et al., 1999) compels the consideration of alternative mechanisms of termination. Indeed, Mischo et al. (2011) found that a mutation in yeast Sen1 that inhibits Pol II termination results in accumulation of RNA/DNA hybrids (R-loops) along genes in vivo, indicating that unwinding of R-loops may be an important step in termination. Skourti-Stathaki and coworkers obtained similar results with Senataxin-depleted HeLa cells, leading them to propose that R-loops are more common in vivo than previously believed, and that their resolution by Sen1/Senataxin is a necessary step in transcription termination by Pol II. R-loops form when the nascent transcript invades duplex DNA after both emerge from RNA polymerase, but it is generally thought that RNA-binding proteins coat the nascent transcript and prevent it from base-pairing with DNA. The method of R-loop detection used in these studies does not allow determination of the fraction of cells that have R-loops on a given gene, so it is possible that some or most termination events occur without R-loop formation and resolution. Further studies will be required to resolve this issue.
Many other questions still remain to be answered. The human homologs of Nrd1 and Nab3 have not been identified. In yeast, substitutions in the RNA-binding domains of Nrd1 and Nab3 result in read-through of some Sen1-dependent terminators. Are mutations in the genes for human homologs of Nrd1 and Nab3 responsible for related neurodegenerative disorders, or do the AOA2/ALS4 mutations affect a function of Senataxin that is independent of such factors? The genome-wide targets of Senataxin are as yet unknown. Most human snoRNAs are processed from pre-mRNA introns and thus do not have transcription terminators, and human snRNAs use a specialized termination pathway distinct from the Sen1 pathway (Kuehner et al., 2011). But we now know that there are many other non-coding RNAs in humans, which are potential targets for Senataxin. Are genes for any such transcripts adjacent to genes important for motor neuron survival, or to the alpha-fetoprotein gene that appears to be misregulated in AOA2? What is the mechanism of Sen1-dependent transcription termination, and how do the AOA2/ALS4 substitutions interfere with this mechanism (if indeed they do)? Among the rewards of the challenging studies required to answer such questions will be new insights into, and possible therapeutic approaches for, crippling neurodegenerative disorders.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anheim M, Monga B, Fleury M, Charles P, Barbot C, et al. Brain. 2009;132:2688–2698. doi: 10.1093/brain/awp211. [DOI] [PubMed] [Google Scholar]
- Kim HD, Choe J, Seo YS. Biochemistry. 1999;38:14697–14710. doi: 10.1021/bi991470c. [DOI] [PubMed] [Google Scholar]
- Kuehner JN, Pearson EL, Moore C. Nature Rev Molec Cell Biol. 2011;12:1–12. doi: 10.1038/nrm3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmens R, Moore MJ, Al-Chalabi A, Brown RH, Jr, Robberecht W. Trends Neurosci. 2010;33:249–258. doi: 10.1016/j.tins.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Mischo HE, Gomez-Gonzalez B, Grzechnik P, Rondon AG, Wei W, Steinmetz L, Aguilera A, Proudfoot NJ. Mol Cell. 2011;41:21–32. doi: 10.1016/j.molcel.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JM, Mooney RA, Kuan PF, Rowland JL, Keles S, Landick R. Proc Natl Acad Sci USA. 2009;106:15406–15411. doi: 10.1073/pnas.0903846106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondon AG, Mischo HE, Kawauchi J, Proudfoot NJ. 2009;36:88–98. doi: 10.1016/j.molcel.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skourti-Stathaki, et al. Mol Cell. 2011 doi: 10.1016/j.molcel.2011.04.026. [THIS ISSUE] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz EJ, Conrad NK, Brow DA, Corden JL. Nature. 2001;413:327–331. doi: 10.1038/35095090. [DOI] [PubMed] [Google Scholar]
- Steinmetz EJ, Ng SBH, Cloute JP, Brow DA. Molec Cell Biol. 2006a;26:2688–2696. doi: 10.1128/MCB.26.7.2688-2696.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz EJ, Warren CL, Kuehner JN, Panbehi B, Ansari AZ, Brow DA. Mol Cell. 2006b;24:735–746. doi: 10.1016/j.molcel.2006.10.023. [DOI] [PubMed] [Google Scholar]