Abstract
Parenteral nutrition–associated liver disease (PNALD) is a complex disease that is diagnosed by clinical presentation, biochemical markers of liver injury, concurrent use of parenteral nutrition (PN), and negative workup for other causes of liver disease. For the past 30 years, clinicians have had few effective treatments for PNALD and when disease progressed to liver cirrhosis it was historically associated with poor outcomes. Within the past 5 years there has been some encouraging evidence for the potential benefits of fish oils, rich in omega-3 long-chain polyunsaturated fatty acids (ω3PUFA), in reversing liver injury associated with PN. This article reviews the current literature relating to ω3PUFA and PNALD.
Keywords: cholestasis, fish oil, pediatrics, ω3PUFA
INTRODUCTION
Parenteral nutrition–associated liver disease (PNALD) occurs in approximately one quarter to one half of pediatric patients maintained on long-term parenteral nutrition.1 PNALD is more common in infants born prematurely, especially very low birth weight (VLBW) infants, and those with short bowel syndrome (SBS).2 PNALD can develop within the first month of PN therapy.1 This article reviews the pathophysiology and traditional treatments for PNALD and evaluate the current literature regarding ω3PUFA for the treatment of PNALD.
PATHOPHYSIOLOGY
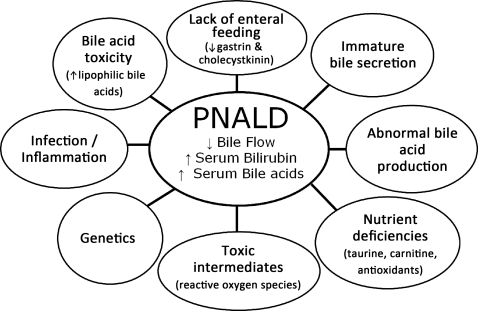
The etiology of PNALD is not well understood but is thought to be multifactorial.1–4 Potential contributing factors to PNALD include lack of enteral nutrition (EN), immature bile secretion, inflammation, oxidative stress, infection, nutrient deficiencies, and impurities in parenteral products including lipids or amino acids.1–4 More recently, cholestasis has been divided into infectious and noninfectious etiologies. Sepsis often results in inflammation and oxidative stress, but both of these processes can also occur without an underlying infectious etiology and are thought to have a role in the development of PNALD. In the presence of inflammation, proinflammatory cytokines are released. These cytokines are potent inhibitors of hepatobiliary transporter gene expression, which may be responsible for impaired bile secretion resulting in hyperbilirubinemia and cholestasis.5 In addition, highly reactive oxygen species are capable of cell injury through lipid peroxidation, oxidation of proteins, and destruction of DNA. Because the antioxidant defense mechanisms during early infancy are poorly developed, reactive oxygen species may be an important factor in the development of PNALD in this population.6,7 Inhibition of reactive oxygen species during cholestasis has been shown to reduce fibrosis.8
Lack of EN can lead to a decrease in the release of counterregulatory hormones secreted from the gut, thereby decreasing bile flow. In prematurity, organ systems are not developed to the extent they are in a term infant and/or adult, which can result in a build-up of toxic intermediates from parenteral products, which in turn can have harmful effects on the hepatobiliary system. Methionine, as an example, is known to be hepatotoxic in immature rabbit pups, and the very high concentrations of methionine in the plasma of neonates and infants receiving standard adult amino acid formulations may result in liver injury in the neonate.9 Composition and dose of soybean-based intravenous fat emulsion (IVFE) have been associated as risk factors for cholestasis, and recent packaging changes of IVFE have been associated with fat globules > 5 μm.10,11 In animal studies, unstable IVFE administration resulted in increased oxidative stress and liver injury.12 There also seems to be a potential genetic predisposition in the pathophysiology of PNALD, as it is possible to have 2 patients with a very similar clinical and surgical course and 1 patient will develop PNALD while the other patient does not.
Figure 1 is a representation of the complex multifactorial pathophysiology of PNALD. The development of PNALD is associated with increased morbidity and mortality, and, if not reversed, PNALD can progress to liver fibrosis, hepatic failure, and death. PNALD improves with the initiation of enteral feeding. Although most PNALD reverses with advancement of enteral feeds and discontinuation of parenteral nutrition (PN), this is not an option in many patients with anatomic or functional SBS.
Figure 1.
Multifactorial pathophysiology associated with parenteral nutrition–associated liver disease (PNALD).
