Abstract
The lipid A moiety of Escherichia coli lipopolysaccharide is a hexa-acylated disaccharide of glucosamine that makes up the outer monolayer of the outer membrane. Arabidopsis thaliana contains nuclear genes encoding orthologs of key enzymes of bacterial lipid A biosynthesis, including LpxA, LpxC, LpxD, LpxB, LpxK and KdtA. Although structurally related lipid A molecules are found in most other Gram-negative bacteria, lipid A and its precursors have not been directly detected in plants previously. However, homozygous insertional knockout mutations or RNAi knock-down constructs of Arabidopsis lpx and kdtA mutants revealed accumulation (or disappearance) of the expected monosaccharide or disaccharide lipid A precursors by mass spectrometry of total lipids extracted from 10-day old seedlings of these mutants. In addition, fluorescence microscopy of lpx-gfp fusions in transgenic Arabidopsis plants suggests that the Lpx and KdtA proteins are expressed and targeted to mitochondria. Although the structure of the lipid A end product generated by plants is still unknown, our work demonstrates that plants synthesize lipid A precursors using the same enzymatic pathway present in E. coli.
Keywords: glucosamine-based lipids, Lpx genes, higher plants, electrospray ionization
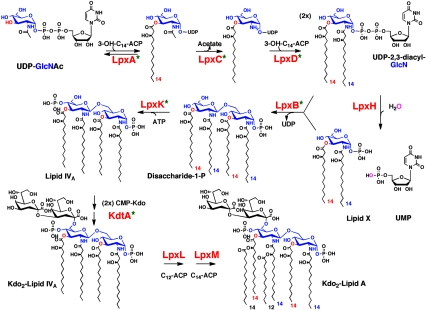
Lipid A, the hydrophobic portion of LPS, is a glucosamine-based lipid that makes up the outer monolayer of the outer membrane of Gram-negative bacteria (1, 2). Kdo2-lipid A (Fig. 1) usually represents the minimal LPS substructure required for growth (1, 2). In a few systems, like Neisseria meningitides, lipid A biosynthesis is not essential (3). However, in most cases lipid A biosynthesis inhibitors that target LpxC (Fig. 1) are potent Gram-negative-selective antibiotics (4). The structure of lipid A is relatively conserved in diverse Gram-negative pathogens (1, 2). Picomolar Kdo2-lipid A is recognized by the TLR4/MD2 receptor complex of animal cells (5), triggering cytokine production that can contribute to the complications of Gram-negative sepsis (6).
Fig. 1.
Enzymatic synthesis of lipid A in E. coli and evidence for a similar pathway in A. thaliana. The green asterisks indicate that significant orthologs of the E. coli enzymes are present in A. thaliana. These proteins show 27–41% sequence identity over their full-lengths (Table 1).
Most Gram-negative bacteria encode single copy homologues of the nine Escherichia coli enzymes that assemble the Kdo2-lipid A moiety of E. coli LPS (Fig. 1) (1, 2). Gram-positive bacteria, Archaea, fungi, insects, worms, and vertebrates do not contain these genes. Although lipid A-like molecules have not been reported in plants, many plants, including Arabidopsis thaliana, encode full-length nuclear orthologs of six of the nine enzymes of the E. coli system (Fig. 1, green asterisks; and Table 1) (1, 7). A homologue of the UDP-2,3-diacylglucosamine pyrophosphatase LpxH of E. coli (Fig. 1) is missing in plants, but it is also missing in some Gram-negative bacteria in which it may be replaced with a different pyrophosphatase (8).
Table 1.
