Abstract
OBJECTIVES
The purpose of this study was to assess the appropriateness of weight-based dosing of continuous intravenous infusion of fentanyl in overweight/obese versus normal-weight children admitted to the pediatric intensive care unit (PICU).
METHODS
This retrospective, pilot study included 5- to 12-year-old children admitted to the PICU over a 2-year period who received continuous intravenous infusion fentanyl for ≥ 4 days. The overweight/obese group included children with a body mass index (BMI) ≥ 85th percentile, while the control group included children with BMI < 85th percentile. The primary objective was to compare the number of fentanyl continuous intravenous infusion dosage changes required per day to achieve adequate sedation between groups. Secondarily, opioid withdrawal symptoms following the discontinuation of fentanyl and concomitant sedative/analgesic regimens were analyzed between groups. Student t tests and chi-square analyses were performed as appropriate, with an a priori alpha of p≤0.05.
RESULTS
Sixteen normal-weight and 15 overweight/obese patients with 18 and 16 individual infusions were identified, respectively. No statistical difference was found between groups for the number of dosage changes per day, 0.92 versus 0.69 (p=0.16). Five patients in each group experienced withdrawal (p=0.71). The total number of concomitant bolus doses received was greater in the overweight/obese group but did not reach statistical significance.
CONCLUSIONS
There was a numerical, but statistically nonsignificant difference in the number of sedative/analgesic bolus doses and dosing changes per day between groups. Larger studies are warranted to determine the optimal dosing strategy for continuous intravenous infusion fentanyl in overweight/obese children.
Keywords: fentanyl, obese, overweight, pediatric
INTRODUCTION
Obesity is an increasing problem not only in adults but also in children.1,2 The American Academy of Pediatrics and Centers for Disease Control and Prevention define obesity as a body mass index (BMI) ≥ 95th percentile based on age and sex, and overweight as a BMI ≥ 85th to 94th percentiles in patients 2 to 20 years of age.3,4 Data from the most recent National Health and Nutrition Examination Survey show that 31.7% of all children 2 to 19 years of age have a BMI ≥ 85th percentile based on age and gender.2 With such a large portion of the pediatric population now classified as overweight, a reevaluation of appropriate medication dosing in children is necessary. A number of pharmacokinetic changes have been reported in obese adults including increased volume of distribution for lipophilic drugs and an increased rate of elimination for some medications, such as vancomycin.5,6 To date, few studies have evaluated pharmacokinetic changes in overweight children.7 Koshida et al7 noted that 6 obese children receiving tobramycin had a larger than expected volume of distribution for the patients' ideal body weight due to an increased water volume in adipose tissue. Additionally, the clearance of antineoplastic agents was found to be similar between normal weight and overweight/obese children.8,9 However, there are limited pharmacokinetic data regarding lipophilic medications in the overweight/obese pediatric population.
One medication with lipophilic properties commonly used for sedation and analgesia in pediatric patients is fentanyl. According to a survey of pediatric intensive care unit (PICU) fellowship programs, fentanyl is the fourth most common agent used for sedation or analgesia in the United States.10 In obese adults, fentanyl is thought to distribute throughout the body, including adipose tissue.6 Although these patients may require a larger loading dose at the initiation of therapy, studies of other lipophilic synthetic opioids in obese adults have noted that obese patients may require decreased maintenance dosing.11 This is due to a longer elimination half-life that results from a larger volume of distribution in this population.11 These possible pharmacokinetic differences in obesity have not been studied in children. However, with the increasing number of overweight children, there is potential for complications with fentanyl continuous infusions due to these pharmacokinetic alterations. Children who receive inappropriate doses may be at greater risk for adverse events due to accumulation of fentanyl and/or opioid dependence upon rapid discontinuation. There also may be concern for underdosing resulting in reduced efficacy. To date, no studies have evaluated dosing requirements of fentanyl continuous infusions in overweight children. Based on the aforementioned pharmacokinetic alterations noted in obese adults, we assessed the clinical impact of these alterations in children admitted to the PICU who were receiving a continuous intravenous infusion of fentanyl. The objective of this study was to assess the number of infusion rate changes needed per day to achieve adequate sedation for children 5 to 12 years of age with a BMI ≥ 85th percentile compared with patients of normal weight.