TRADITIONAL TREATMENTS
Current treatments for PNALD have been reported to have limited success and they include PN cycling, drug therapy with ursodeoxycholic acid, cholecystokinin, oral antibiotics, and nutrient restriction including limiting soybean-based lipids and providing conservative protein and dextrose calories to prevent overfeeding.13–17 Surgical approaches such as the serial transverse enteroplasty (STEP) bowel lengthening procedures have been used to decrease need for PN.18 Intestine and intestine plus liver transplantation have also been lifesaving for patients with progressive PNALD.19,20 However, these are invasive surgical procedures that have a high risk of complications including bleeding, infection, and rejection (in transplant).19,20 Investigators have reported some success with parenteral fish oil–based intravenous fat emulsion (IVFE) (Omegaven, Fresenius Kabi AG, Bad Homburg v.d.h., Germany) for the treatment of PNALD.21,22 These initial case reports were followed by small studies in humans and animal research using fish oil for the treatment of PNALD.
ANIMAL STUDIES OF ω3PUFA FOR THE TREATMENT OF LIVER DISEASE
Several animal models have been used to study the effects of omega-3 long-chain polyunsaturated fatty acids (ω3PUFA) supplementation on liver injury. Alwayn et al23 report the use of both enteral and parenteral ω3PUFA in a mouse model of nonalcoholic fatty liver disease (NAFLD). Animals were randomized to receive standard chow or an enteral fat-free, high carbohydrate diet identical to parenteral nutrition solutions given to pediatric patients, with and without ω3PUFA supplementation. Mice fed standard chow had the lowest hepatic fat content and served as controls. Hepatic fat content was the highest in mice fed the liquid high carbohydrate diet and was significantly decreased by both enteral and parenteral ω3PUFA supplementation.23
In a surgical model of liver injury, mice underwent a common bile duct ligation and were then randomized to receive a control soy diet rich in ω6PUFA or a Menhaden diet rich in ω3PUFA.24 In this study, there were trends that a Menhaden diet was hepato-protective against injury, but this was not statistically significant.24
A study in rabbits receiving a balanced PN regimen with 3 g/kg/day IVFE evaluated the effects of a soybean oil emulsion alone, an olive oil emulsion alone, and a combination soybean (2.8 g/kg/day) and fish oil (0.2 g/kg/day) emulsion. Animals that received the fish oil were found to have more extensive hepatic fibrosis than those that received the soybean or olive oil regimens.25 The authors speculated that this finding might be due to the omega-3 to omega-6 ratio of 1:6 in the emulsion studied, whereas optimal omega-3 to omega-6 ratios in animal models have ranged from 1:2 to 1:4.26,27
HUMAN STUDIES OF INTRAVENOUS ω3PUFA
Children's Hospital Boston reported the case of a 17-year-old male who developed essential fatty acid deficiency (EFAD) due to a soy allergy preventing administration of soybean-based IVFE.21 The patient was treated with parenteral fish oil composed primarily of ω3PUFA and limited ω6PUFA; EFAD was reversed. Clinicians also observed decreases in aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin, and direct bilirubin. In 2006, the Boston group reported the complete reversal of PNALD in 2 infants with intestinal failure treated with fish oil–based IVFE at 1 g/kg/day.22 Cholestasis resolved in both infants within the first 60 days of treatment.
The same group published safety and efficacy data for 18 patients treated with fish oil–based IVFE at 1 g/kg/day infused over 12 hours per day from September 2004 to August 2006 compared with 21 historical control patients who received standard soy-based lipid emulsions 1 to 4 g/kg/day infused over 24 hours per day between 1986 and 1996.28 They reported the time to reversal of cholestasis of 9.4 weeks in the fish oil–based IVFE group compared with 44 weeks in the control group (p=0.002). There were 2 deaths and no liver transplants reported in the fish oil–based IVFE group compared with 7 deaths and 2 liver transplants in the control group. Death occurred at 6.7 and 10.7 weeks, respectively, in the 2 patients in the fish oil group, whereas the time to death in the historic control group was reported as a median of 41 weeks. Although the initial findings are interesting, this study is limited by the small sample size and a control group that is from an observation period 10 to 20 years prior to the treatment group. Standards of care and infant survival have changed in the last 20 years. Also, the dose of lipids was significantly decreased in the treatment group. These results could be interpreted as suggesting that reduction of fats resulted in the improved outcomes.
Puder et al29 published results of an open-label study of 42 infants treated with fish oil–based IVFE between August 2006 and November 2007 compared with a historical cohort of 49 patients with SBS and PNALD treated with soybean-based IVFE from 1999 to 2006. This report is an extension of the previously published work from this group with a slightly more contemporary historical cohort. Death occurred in 3 of 42 (7%) patients in the fish oil group and 12 of 49 (24%) in the control group. One patient receiving fish oil underwent liver transplant compared with 6 patients in the control group.29 The median time to discontinuation of PN was 4 weeks in the fish oil group as compared with 20 weeks in the control group. PNALD resolved while patients were still receiving PN in 19 patients in the fish oil group and only 2 patients in the control group.29 Patients in the fish oil group had lower international normalized ratio (INR) values and increased platelet counts compared with the control group, suggesting that fish oil has a limited effect on coagulation, but this conclusion is confounded by the higher incidence of advanced liver disease in the control group.