Full-length orthologs of E. coli lipid A biosynthetic enzymes in Arabidopsis
| Gene names | Locus tags | Splice variants | Protein names | Lengths* (Ec/At) | Sequence similarity† | Paired E values | Identity (%) |
| AtLpxA | AT4G29540 | AT4G29540.1‡ | AtLpxA1 | 262/334 | 108/156/291 | 1 × 10-50 | 38 |
| AT4G29540.2‡ | AtLpxA2 | 262/336 | 112/157/293 | 3 × 10-50 | 39 | ||
| AtLpxC1 | AT1G24793 | AT1G24793.1‡ | AtLpxC1.1 | 305/326 | 94/137/289 | 9 × 10-32 | 33 |
| AT1G24793.2‡ | AtLpxC1.2 | 305/266 | 71/109/230 | 1 × 10-21 | 31 | ||
| AtLpxC2 | AT1G24880 | AT1G24880.1§ | AtLpxC2.1 | 305/369 | 94/138/289 | 1 × 10-31 | 33 |
| AtLpxC3 | AT1G25054 | AT1G25054.1¶ | AtLpxC3.1 | 305/369 | 94/138/289 | 1 × 10-31 | 33 |
| AT1G25054.2¶ | AtLpxC3.2 | 305/326 | 94/137/289 | 9 × 10-32 | 33 | ||
| AtLpxC4 | AT1G25145 | AT1G25145.1§ | AtLpxC4 | 305/326 | 94/137/289 | 9 × 10-32 | 33 |
| AtLpxC5 | AT1G25210 | AT1G25210.1¶ | AtLpxC5.1 | 305/371 | 94/137/289 | 1 × 10-31 | 33 |
| AT1G25210.2¶ | AtLpxC5.2 | 305/311 | 71/109/230 | 1 × 10-21 | 31 | ||
| AtLpxD1 | AT4G05210 | AT4G05210.1¶ | AtLpxD1 | 341/299 | 85/117/211 | 5 × 10-36 | 41 |
| AtLpxD2 | AT4G21220 | AT4G21220.1¶ | AtLpxD2 | 341/304 | 96/144/286 | 5 × 10-38 | 34 |
| AtLpxB | AT2G04560 | AT2G04560.1‡ | AtLpxB | 382/455 | 118/179/366 | 3 × 10-43 | 33 |
| AtLpxK | AT3G20480 | AT3G20480.1‡ | AtLpxK | 328/395 | 88/148/336 | 2 × 10-27 | 27 |
| AtKdtA | AT5G03770 | AT5G03770.1‡ | AtKdtA | 425/447 | 146/219/411 | 2 × 10-56 | 36 |
*The full-length E. coli (Ec) lipid A biosynthesis proteins were compared to their predicted A. thaliana (At) counterparts, as currently annotated in The Arabidopsis Information Resource (TAIR) database. The number of amino acid residues for each protein is indicated.
†Sequence similarity is shown as identities/positives/length of aligned segments (gaps not indicated). Pairwise BLASTp alignment (40) was used to determine the E values.
‡These sequences are supported by the identification of full-length mRNA transcripts and EST records.
§These protein sequences are deduced from the genomic DNA sequences without documentation of full-length mRNA transcripts or EST records.
¶These protein sequences are deduced from the genomic DNA sequences together with some EST records.
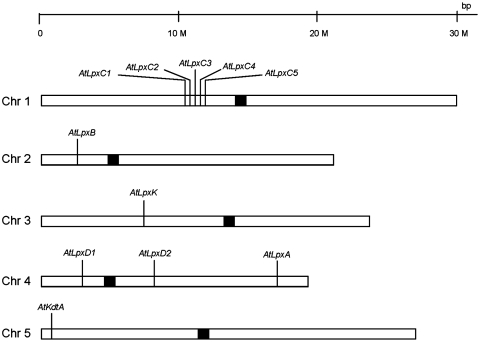
We now report the isolation and characterization of homozygous insertional knockout mutants in the A. thaliana genes encoding LpxA, LpxD, LpxB, LpxK, and KdtA (Table 1 and Fig. 2). Given the presence of multiple genes encoding LpxC-like proteins in Arabidopsis (Fig. 2), we chose an RNAi strategy to suppress AtLpxC expression. Mutants generated from both gene inactivation strategies are viable under laboratory conditions. However, they accumulate (or lose) the expected lipid A precursors (Fig. 1) when analyzed by sensitive liquid chromatography–electrospray ionization/mass spectrometry (LC–ESI/MS) protocols. The lipid A biosynthetic genes of higher plants may have been acquired from Gram-negative bacteria with the endosymbiosis of mitochondria. Plant lipid A may therefore have a structural role in mitochondrial or perhaps chloroplast outer membranes. Alternatively, it may serve a regulatory role, or it may participate in signal transduction, for instance, in the plant response to infection by bacterial pathogens (9). This work definitively demonstrates the presence of lipid A precursors in a eukaryotic system.