METHODS
Study Design
This retrospective cohort study included data from children 5 to 12 years of age admitted to a tertiary care, academic medical center from January 1, 2007 through December 31, 2008 who received a continuous intravenous fentanyl infusion for ≥ 4 days. To assess for sign or symptoms of withdrawal, patients receiving fewer than 4 days of fentanyl infusions were excluded because the half-life of fentanyl is approximately 21 hours (range, 11–36 hours).12 Katz and colleagues13 demonstrated that 50% of children given a continuous fentanyl infusion for ≥ 5 days experienced withdrawal following discontinuation of the medication. Patients were identified using the electronic medical record (EMR) (Meditech database. Medical Information Technology, Inc, Westwood, MA). Patients younger than 5 and older than 12 years were excluded due to large physiologic variations in the pharmacokinetic and pharmacodynamic properties of many medications in these populations.14 The volume of distribution for some medications is markedly larger in neonates and infants due to differences in body composition.11 Oftentimes nonobese children require larger milligram per kilogram doses than adolescents and adults due to increased metabolism and elimination.14 Thus, children 5 to 12 years of age would provide the most homogeneous group for analysis. Additionally, patients were excluded if they had incomplete EMR or if they received fentanyl via a patient controlled analgesia device.
Height, weight, age, and sex were collected for all identified patients and inputted into the Centers for Disease Control and Prevention pediatric BMI calculator.15 Patients with a BMI ≥ 85th percentile were included in the overweight/obese group, while those with a BMI < 85th percentile were included in the normal weight (i.e., control) group. Additionally, patients were further divided into 4 BMI subgroups reflecting underweight (< 5th percentile), normal weight (5th-84th percentile), overweight (85th-94th percentile), and obese (≥ 95th percentile).
Study Objectives and Data Collection
Baseline demographic data were collected for all patients using the hospital EMR. Paper charts were used as needed to collect data not found in the EMR (e.g., progress notes and nursing records). In addition to the previously mentioned demographic data, race, admission diagnosis, and length of stay were also collected. A Pediatric Risk of Mortality (PRISM III) score, a common tool used to assess pediatric mortality based on physiologic status, was calculated for each patient.16 This score was used to evaluate a patient's initial mortality risk and to assess homogeneity between the overweight/obese and control group.
Additional information was collected regarding each patient's sedation regimen. Supplementary sedative agents were recorded for each patient throughout the duration of their fentanyl regimen, including continuous infusions of benzodiazepines, dexmedetomidine, ketamine, neuromuscular blockers, and pentobarbital. The number of concomitant and as needed sedative boluses per patient was also collected. Each patient's initial fentanyl loading (mcg/kg; total mcg) and infusion rate (mcg/kg/hr; total mcg/hr) doses, peak fentanyl dose (mcg/kg/hr), number of dosing adjustments per day, cumulative fentanyl dose (mcg), and duration of fentanyl infusion (days) were recorded. Dosing was evaluated based on recommendations included in the Pediatric Dosage Handbook17 and from the adult literature compared with the control group.18
The primary outcome for each group was defined as the number of fentanyl dosing changes per day that were necessary to maintain adequate sedation in each patient as determined by clinician judgment. One secondary objective included the use of concomitant sedatives and analgesics to maintain adequate sedation. Withdrawal symptoms have been shown to increase as the duration of infusion and total cumulative dose increases.13 It is unknown if there is a difference between the incidence of withdrawal for normal weight and overweight/obese children; hence, another secondary outcome included withdrawal symptoms following the discontinuation of fentanyl.