Similar success has also been observed by Italian clinicians in a case report of an infant with severe PNALD.30 The patient was started at 0.2 g/kg/day of fish oil–based IVFE and advanced by 0.2 g/kg/day to achieve a dose of 1.5 g/kg/day, which is higher than has been previously reported.30 Chinese clinicians report the reversal of PNALD in 3 of 4 infants with intestinal failure treated with fish oil–based IVFE.31 In these patients, standard soybean oil–based IVFE was discontinued and fish oil–based IVFE was initiated at 1 g/kg/day.31 Calhoun and Sullivan32 report successful resolution of PNALD in a 17-month-old infant with SBS after treatment with fish oil–based IVFE. Chung et al.33 reported the use of fish oil–based IVFE to treat 4 patients with SBS and PNALD.
A study from Toronto recently reported the reversal of PNALD in 9 of 12 infants with SBS that were treated with a combination of a soybean oil–based IVFE and fish oil–based IVFE. Four patients had complete reversal of PNALD while receiving a combination of soybean-based IVFE and fish oil–based IVFE, whereas the other 5 patients did not have reversal of disease until after soybean-based IVFE was discontinued.34
Early reports of fish oil–based IVFE used outcomes based on clinical presentation and biochemical markers of cholestasis (AST, ALT, total bilirubin, and direct bilirubin) and not based on liver biopsy results. Investigators from Boston have now reported 83 biopsy results taken in 66 children.35 Of the 83 biopsies, 74 demonstrated fibrosis; 8 of these also demonstrated cirrhosis. Forty-one of the 74 fibrotic biopsies (55%) were obtained in patients without biochemical evidence of cholestasis. Seventy percent of these patients were treated with fish oil–based IVFE during their clinical course. Soden et al36 reports similar findings with 2 infants who had improvement in biochemical markers of cholestasis but persistent portal fibrosis despite treatment with fish oil–based IVFE. Hepatocellular injury associated with PNALD has been reported to occur within the first few weeks of PN and the reversal of fibrosis associated with any type of liver injury may never occur, but, if it does, reversal often takes a long time.37,38
ELIMINATION OF SOYBEAN-BASED IVFE AND ENTERAL FISH OIL
Although fish oil–based IVFE appears to be a promising therapy for PNALD, its use in the United States is hindered by lack of FDA approval, thereby necessitating the use of an investigational new drug (IND) application. Because of the difficulty in obtaining the parenteral fish oil product, some institutions have restricted or eliminated soybean-based IVFE and used enteral fish oil products to treat infants with PNALD. Rollins et al39 retrospectively evaluated 26 patients with SBS receiving soybean-based IVFE 2 to 3 g/kg/day: 23 patients developed PNALD. PNALD resolved in 10 patients with the advancement of EN, 1 patient developed PNALD and remained on 2 g/kg/day of soybean-based IVFE, 3 patients required liver transplant, 3 patients died, and 6 patients had complete resolution of PNALD with the removal of soybean-based IVFE. Four of these 6 patients also received enteral fish oil.
Tillman et al40 reported 6 infants with PNALD treated with enteral fish oil. PNALD completely reversed in 4 of the 6 infants supplemented with enteral fish oil within a mean of 5 weeks after initiation of therapy. The 2 infants that did not have complete resolution of disease had some improvement in bilirubin and liver enzymes while on enteral fish oil. One of these nonresponding infants had extreme short gut, estimated as 8 cm of small bowel remaining, and no ileocecal valve. This would suggest that absorption is limited in the extremely short gut patient but does not rule out an intraluminal effect of fish oil. Similar results were observed by Sharma41 when 4 infants had resolution of PNALD after being treated with enteral fish oil.
USE OF OTHER CONTEMPORARY LIPIDS
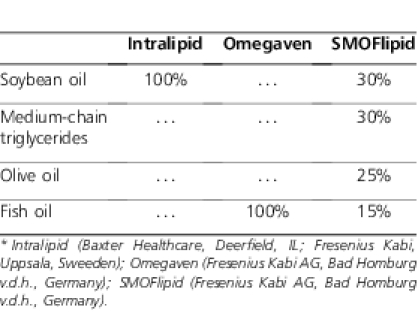
European clinicians and researchers have used other lipid products in addition to fish oil–based IVFE. An IVFE composed of soybean oil, medium-chain triglycerides, olive oil, and fish oil (SMOFlipid 20%, Fresenius Kabi AG) has also been used for the treatment of PNALD (Table). In a randomized, double-blind trial of 22 patients treated with SMOFlipid and 22 patients treated with olive oil and soybean oil IVFE for 5 days postoperatively, there was no significant difference in baseline AST and ALT between the 2 groups; however, significantly lower AST and ALT values were observed at day 2 and day 5 in the SMOFlipid group as compared with the olive oil and soybean oil group.42
TABLE.