Fig. 2.
Chromosomal locations of the putative Arabidopsis lipid A genes. The gene locations were obtained from The Arabidopsis Information Resource database. Multiple AtLpxC genes may have arisen by duplication.
Results
Identification of Orthologs of Lipid A Biosynthesis Genes in Arabidopsis.
A position-specific iterated-BLAST search of the nonredundant protein sequences database was conducted using the E. coli protein sequences of LpxA, LpxC, LpxD, LpxB, LpxH, LpxK, KdtA, LpxL, and LpxM as probes (Fig. 1). Eleven nuclear sequences were identified in A. thaliana, encoding proteins with significant homology to E. coli LpxA, LpxC, LpxD, LpxB, LpxK, or KdtA (Table 1). Homologues of LpxH, LpxL, and LpxM were not found in Arabidopsis, but these are also absent in some Gram-negative bacteria (10). The AtLpxA gene (Table 1) encodes two mRNA splice variants, one of which directs the synthesis of a protein with two additional amino acid residues inserted in the C-terminal region. These protein variants, designated AtLpxA1 and AtLpxA2, are otherwise identical to each other; they share 38 and 37% sequence identity with E. coli LpxA, respectively, (Table 1), with conservation of all active site residues (11). There are five AtLpxC orthologs located in a 68-kb region on chromosome one (Fig. 2). They have very similar nucleotide sequences, suggesting possible gene duplications from a common ancestor. Expressed sequence tag (EST) and deduced cDNA databases indicate these AtLpxC genes generate at least eight different but related mRNA transcripts (Table 1). Previous studies of E. coli LpxC had shown that three amino acid residues (H238, D246, and H265) in the C-terminal region of the protein are critical for activity (12). These residues are truncated in two of the eight AtLpxC proteins, specifically in AtLpxC1.2 and AtLpxC5.2 (Table 1), suggesting that these two proteins are not catalytically active.
Two AtLpxD orthologs (designated AtLxpD1 and AtLpxD2) were encoded on chromosome 4 (Fig. 2 and Table 1); these proteins share 41 and 34% identity with E. coli LpxD (Table 1), with conservation of key catalytic residues (13); however, only AtLpxD2 contains the putative N-terminal nucleotide binding domain of LpxD (13), which is required for E. coli LpxD activity. Single copy genes encoding AtLpxB (AT2G04560), AtLpxK (AT3G20480) and AtKdtA (AT5G03770) (Table 1) were also found, with 31, 26, and 34% sequence identity to the corresponding E. coli proteins, respectively (Table 1).
Putative full-length genes encoding enzymes of lipid A biosynthesis are present in many other plants, including Oryza sativa japonica, Physcomitrella patens, Populus trichocarpa, Ricinus communis, Selaginella moellendorffii, Sorghum bicolor, Triticum aestivum, Vitis vinifera, and Zea mays. However, no lipid A-like molecules have previously been identified in plants by physical or chemical methods. Histochemical evidence for lipid A in green algae and peas was recently reported (14).
Insertional Knockout Mutants and RNAi Knock-Down Constructs in the Plant Lipid A System.