The incidence of withdrawal was analyzed retrospectively after the discontinuation of the fentanyl infusion using the Modified Narcotic Withdrawal Scale (MNWS),19 as currently no standardized tool is used to assess withdrawal at the bedside. The MNWS includes 19 different symptoms that are weighted for the severity (e.g., seizures = 6, lacrimation = 1). Significant withdrawal symptoms were scored as > 8 on the MNWS.19 The MNWS scale was performed daily for 4 days following discontinuation of fentanyl. Based on a fentanyl half-life of 21 hours this allowed 4 to 5 half-lives for elimination of a majority of the drug.12 To provide descriptive data pertaining to treatment of withdrawal, the number of additional PRN opioid analgesic doses was noted. Additionally, the number of patients receiving preventative treatment for drug withdrawal (e.g., methadone) due to extended duration of therapy or elevated cumulative dose was collected and analyzed.
Statistical Methods
Descriptive and inferential statistics were performed. To assess the relationship between group (BMI ≥ 85th vs < 85th percentile) and the primary and secondary outcome variables, independent sample t tests and chi-square tests were used for continuous and categorical data, respectively. Data management and analysis was conducted using STATA for Windows version 10.1 (StataCorp, College Station, TX).20 The a priori alpha was set at p≤0.05. Data that were not normally distributed, due to a small sample size, were transformed in an ad hoc analysis. A square root transformation was performed on the individual data points for each variable in question.
RESULTS
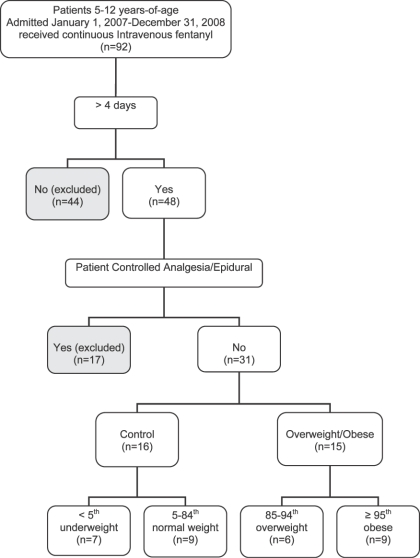
A total of 31 patients were identified representing 34 infusions (Figure). Fifteen patients with a total of 16 courses of continuous intravenous fentanyl infusions were identified in the overweight/obese group, of which 9 patients were classified as obese (≥ 95th percentile). Sixteen patients with 18 total courses were identified in the normal weight group. The groups were well matched for age (Table 1). There were no differences in length of stay, PRISM III score, and duration of fentanyl infusions between the 2 groups (Tables 1 and 2).
Figure.
Flow chart of sample selection process.
Table 1.
Baseline Demographics
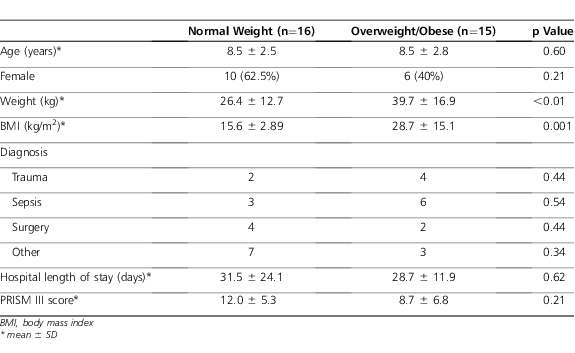
Table 2.