Lipid Product Comparison*
ADVERSE SIDE EFFECTS ASSOCIATED WITH FISH OIL
As with any new therapy, there may be adverse side effects. In the initial Boston cohort study, only 1 of 18 patients and 2 of 42 patients in the second cohort exhibited biochemical evidence of EFAD based on triene:tetraene ratios (T/T); however, total fatty acid profiles were not reported in either study.28,29 With decreased lipid dosing, it is possible to have T/T within the reference range but have concentrations of individual fatty acids below the age-related reference range. A prospective evaluation of 10 patients receiving fish oil–based IVFE as a sole fat source at a dose of 1 g/kg/day resulted in T/T within the reference range in all 10 patients, but 50% of patients had linoleic acid concentrations below the reference range and 90% had at least a 17% reduction in linoleic acid concentration after 6 weeks of treatment with fish oil–based IVFE.43 Animal studies have not clarified the adequacy of linoleic acid intake with fish oil. A study of pair-fed mice provided with chow containing either soybean-based or fish oil–based fat showed fish oil both prevents EFAD and enhances growth in mice. EFAD was determined based on T/T. All T/T were within the reference range, but linoleic acid concentrations were not reported.44
One infant with SBS and PNALD treated with fish oil–based IVFE developed burr cell anemia that resolved upon discontinuation of fish oil–based IVFE.45 The authors speculated that incorporation of ω3PUFA within red blood cell membranes made erythrocytes susceptible to trapping and destruction within the spleen. The product labeling for the fish oil–based IVFE suggests that the product could increase bleeding risk.46 In contrast, Bays47 and Harris48 both conclude that ω3PUFA does not increase bleeding risk.
POTENTIAL MECHANISM OF ACTION OF ω3PUFA
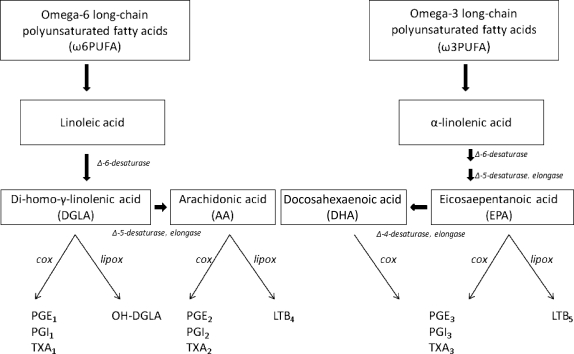
ω3PUFA supplementation has been used for the treatment of many inflammatory diseases including cardiovascular disease, arthritis, asthma, sepsis, autoimmune disease, and malignancy.47 ω3PUFA are thought to exhibit anti-inflammatory effects by causing a shift from ω6PUFA-derived proinflammatory eicosanoids to the anti-inflammatory variety derived from ω3PUFA.49 This pathway is regulated by the Δ-5-desaturase enzyme system, which preferentially metabolizes ω3PUFA (Figure 2). A number of studies have suggested that positive changes in membrane phospholipid content as well as in clinical outcomes can be achieved with relatively low intakes of fish oil.50–53 This suggests that ω3PUFA compete very effectively as substrates for the Δ-5-desaturase enzyme system despite the presence of large amounts of ω6PUFA.
Figure 2.
This is a simplified diagram of the omega-6 long-chain polyunsaturated fatty acid (ω6PUFA) and omega-3 long-chain polyunsaturated fatty acid (ω3PUFA) metabolism pathways leading to both proinflammatory and anti-inflammatory eicosanoids. Both pathways compete for the same desaturase and elongase enzymes. COX, cyclooxygenase; lipox, lipoxygenase; LTB, leukotriene beta; PGE, prostaglandin E; TXA, thromboxane.
Tumor necrosis factor-α (TNF-α) is one of the major proinflammatory cytokines involved in liver disease.54 In a murine macrophage model, the reduction of TNF-α gene transcription, via inactivation of the NF-κB signal transduction cascade secondary to decreased IκB phosphorylation at serine 32, has been proposed as a mechanism for the anti-inflammatory effects of ω3PUFA.55 TNF-α and other proinflammatory cytokines transcribed via NF-κB, as well as stimulation of death receptors on the hepatocellular surface, have been linked to liver injury, causing both apoptosis and necrosis in many types of liver diseases (cholestasis, NAFLD, alcoholic cirrhosis, and hepatitis).56 Although the exact mechanism of action of ω3PUFA in PNALD is unclear, this anti-inflammatory pathway would seem to make sense based on potential etiologies of the disease.