To evaluate the functions of the putative Arabidopsis lipid A biosynthetic enzymes, insertional mutants for AtLpxA, AtLpxD1, AtLpxD2, AtLpxB, AtLpxK, and AtKdtA were obtained as seeds from the Arabidopsis Biological Resource Center at Ohio State University and other facilities (15–18) (Fig. S1). After characterizing 30-day old plants by PCR-based genotyping and RT-PCR (Fig. S2), homozygous null mutants of the above genes were identified and used for further experiments. To suppress transcription of the five closely linked AtLpxC genes (Fig. 2 and Table 1), independent AtLpxC-RNAi transgenic lines were generated in Columbia-0 (Col-0) wild-type Arabidopsis. In nine selected T1 transgenic lines, the expression levels of AtLpxC mRNA transcripts were characterized by RT-PCR using primers designed from regions of sequence identity (Fig. S2G, and Table S1). T3 homozygous AtLpxC-RNAi lines with the lowest expression of AtLpxC mRNA were used for further studies. All null mutants and RNAi knock-down lines were viable and showed no obvious phenotypic differences compared to wild-type plants.
Detection of 2,3-Diacylglucosamine 1-Phosphate in Arabidopsis and its Accumulation in an atlpxb-1 Mutant.
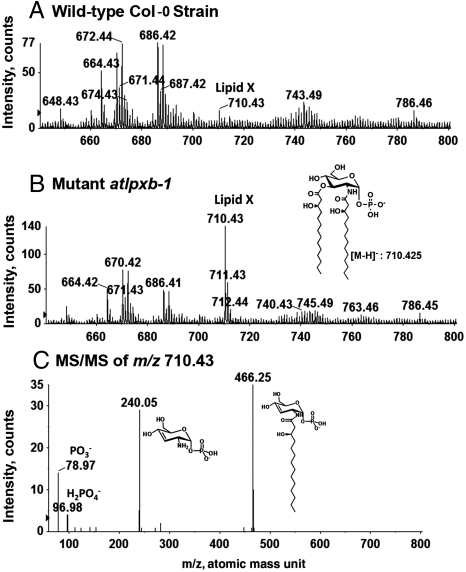
To detect lipid A-like molecules or lipid A precursors in Arabidopsis, total lipids were extracted from homogenates of 10-day old plants and subjected to normal phase LC separation coupled with online ESI/MS/MS in the negative-ion mode. In E. coli, 2,3-diacylglucosamine 1-phosphate (lipid X) is a key precursor of lipid A (Fig. 1). It is detectable at low levels in wild-type cells and accumulates in lpxB mutants (19, 20). To determine if lipid X exists in Arabidopsis, total lipids from the atlpxb-1 mutant and its parental Col-0 wild-type plant were compared by LC–ESI/MS/MS. As shown in Fig. 3A, a small peak at m/z 710.43, consistent with the [M-H]- ion of a lipid X molecule bearing two R-3-hydroxymyristoyl chains (Fig. 1) (8, 19), was present in the wild type. It accumulated more than 10-fold in the atlpxb-1 mutant (Fig. 3B). No other lipid X molecular species were detected. ESI/MS/MS analysis of the m/z 710.43 ion in the atlpxb-1 mutant revealed the fragmentation pattern expected for lipid X (Fig. 3C).
Fig. 3.
Detection of the lipid A precursor 2,3-diacylglucosamine 1-phosphate in A. thaliana. (A) Negative-ion LC–ESI/MS analysis of lipids, eluting from a normal phase LC column between minutes 22.7 and 23.3, from 10-day old Col-0 wild-type seedlings. (B) Corresponding analysis of the lipids from 10-day old seedlings of the atlpxb-1 mutant. The m/z 710.43 ion peak that accumulates in this mutant corresponds to the [M-H]- ion of 2,3-diacylglucosamine 1-phosphate (lipid X) with the same acyl chain composition as E. coli lipid X (Fig. 1). (C) ESI/MS/MS analysis of the lipid X [M-H]- ion at m/z 710.43, which accumulates in the atlpxb-1 mutant. The fragment ions are the same as those seen for E. coli lipid X. The mass spectra were acquired using a high resolution QSTAR XL quadrupole time-of-flight tandem mass spectrometer (Applied Biosystems).