Fentanyl Continuous Infusion Dosing
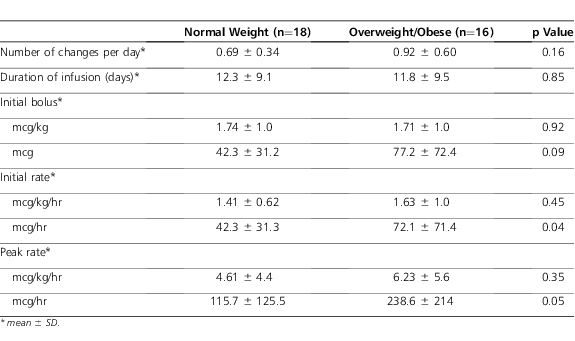
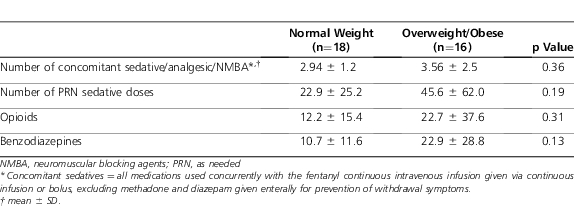
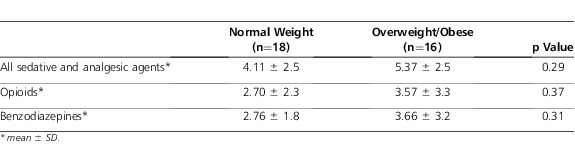
There was a numerical but nonstatistical difference between groups for the primary outcome of dose changes per day (Table 2). Despite appropriate weight-based dosing, it should be noted that the initial infusion rate of 2 patients in the overweight/obese group exceeded the maximal initial rate of 100 mcg/hr for adults.18 One of these patients was opioid-naïve and received 2 mcg/kg/hr; the other was opioid-experienced and received 5 mcg/kg/hr. There were no statistical differences between other variables, including number of concomitant sedative/analgesic agents and peak infusion dose. In addition, the average infusion dose and duration were comparable between groups. There was a numerical difference in the total number of bolus doses given, during the continuous intravenous fentanyl infusion, between the overweight/obese and control groups (Table 3).
Table 3.
Concomitant Sedative Regimens
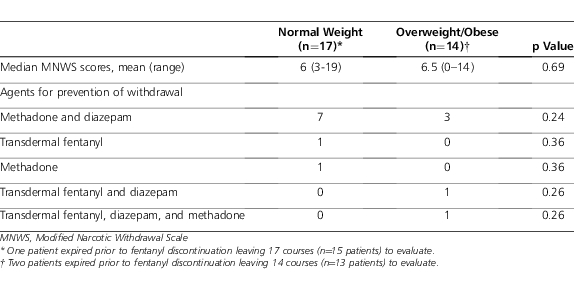
In the assessment of withdrawal, 3 patients (i.e., 1 control and 2 overweight/obese patients) expired prior to discontinuation of fentanyl and were excluded for evaluation with the MNWS (Table 4). The median score was similar between groups. There was no statistical difference between groups with a MNWS score > 8 (p=0.71). The use of agents to prevent withdrawal was also similar between groups (Table 4). Following discontinuation of intravenous fentanyl, there was no statistical difference in the mean PRN sedative or analgesic bolus doses required between the overweight/obese and control groups, 1.53 versus 1.47 doses (p=0.92).
Table 4.
Withdrawal Assessment and Treatment
An ad hoc analysis was performed. In this analysis, the square root transformation of several variables was performed due to the nonnormal distribution of the data (Table 5). No statistical significance was detected with these analyses between groups.
Table 5.
Ad Hoc Analysis: Square Root of Number of As Needed Sedative Doses
DISCUSSION
Few studies have specifically evaluated pharmacokinetic changes and dosing requirements in medications like fentanyl in overweight/obese children. In a recent study, Miller and colleagues23 reported the prevalence of overweight children (5-12 years of age) admitted to an inpatient institution over a 6-month period and the likelihood of inappropriate medication dosing in this population. This study found that 33.1% of children were classified as overweight/obese, in comparison to the patient population of the current study where 48.4% were classified as overweight/obese. The authors noted considerable medication dosing errors in overweight/obese patients receiving analgesics, which have a high risk for adverse effects when dosed inappropriately.23
In obese adults, pharmacokinetic properties of medications have been noted. First, increased adipose tissue and decreased lean body mass alter the volume of distribution of lipophilic medications in obese patients. In addition, phase I and phase II metabolic reactions can be heightened in this patient population. Last, increased kidney size leads to higher glomerular filtration rates and increased excretion of renally eliminated medications.6 With these possible changes in mind, it is prudent to adjust dosing to avoid toxicity or therapeutic failure.