CONCLUSION
The current data suggest that fish oil given either parenterally or enterally may be a potential treatment for patients with PNALD. It is clear from the treatment failures reported from many centers across the world that fish oil is not a universal cure for all patients. Recent biopsy reports from patients treated with fish oil–based IVFE that showed hepatic fibrosis with no biochemical evidence of cholestasis is concerning.35,36 Contemporary lipid solutions, such as the SMOFlipid product that offers a blend of ω3PUFA, ω6PUFA, and ω9PUFA, may have an advantage in the treatment of PNALD. A well-balanced lipid product may have advantages over a single source product. Several centers leading research efforts against intestinal failure and PNALD have established intraprofessional teams to attack this multifactorial disease from all angles.57–59 This approach to the care of this disease will likely result in the most favorable patient outcomes. More research in the form of randomized controlled trials evaluating dosing of ω3PUFA, as well as comparison of enteral and parenteral fish oil, are needed to adequately determine if fish oil is both safe and effective in treating PNALD.
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- EFAD
essential fatty acid deficiency
- EN
enteral nutrition
- IND
investigational new drug
- INR
international normalized ratio
- IVFE
intravenous fat emulsion
- NAFLD
nonalcoholic fatty liver disease
- ω3PUFA
omega-3 long-chain polyunsaturated fatty acids
- PN
parenteral nutrition
- PNALD
parenteral nutrition-associated liver disease
- SBS
short bowel syndrome
- STEP
serial transverse enteroplasty
- TNF-α
tumor necrosis factor-α
- T/T
triene:tetraene ratio
- VLBW
very low birth weight
Footnotes
DISCLOSURE Dr Tillman has grant support for Le Bonheur Children's Hospital, Pediatric Pharmacy Advocacy Group, the University of Tennessee Health Science Center, Center for Translational Science and the University of Tennessee Research Foundation. Dr Helms has grant support from the State of Tennessee Center of Excellence in Pediatric Pharmacokinetics and Therapeutics, Le Bonheur Children's Hospital and the University of Tennessee Research Foundation.
REFERENCES
- 1.Merritt RJ. Cholestasis associated with total parenteral nutrition. J Pediatr Gastroenterol Nutr. 1986;5(1):9–22. doi: 10.1097/00005176-198601000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Beale EF, Nelson RM, Bucciarelli RL, et al. Intrahepatic cholestasis associated with parenteral nutrition in premature infants. Pediatrics. 1979;64(3):342–347. [PubMed] [Google Scholar]
- 3.Whitington PF. Cholestasis associated with total parenteral nutrition in infants. Hepatology. 1985;5(4):693–696. doi: 10.1002/hep.1840050428. [DOI] [PubMed] [Google Scholar]
- 4.Sinatra FR. Cholestasis in infancy and childhood. Curr Probl Pediatr. 1982;12(12):1–54. doi: 10.1016/0045-9380(82)90018-4. [DOI] [PubMed] [Google Scholar]
- 5.Trauner M, Fickert P, Stauber RE. Inflammation-induced cholestasis. J Gastroenterol Hepatol. 1999;14(10):946–959. doi: 10.1046/j.1440-1746.1999.01982.x. [DOI] [PubMed] [Google Scholar]
- 6.Warner BB, Wispe JR. Free radical-mediated diseases in pediatrics. Semin Perinatol. 1992;16(1):47–57. [PubMed] [Google Scholar]
- 7.Saugstad OD. Update on oxygen radical disease in neonatology. Curr Opin Obstet Gynecol. 2001;13(2):147–153. doi: 10.1097/00001703-200104000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Copple BL, Jaeschke H, Klaassen CD. Oxidative stress and the pathogenesis of cholestasis. Semin Liver Dis. 2010;30(2):195–204. doi: 10.1055/s-0030-1253228. [DOI] [PubMed] [Google Scholar]
- 9.Moss RL, Haynes AL, Pastuszyn A, Glew RH. Methionine infusion reproduces liver injury of parenteral nutrition cholestasis. Pediatr Res. 1999;45(5 Pt 1):664–668. doi: 10.1203/00006450-199905010-00009. [DOI] [PubMed] [Google Scholar]
- 10.Colomb V, Jobert-Giraud A, Lacaille F, et al. Role of lipid emulsions in cholestasis associated with long-term parenteral nutrition in children. JPEN J Parenter Enteral Nutr. 2000;24(6):345–350. doi: 10.1177/0148607100024006345. [DOI] [PubMed] [Google Scholar]