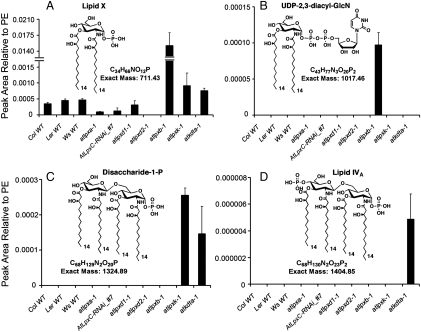
To confirm the presence of lipid X and to quantify its level in the mutants (Fig. 4), a sensitive multiple reaction monitoring (MRM) protocol was developed in conjunction with LC prefractionation. Singly charged precursor/product ion pairs (710.4/240.0) were used to detect lipid X selectively (Figs. S3 and S4). The MRM peak area of the lipid X ion was normalized to that of the major species of Arabidopsis phosphatidylethanolamine (precursor [M-H]- ion at m/z 714.5 and product ion at m/z 279.2), which is acylated with palmitate (C16∶0) and linoleate (C18∶2) (Fig. S3). Lipid X accumulates 42-fold in the atlpxb-1 mutant compared to the Col-0 wild type by this criterion (Fig. 4A).
Fig. 4.
Quantification of 2,3-diacylglucosamine 1-phosphate and other lipid A precursors in Arabidopsis mutants. MRM analysis and quantification of 2,3-diacylglucosamine 1-phosphate (lipid X) (A), UDP-2,3-diacyl-GlcN (B), disaccharide 1-phosphate (C), and lipid IVA (D) was carried out using lipids extracted from the indicated 10-day old seedlings of three parental wild types, six Arabidopsis insertional mutants, and one RNAi transgenic line. These Arabidopsis lipid A precursors, which have the same acyl chain compositions and molecular weights as their E. coli counterparts (Fig. 1), were detected using the precursor/product ion pairs shown in Fig. S3. Peak areas were normalized to the major phosphatidylethanolamine molecular species (C16∶0/C18∶2) present in the same sample (Fig. S3). Error bars represent standard deviations of three biological replicates. The full LC–MRM tracings for each precursor/product ion pair are shown in Figs. S4, S6, S7, and S8. All LC–MRM experiments were performed using a 4,000 Q-Trap hybrid triple quadrupole linear ion trap mass spectrometer, equipped with a Turbo V ion source (Applied Biosystems).
Quantification of Lipid X in Other Arabidopsis Lipid A Mutants.
The level of lipid X in the atlpxa-1 insertion mutant was approximately 21% of its matched wild-type control [Wassilewskija (Ws)], indicating that the loss of function of AtLpxA did not completely eliminate the production of lipid X (Fig. 4A). This result implies that plants may have a second pathway for the acylation of UDP-GlcNAc not present in bacteria.
The lipid X level decreased in AtLpxC-RNAi transgenic plants to approximately 37% of the amount present in the Col-0 wild type (Fig. 4A). To confirm the function of the AtLpxC proteins in production of lipid X and the order of reactions in Fig. 1, the AtLpxC-RNAi transgenes were introduced into the atlpxb-1 mutant by pollination to generate AtLpxC-RNAi atlpxb-1 F2 homozygous plants. Fig. S5 shows that accumulation of lipid X in the atlpxb-1 mutant was greatly attenuated by suppressing the transcription of the AtLpxC genes, consistent with the proposal that LpxC functions before LpxB (Fig. 1).