To date, 2 studies have been conducted to evaluate the impact of the pharmacokinetic changes of obesity in adults receiving continuous fentanyl infusions. Shibutani et al21,22 observed that optimal dosing of continuous intravenous infusions of fentanyl was derived from the “pharmacokinetic mass” rather than actual, ideal, or adjusted body weights. Pharmacokinetic mass was determined based on observations of the investigators including effect, serum fentanyl levels, and dose. As actual body weight increases, pharmacokinetic mass increases logarithmically, suggesting there may be an upper limit to the weight by which fentanyl is dosed.21,22 Thus, if fentanyl is dosed based on actual body weight in obese patients, there is a greater risk for overdosing, oversedation, and possibly development of withdrawal upon rapid discontinuation. In our study, we found a trend in the number of dosing changes per day in the overweight/obese group versus control. Although not statistically significant, we also noted a trend toward more sedative/analgesic PRN doses in overweight/obese group to attain adequate sedation as determined by the medical team. Without a prospective study evaluating serum concentrations, it is difficult to draw a significant number of conclusions from our pilot study.
Although our study did not find a significant difference in dosing changes between groups and did not evaluate serum concentrations, our results do highlight some considerations for clinicians caring for critically ill overweight/obese children receiving fentanyl. One particular concern that we noted in our study was the use of weight-based dosing for the overweight/obese pediatric population. In our study, we noted that 9 children (29.0%) were obese and received continuous infusion dosing in mcg/kg/hr. For children with adult-sized weights (e.g., >70 kg), there may be an increased risk of severe adverse effects (i.e., respiratory depression) due to the potential for overdosing of agents such as fentanyl. In the current study, analysis of adverse effects beyond withdrawal was not assessed. To ensure that medication errors are minimized in the pediatric population, the Academy of Pediatrics recommends that pediatric weight-based dose should not exceed recommended adult maximum doses.24 In this study, the initial infusion rate of 2 patients in the overweight/obese group exceeded 100 mcg/hr, which is cited as the maximal initial rate; however, rates did not exceed the maximum weight based dosing of 0.7 to 10 mcg/kg/hr.18,25 Consistent with the report of Miller et al,23 our results highlight that obese children would receive larger doses than those recommended for typical adult regimens.
Until further studies can be performed to evaluate the pharmacokinetic differences in overweight/obese children for fentanyl continuous infusions, clinicians may consider some general dosing guidelines. For children and adolescents < 40 kg, clinicians should use weight-based dosing in mcg/kg/hr. Generally, this dose may be increased by 1 mcg/kg/hr every 30 to 60 minutes and titrated to effect. Conversely, for patients ≥ 40 kg, it may be prudent to initiate fentanyl infusions at the recommended adult dosing in mcg/hr (i.e., 100 mcg/hr).18 To achieve adequate sedation, this dose should be increased by 50 to 100 mcg/hr every 30 to 60 minutes. These are general recommendations to be used to avoid potential accumulation of fentanyl and adverse events while maintaining adequate analgesia.
There are several limitations to this study. The use of the MNWS, which has only been validated prospectively, has questionable use in retrospective analyses. To address this within the study design, we analyzed the use of PRN sedative and analgesic agents following discontinuation of fentanyl to determine if these agents were used to treat symptoms of withdrawal. Another limitation was the inconsistent recording of PRN boluses from paper chart to EMR. The EMR was used as the main source of charting PRN doses due to the patient safety checks implemented by the hospital. Each patient and medication was scanned before administration to ensure that the appropriate drug was administered to the correct patient. Retrospectively, there was no way to discern which record was most accurate. Another limitation relates to using the number of dose changes per day to determine the appropriateness of dosing. Because it is difficult to retrospectively identify underdosing or overdosing during a fluctuating dose of continuous infusion fentanyl, we used a surrogate endpoint to determine the dose needed to achieve the therapeutic endpoint as determined by the medical team. Future studies should be conducted to determine more direct endpoints to account for pharmacokinetic alterations with fentanyl in overweight/obese children.