- 11.Driscoll DF, Ling PR, Bistrian BR. Physical stability of 20% lipid injectable emulsions via simulated syringe infusion: effects of glass vs plastic product packaging. JPEN J Parenter Enteral Nutr. 2007;31(2):148–153. doi: 10.1177/0148607107031002148. [DOI] [PubMed] [Google Scholar]
- 12.Driscoll DF, Ling PR, Andersson C, Bistrian BR. Hepatic indicators of oxidative stress and tissue damage accompanied by systemic inflammation in rats following a 24-hour infusion of an unstable lipid emulsion admixture. JPEN J Parenter Enteral Nutr. 2009;33(3):327–335. doi: 10.1177/0148607108327155. [DOI] [PubMed] [Google Scholar]
- 13.Balistreri WF. Bile acid therapy in pediatric hepatobiliary disease: the role of ursodeoxycholic acid. J Pediatr Gastroenterol Nutr. 1997;24(5):573–589. doi: 10.1097/00005176-199705000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Teitelbaum DH, Tracy TF, Jr, Aouthmany MM, et al. Use of cholecystokinin-octapeptide for the prevention of parenteral nutrition-associated cholestasis. Pediatrics. 2005;115(5):1332–1340. doi: 10.1542/peds.2004-1014. [DOI] [PubMed] [Google Scholar]
- 15.Kaufman SS. Prevention of parenteral nutrition-associated liver disease in children. Pediatr Transplant. 2002;6(1):37–42. doi: 10.1034/j.1399-3046.2002.1o061.x. [DOI] [PubMed] [Google Scholar]
- 16.Lambert JR, Thomas SM. Metronidazole prevention of serum liver enzyme abnormalities during total parenteral nutrition. JPEN J Parenter Enteral Nutr. 1985;9(4):501–503. doi: 10.1177/0148607185009004501. [DOI] [PubMed] [Google Scholar]
- 17.Jensen AR, Goldin AB, Koopmeiners JS, et al. The association of cyclic parenteral nutrition and decreased incidence of cholestatic liver disease in patients with gastroschisis. J Pediatr Surg. 2009;44(1):183–189. doi: 10.1016/j.jpedsurg.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 18.Javid PJ, Kim HB, Duggan CP, Jaksic T. Serial transverse enteroplasty is associated with successful short-term outcomes in infants with short bowel syndrome. J Pediatr Surg. 2005;40(6):1019–1023. doi: 10.1016/j.jpedsurg.2005.03.020. ; discussion 1023-1014. [DOI] [PubMed] [Google Scholar]
- 19.Kim HB, Fauza D, Garza J, et al. Serial transverse enteroplasty (STEP): a novel bowel lengthening procedure. J Pediatr Surg. 2003;38(3):425–429. doi: 10.1053/jpsu.2003.50073. [DOI] [PubMed] [Google Scholar]
- 20.Fishbein TM. Intestinal transplantation. N Engl J Med. 2009;361(10):998–1008. doi: 10.1056/NEJMra0804605. [DOI] [PubMed] [Google Scholar]
- 21.Gura KM, Parsons SK, Bechard LJ, et al. Use of a fish oil-based lipid emulsion to treat essential fatty acid deficiency in a soy allergic patient receiving parenteral nutrition. Clin Nutr. 2005;24(5):839–847. doi: 10.1016/j.clnu.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 22.Gura KM, Duggan CP, Collier SB, et al. Reversal of parenteral nutrition-associated liver disease in two infants with short bowel syndrome using parenteral fish oil: implications for future management. Pediatrics. 2006;118(1):e197–e201. doi: 10.1542/peds.2005-2662. [DOI] [PubMed] [Google Scholar]
- 23.Alwayn IP, Gura K, Nose V, et al. Omega-3 fatty acid supplementation prevents hepatic steatosis in a murine model of nonalcoholic fatty liver disease. Pediatr Res. 2005;57(3):445–452. doi: 10.1203/01.PDR.0000153672.43030.75. [DOI] [PubMed] [Google Scholar]
- 24.Lee S, Kim S, Le HD, et al. Reduction of hepatocellular injury after common bile duct ligation using omega-3 fatty acids. J Pediatr Surg. 2008;43(11):2010–2015. doi: 10.1016/j.jpedsurg.2008.05.030. [DOI] [PubMed] [Google Scholar]
- 25.Kohl M, Wedel T, Entenmann A, et al. Influence of different intravenous lipid emulsions on hepatobiliary dysfunction in a rabbit model. J Pediatr Gastroenterol Nutr. 2007;44(2):237–244. doi: 10.1097/01.mpg.0000252193.99331.03. [DOI] [PubMed] [Google Scholar]
- 26.Grimm H, Tibell A, Norrlind B, et al. Immunoregulation by parenteral lipids: impact of the n-3 to n-6 fatty acid ratio. JPEN J Parenter Enteral Nutr. 1994;18(5):417–421. doi: 10.1177/0148607194018005417. [DOI] [PubMed] [Google Scholar]
- 27.Furst P, Kuhn KS. Fish oil emulsions: what benefits can they bring? Clin Nutr. 2000;19(1):7–14. doi: 10.1054/clnu.1999.0072. [DOI] [PubMed] [Google Scholar]