The lipid X level in the atlpxd1-1 mutant was not reduced from that of wild type (Fig. 4A). Introducing the atlpxd1-1 mutation into the atlpxb-1 mutant background did not lower its elevated lipid X levels (Fig. S5). However, lipid X was undetectable in the atlpxd2-1 mutant compared with its parental plant [Landsberg erecta (Ler)] (Fig. 4A), indicating that AtLpxD2 is the predominant UDP-3-O-acyl-GlcN N-acyltransferase (Fig. 1). Introducing the atlpxd2-1 mutation into the atlpxb-1 mutant background significantly lowered lipid X levels (Fig. S5). The presence of some residual lipid X in this setting leaves open the possibility that AtLpxD1 generates a small portion of the lipid X pool. In the atlpxk-1 and atkdta-1 mutants, lipid X levels were 2.6- and 2.2-fold higher than in the Col-0 wild type, respectively (Fig. 4A), consistent with the pathway (Fig. 1).
Detection of Additional Lipid A Precursors in Arabidopsis Mutants.
UDP-2,3-diacyl-GlcN is the donor substrate for LpxB in E. coli (Fig. 1) (20, 21). By monitoring appropriate pairs of precursor/product ions (1,016.5/385.0) (Fig. S3), UDP-2,3-diacyl-GlcN could be detected by LC–MRM in the atlpxb-1 mutant lipids (Fig. 4B and Fig. S6) at levels that are approximately 100 times lower than those of lipid X. UDP-2,3-diacyl-GlcN was not detectable in the Arabidopsis wild types or in any of the mutants (Fig. 4B).
The disaccharide-1-phosphate intermediate generated by LpxB (Fig. 1) accumulated in the atlpxk-1 and atkdta-1 mutants (Fig. 4C and Fig. S7), as judged by MRM analysis, but was not detectable in wild type or other mutant strains. Lipid IVA (Fig. 1) was detected only in the atkdta-1 mutant (Fig. 4D and Fig. S8), but was not seen in the wild type and other mutants. The independently isolated atlpxb-2, atlpxk-2, and atlpxk-3 mutants showed similar lipid accumulation patterns as the atlpxb-1 and atlpxk-1 mutants.
Lipid A Pathway Enzymes are Localized in Arabidopsis Mitochondria.
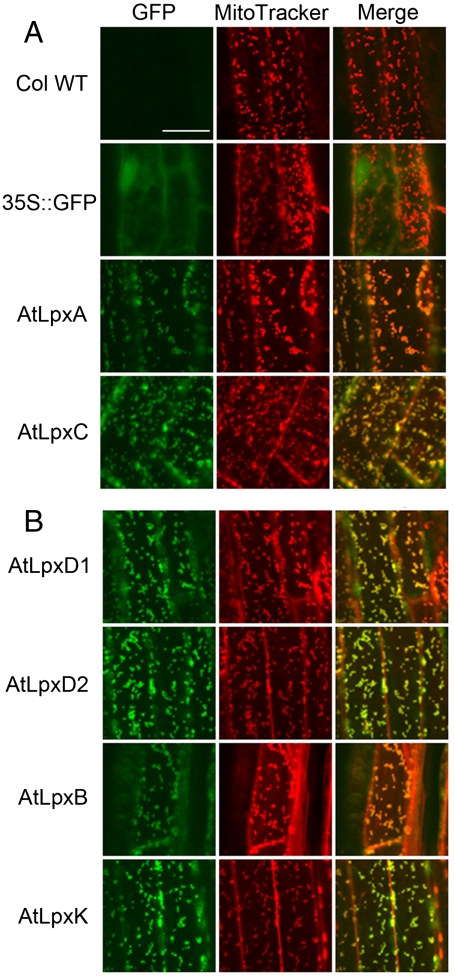
The AtKdtA protein was recently localized to mitochondria of Arabidopsis (22), although no characterization of its activity was reported. To determine the subcellular localization of AtLpxA, AtLpxC1.1, AtLpxD1, AtLpxD2, AtLpxB, and AtLpxK, C-terminal GFP fusions were generated by cloning either the full-length coding sequences or the 5′ termini of the coding sequences in-frame with GFP under the control of the CaMV 35S promoter (see SI Materials and Methods). These constructs were transformed into Arabidopsis wild-type Col-0, and T2 generation plants were analyzed by confocal microscopy. In mesophyll cells, GFP signals were shown as 1 μm dots, but were not colocalized with chloroplasts (data not shown). To avoid interference from chlorophyll auto-fluorescence, only root cells of the transgenic lines were examined. As shown in Fig. 5, the fluorescence of the GFP fusion proteins was localized to small but distinct regions. To confirm that this fluorescence reflected mitochondrial localization, MitoTracker stain (23) was used in parallel. GFP and MitoTracker fluorescence signals were recorded simultaneously. The GFP fluorescence from the full-length GFP fusions of AtLpxA and AtLpxC1.1 localized in mitochondria (Fig. 5A). Full-length GFP fusions of AtLpxD1, AtLpxD2, AtLpxB, and AtLpxK did not emit sufficient GFP fluorescence to permit localization. However, 5′-terminal fusions of AtLpxD1, AtLpxD2, AtLpxB, and AtLpxK to GFP showed the same mitochondrial pattern as full-length AtLpxA and AtLpxC (Fig. 5B).