We did not note a statistical difference in the number of dosing changes per day of fentanyl continuous infusions. This could likely be explained by the small sample size as evidenced by the extreme nonnormal distribution of the data points seen with many variables. For example, the number of bolus doses during the continuous fentanyl infusions is almost doubled in the overweight/obese group versus control group despite no statistical difference being determined. Also, the ad hoc analysis used to normalize the distribution of these data points did not find any statistically significant difference between groups. Larger studies are necessary to determine whether or not variables such as this could indicate a need for larger doses of fentanyl to adequately maintain sedation in overweight/obese children. A post hoc power calculation was performed and estimated that 69 patients in each group would be necessary to reach 28% power to show a difference in the primary outcome. With this in mind, a much larger study would be necessary to truly assess the differences in dosing requirements of fentanyl continuous intravenous infusions.
CONCLUSION
In this retrospective analysis, there was a numerical, but not statistically significant, difference in the number of mean dosing changes per day in continuous fentanyl infusions required as part of the sedation/analgesia regimen in overweight/obese and normal-weight, critically ill children. Clinicians should be aware of the pharmacokinetic differences in overweight/obese patients and how these may affect therapeutic outcomes. The pharmacokinetic differences in overweight/obese children should be the focus of future studies to determine the optimal safe and effective doses of analgesic agents like fentanyl.
Acknowledgments
Platform presentations at the ALCALDE Residency Conference in Austin, Texas in April 2009 and poster presentation at ASHP Midyear Clinical Meeting in Orlando, Florida in December 2008 were made.
Abbreviations
- BMI
body mass index
- EMR
electronic medical record
- MNWS
Modified Narcotic Withdrawal Scale
- PICU
pediatric intensive care unit
Footnotes
DISCLOSURE The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303(3):235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA. 2010;303(3):242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 3.Barlow SE. and the Expert Committee. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(suppl 4):S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Defining childhood overweight and obesity. 2010 www.cdc.gov/obesity/childhood/defining.html. Accessed November 19. [Google Scholar]
- 5.Wurtz R, Itokazu G, Rodvold K. Antimicrobial dosing in obese patients. Clin Infect Dis. 1997;25(1):112–118. doi: 10.1086/514505. [DOI] [PubMed] [Google Scholar]
- 6.Cheymol G. Effects of obesity on pharmacokinetics: implications for drug therapy. Clin Pharmacokinet. 2000;39(3):215–231. doi: 10.2165/00003088-200039030-00004. [DOI] [PubMed] [Google Scholar]
- 7.Koshida R, Nakashima E, Taniguchi N, et al. Prediction of the distribution volumes of cefazolin and tobramycin in obese children based on physiological pharmacokinetic concepts. Pharm Res. 1989;6(6):486–491. doi: 10.1023/a:1015968407226. [DOI] [PubMed] [Google Scholar]
- 8.Ritzmo C, Soderhall S, Karlen J, et al. Pharmacokinetics of doxorubicin and etoposide in a morbidly obese pediatric patient. Pediatr Hematol Oncol. 2007;24(6):437–445. doi: 10.1080/08880010701451343. [DOI] [PubMed] [Google Scholar]