- 28.Gura KM, Lee S, Valim C, et al. Safety and efficacy of a fish-oil-based fat emulsion in the treatment of parenteral nutrition-associated liver disease. Pediatrics. 2008;121(3):e678–e686. doi: 10.1542/peds.2007-2248. [DOI] [PubMed] [Google Scholar]
- 29.Puder M, Valim C, Meisel JA, et al. Parenteral fish oil improves outcomes in patients with parenteral nutrition-associated liver injury. Ann Surg. 2009;250(3):395–402. doi: 10.1097/SLA.0b013e3181b36657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ekema G, Falchetti D, Boroni G, et al. Reversal of severe parenteral nutrition-associated liver disease in an infant with short bowel syndrome using parenteral fish oil (omega-3 fatty acids) J Pediatr Surg. 2008;43(6):1191–1195. doi: 10.1016/j.jpedsurg.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 31.Cheung HM, Lam HS, Tam YH, et al. Rescue treatment of infants with intestinal failure and parenteral nutrition-associated cholestasis (PNAC) using a parenteral fish-oil-based lipid. Clin Nutr. 2009;28(2):209–212. doi: 10.1016/j.clnu.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 32.Calhoun AW, Sullivan JE. Omegaven for the treatment of parenteral nutrition associated liver disease: a case study. J Ky Med Assoc. 2009;107(2):55–57. [PubMed] [Google Scholar]
- 33.Chung PH, Wong KK, Wong RM, et al. Clinical experience in managing pediatric patients with ultra-short bowel syndrome using omega-3 fatty acid. Eur J Pediatr Surg. 2010;20(2):139–142. doi: 10.1055/s-0029-1238283. [DOI] [PubMed] [Google Scholar]
- 34.Diamond IR, Sterescu A, Pencharz PB, et al. Changing the paradigm: omegaven for the treatment of liver failure in pediatric short bowel syndrome. J Pediatr Gastroenterol Nutr. 2009;48(2):209–215. doi: 10.1097/MPG.0b013e318182c8f6. [DOI] [PubMed] [Google Scholar]
- 35.Fitzgibbons SC, Jones BA, Hull MA, et al. Relationship between biopsy-proven parenteralnutrition-associated liver fibrosis and biochemical cholestasis in children with short bowel syndrome. J Pediatr Surg. 2010;45(1):95–99. doi: 10.1016/j.jpedsurg.2009.10.020. ; discussion 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soden JS, Lovell MA, Brown K. et al Failure of resolution of portal fibrosis during omega-3 fatty acid lipid emulsion therapy in two patients with irreversible intestinal failure. J Pediatr. 2010;156(2):327–331. doi: 10.1016/j.jpeds.2009.08.033. [DOI] [PubMed] [Google Scholar]
- 37.Moss RL, Das JB, Raffensperger JG. Total parenteral nutrition-associated cholestasis: clinical and histopathologic correlation. J Pediatr Surg. 1993;28(10):1270–1274. doi: 10.1016/s0022-3468(05)80311-2. ; discussion 1274–1275. [DOI] [PubMed] [Google Scholar]
- 38.Hammel P, Couvelard A, O'Toole D, et al. Regression of liver fibrosis after biliary drainage in patients with chronic pancreatitis and stenosis of the common bile duct. N Engl J Med. 2001;344(6):418–423. doi: 10.1056/NEJM200102083440604. [DOI] [PubMed] [Google Scholar]
- 39.Rollins MD, Scaife ER, Jackson WD, et al. Elimination of soybean lipid emulsion in parenteral nutrition and supplementation with enteral fish oil improve cholestasis in infants with short bowel syndrome. Nutr Clin Pract. 2010;25(2):199–204. doi: 10.1177/0884533610361477. [DOI] [PubMed] [Google Scholar]
- 40.Tillman EM, Crill CM, Black DD, et al. Enteral Fish Oil for Treatment of Parenteral Nutrition-Associated Liver Disease in Six Infants with Short-Bowel Syndrome. Pharmacotherapy. 2011;31(5):503–509. doi: 10.1592/phco.31.5.503. [DOI] [PubMed] [Google Scholar]
- 41.Sharma AA. Supplementing enteral feeds with fish oil improves or reverses the total parenteral nutrition induced cholestasis in 4 infants in neonatal/pediatric intensive care units [abstract] JPEN J Parenter Enteral Nutr. 2010;34(2):231. [Google Scholar]
- 42.Piper SN, Schade I, Beschmann RB, et al. Hepatocellular integrity after parenteral nutrition: comparison of a fish-oil-containing lipid emulsion with an olive-soybean oil-based lipid emulsion. Eur J Anaesthesiol. 2009;26(12):1076–1082. doi: 10.1097/eja.0b013e32832e08e0. [DOI] [PubMed] [Google Scholar]