Fig. 5.
Subcellular localization of GFP-fusion proteins of AtLpxA, AtLpxC1.1, AtLpxD1, AtLpxD2, AtLpxB, and AtLpxK. Roots from seven-day old Arabidopsis plants expressing the indicated GFP fusion proteins (green) were stained simultaneously with MitoTracker (red). The GFP and MitoTracker images were merged to show the colocalization of the GFP signal and mitochondria. Col WT, the Col-0 WT without the GFP transgene; 35S∷GFP, overexpression of GFP cDNA under the control of 35S CaMV promoter in the Col-0 background. Scale bar: 20 μm.
The mitochondrial localization of the GFP fusion proteins suggested that lipid A-like molecules of plants might also be localized in mitochondria. Subcellular fractions from atlpxb-1 plants were subjected to LC–ESI/MS. Lipid X levels in mitochondria were 3- and 48-fold higher than in chloroplasts or whole cell homogenates, respectively. Lipid X was undetectable in the plasma membrane.
Discussion
The Kdo2-lipid A portion of LPS (Fig. 1) makes up the outer monolayer of the outer membranes of most Gram-negative bacteria (1, 2). The structure and biosynthesis of Kdo2-lipid A are relatively conserved (1, 2). In E. coli, nine enzymes are required for Kdo2-lipid A biosynthesis, designated LpxA, LpxC, LpxD, LpxH, LpxB, LpxK, KdtA, LpxL, and LpxM (Fig. 1) (1, 2). Although Kdo2-lipid A and its precursors have not been identified previously as components of plant lipids, the emerging databases of plant protein, DNA, and EST sequences have revealed the presence of nuclear genes encoding full-length orthologs of LpxA, LpxC, LpxD, LpxB, LpxK, and KdtA in many higher plants (Fig. 1 and Table 1), including A. thaliana. The functions of these genes are unknown, but their presence suggests that plants synthesize lipid A-like molecules. We previously showed that AtLpxA can complement an E. coli mutant defective in its own chromosomal lpxA gene (1, 7), and that all active site residues of E. coli LpxA (11) are conserved in AtLpxA.
By combining the power of Arabidopsis genetics with the sensitivity of ESI mass spectrometry, we have demonstrated the presence of lipid A precursors in Arabidopsis with the same structures as their E. coli counterparts. Of the precursors shown in Fig. 1, only lipid X was detected in the wild type (Figs. 3 and 4). The accumulation of lipid X together with UDP-2,3-diacyl-GlcN in three independently grown atlpxb-1 null mutant plants (Figs. 3 and 4) demonstrates that both compounds are likely substrates for AtLpxB in vivo (Fig. 1). Furthermore, the genes encoding AtLpxA, AtLpxC, and AtLpxD2 are required for the efficient production of lipid X, as judged by analyzing lipids extracted from the single null mutants or from the AtLpxC-RNAi-7 transgenic line (Fig. 4A). Some residual lipid X (∼21% of wild type) is present in atlpxA mutants (Fig. 4A), suggesting the presence of an additional UDP-GlcNAc acyltransferase (see below).