- 9.Hijiya N, Panetta JC, Zhou Y, et al. Body mass index does not influence pharmacokinetics or outcomes of treatment of children with acute lymphoblastic leukemia. Blood. 2006;108(13):3997–4002. doi: 10.1182/blood-2006-05-024414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Twite MD, Rashid A, Zuk J, Friesen RH. Sedation, analgesia, and neuromuscular blockade in the pediatric intensive care unit: survey of fellowship training programs. Pediatr Crit Care Med. 2004;5(6):521–532. doi: 10.1097/01.PCC.0000144710.13710.2E. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz AE, Matteo RS, Ornstein E, et al. Pharmacokinetics of sufentanil in obese patients. Anesth Analg. 1991;73(6):790–793. [PubMed] [Google Scholar]
- 12.Katz R, Kelly HW. Pharmacokinetics of continuous infusions of fentanyl in critically ill children. Crit Care Med. 1993;21(7):995–1000. doi: 10.1097/00003246-199307000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Katz R, Kelly HW, Hsi A. Prospective study on the occurrence of withdrawal in critically ill children who receive fentanyl by continuous infusion. Crit Care Med. 1994;22(5):763–767. doi: 10.1097/00003246-199405000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Carr RR, Ensom MHH. Drug disposition and therapy in adolescence: the effects of puberty. J Pediatr Pharmacol Ther. 2003;8(2):86–96. doi: 10.5863/1551-6776-8.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.BMI percentile calculator for child and teen metric version. Centers for Disease Control; 2010. http://apps.nccd.cdc.gov/dnpabmi/. Accessed November 19. [Google Scholar]
- 16.Pollack MM, Patel KM, Ruttimann UE. PRISM. III: an updated pediatric risk of mortality score. Crit Care Med. 1996;24(5):743–752. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Taketomo CK, Hodding JH, Kraus DM. Pediatric Dosage Handbook. 16th ed. Hudson, OH: Lexi-Comp, Inc;; 2009. [Google Scholar]
- 18.Mirski MA, Lewin JJ. Sedation and pain management in acute neurological disease. Semin Neurol. 2008;28(5):611–630. doi: 10.1055/s-0028-1105970. [DOI] [PubMed] [Google Scholar]
- 19.Darnell CM, Thompson J, Stromberg D, et al. Effect of low-dose naloxone infusion on fentanyl requirements in critically ill children. Pediatrics. 2008;121(5):e1363–e1371. doi: 10.1542/peds.2007-1468. [DOI] [PubMed] [Google Scholar]
- 20.STATA for Windows version 10.1. College Station, TX: StataCorp; 2009. [Google Scholar]
- 21.Shibutani K, Inchiosa MA, Jr, Sawada K, Bairamian M. Accuracy of pharmacokinetic models for predicting plasma fentanyl concentrations in lean and obese surgical patients: derivation of dosing weight (“pharmacokinetic mass”) Anesthesiology. 2004;101(3):603–613. doi: 10.1097/00000542-200409000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Shibutani K, Inchiosa MA, Jr, Sawada K, Bairamian M. Pharmacokinetic mass of fentanyl for postoperative analgesia in lean and obese patients. Br J Anaesth. 2005;95(3):377–383. doi: 10.1093/bja/aei195. [DOI] [PubMed] [Google Scholar]
- 23.Miller JL, Johnson PN, Harrison DL, Hagemann TM. Evaluation of antimicrobial and analgesic dosing errors in overweight children. Ann Pharmacother. 2010;44(1):35–42. doi: 10.1345/aph.1M371. [DOI] [PubMed] [Google Scholar]
- 24.Stucky ER. American Academy of Pediatrics Committee on Drugs and Committee on Hospital Care. Prevention of medication errors in the pediatric inpatient setting. Pediatrics. 2003;112(2):431–436. doi: 10.1542/peds.112.2.431. [DOI] [PubMed] [Google Scholar]
- 25.Fentanyl. DRUGDEX® System [Internet database]. Version 2.0. Greenwood Village, CO: Thomson Healthcare; 2010. In. Updated 7 January 2011. http://thomsonreuters.com/products_services/healthcare/healthcare_products/a-z/drugdex_system/ Accessed November 14. [Google Scholar]