- 43.de Meijer VE, Le HD, Meisel JA, et al. Parenteral fish oil as monotherapy prevents essential fatty acid deficiency in parenteral nutrition-dependent patients. J Pediatr Gastroenterol Nutr. 2010;50(2):212–218. doi: 10.1097/MPG.0b013e3181bbf51e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strijbosch RA, Lee S, Arsenault DA, et al. Fish oil prevents essential fatty acid deficiency and enhances growth: clinical and biochemical implications. Metabolism. 2008;57(5):698–707. doi: 10.1016/j.metabol.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mallah HS, Brown MR, Rossi TM, Block RC. Parenteral fish oil-associated burr cell anemia. J Pediatr. 2010;156(2):324–326. doi: 10.1016/j.jpeds.2009.07.062. e321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Omegaven [package insert] Bad Homburg v.d.h., Germany: Fresenius Kabi AG; 2001. [Google Scholar]
- 47.Bays HE. Safety considerations with omega-3 fatty acid therapy. Am J Cardiol. 2007;99(6A):35C–43C. doi: 10.1016/j.amjcard.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 48.Harris WS. Expert opinion: omega-3 fatty acids and bleeding-cause for concern? Am J Cardiol. 2007;99(6A):44C–46C. doi: 10.1016/j.amjcard.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 49.El-Badry AM, Graf R, Clavien PA. Omega 3 - omega 6: what is right for the liver? J Hepatol. 2007;47(5):718–725. doi: 10.1016/j.jhep.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 50.De Vizia B, Raia V, Spano C, et al. Effect of an 8-month treatment with omega-3 fatty acids (eicosapentaenoic and docosahexaenoic) in patients with cystic fibrosis. JPEN J Parenter Enteral Nutr. 2003;27(1):52–57. doi: 10.1177/014860710302700152. [DOI] [PubMed] [Google Scholar]
- 51.Thienprasert A, Samuhaseneetoo S, Popplestone K, et al. Fish oil n-3 polyunsaturated fatty acids selectively affect plasma cytokines and decrease illness in Thai schoolchildren: a randomized, double-blind, placebo-controlled intervention trial. J Pediatr. 2009;154(3):391–395. doi: 10.1016/j.jpeds.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 52.Vaisman N, Kaysar N, Zaruk-Adasha Y, et al. Correlation between changes in blood fatty acid composition and visual sustained attention performance in children with inattention: effect of dietary n-3 fatty acids containing phospholipids. Am J Clin Nutr. 2008;87(5):1170–1180. doi: 10.1093/ajcn/87.5.1170. [DOI] [PubMed] [Google Scholar]
- 53.Smithers LG, Gibson RA, McPhee A, Makrides M. Effect of long-chain polyunsaturated fatty acid supplementation of preterm infants on disease risk and neurodevelopment: a systematic review of randomized controlled trials. Am J Clin Nutr. 2008;87(4):912–920. doi: 10.1093/ajcn/87.4.912. [DOI] [PubMed] [Google Scholar]
- 54.Schmocker C, Weylandt KH, Kahlke L, et al. Omega-3 fatty acids alleviate chemically induced acute hepatitis by suppression of cytokines. Hepatology. 2007;45(4):864–869. doi: 10.1002/hep.21626. [DOI] [PubMed] [Google Scholar]
- 55.Novak TE, Babcock TA, Jho DH, et al. NF-kappa B inhibition by omega-3 fatty acids modulates LPS-stimulated macrophage TNF-alpha transcription. Am J Physiol Lung Cell Mol Physiol. 2003;284(1):L84–L89. doi: 10.1152/ajplung.00077.2002. [DOI] [PubMed] [Google Scholar]
- 56.Malhi H, Gores GJ. Cellular and molecular mechanisms of liver injury. Gastroenterology. 2008;134(6):1641–1654. doi: 10.1053/j.gastro.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sigalet D, Boctor D, Robertson M, et al. Improved outcomes in paediatric intestinal failure with aggressive prevention of liver disease. Eur J Pediatr Surg. 2009;19(6):348–353. doi: 10.1055/s-0029-1241865. [DOI] [PubMed] [Google Scholar]
- 58.Goulet O, Joly F, Corriol O, Colomb-Jung V. Some new insights in intestinal failure-associated liver disease. Curr Opin Organ Transplant. 2009;14(3):256–261. doi: 10.1097/MOT.0b013e32832ac06f. [DOI] [PubMed] [Google Scholar]
- 59.de Meijer VE, Gura KM, Le HD, et al. Fish oil-based lipid emulsions prevent and reverse parenteral nutrition-associated liver disease: the Boston experience. JPEN J Parenter Enteral Nutr. 2009;33(5):541–547. doi: 10.1177/0148607109332773. [DOI] [PubMed] [Google Scholar]