Five duplicated AtLpxC genes are found in a short region of chromosome 1 (Fig. 2 and Table 1). The lipid X level was significantly reduced (to ∼37% of wild type) by suppressing AtLpxC gene expression with RNAi. However, it remains unclear which of the closely related AtLpxC enzymes is functional in vivo (Table 1).
Although the AtLpxD1 and AtLpxD2 proteins share more than 60% sequence identity, lipid X levels are reduced only in atlpxd2 mutants (Fig. 4). These results suggest that AtLpxD2 is the primary UDP-3-O-acyl-GlcN N-acyltransferase in Arabidopsis. Our previous study of E. coli LpxD revealed that R293 may be required for R-3-OHC14-ACP binding (13, 24). R293 is conserved between E. coli LpxD and AtLpxD2, but is replaced by lysine in AtLpxD1. AtLpxD1 also lacks two aromatic residues in its N-terminal domain that are required for acceptor substrate binding (13, 24).
At present, we cannot exclude the intriguing possibility that AtLpxD1 has LpxA activity, possibly accounting for the residual lipid X seen in atlpxA mutants (Fig. 4). Construction of double mutants lacking AtLpxA and AtLpxD1 should clarify this issue.
In E. coli, LpxK phosphorylates the disaccharide 1-phosphate product made by LpxB to generate lipid IVA (Fig. 1) (1, 2). This step is followed by the transfer of two Kdo residues to lipid IVA by KdtA (Fig. 1) (1, 2). The disaccharide 1-phosphate was not seen in wild-type Arabidopsis, but accumulates in the atlpxk-1 and the atkdta-1 mutants (Fig. 4C). Lipid IVA is detected only in the atkdta-1 mutant (Fig. 4D). We were unable to detect Kdo-lipid IVA, Kdo2-lipid IVA, and putative penta- or hexa-acylated derivatives (Fig. 1). The end products of the plant lipid A pathway remain unknown. The numbers and lengths of additional acyl chains linked to Kdo2-lipid IVA in Arabidopsis might differ from those in E. coli, and other covalent modifications cannot be excluded (2).
The physiological role of lipid A-like molecules in Arabidopsis is unclear. Null mutants of AtLpxA, AtLpxD2, AtLpxB, AtLpxK, and AtKdtA, as well as AtLpxC-RNAi knock-down plants, show no obvious phenotypic differences compared to wild type under laboratory conditions, demonstrating that these genes are not essential for plant growth and development. Our investigations with GFP fusions show that the proteins required for lipid A precursor biosynthesis are targeted to the mitochondria, and the lipid X that accumulates in the atlpxb-1 mutant is mainly located in mitochondria and chloroplasts. These findings suggest that lipid A precursors are synthesized in mitochondria and may be transported from mitochondria to chloroplasts. Conversely, plant digalactosyl-diacylglycerol is synthesized in chloroplasts, but it is found in both chloroplasts and mitochondria (25). Lipid A-like molecules of plants may serve as structural components of the outer membranes of mitochondria and/or chloroplasts, and probably were introduced by association with endosymbiotic bacteria during evolution.
Alternatively, lipid A-like molecules in Arabidopsis may be involved in signal transduction or plant defense responses. LPS or lipid A from some Gram-negative bacteria can induce various plant defense responses, such as an oxidative burst, NO generation, and up-regulation of pathogenesis-related genes (9, 26–31). In some cases, pretreatment with LPS helps plant cells survive subsequent attack by phytopathogens, mainly by suppressing the hypersensitive response (26, 32). Although the mechanisms by which plants detect LPS remain unknown, lipid A-like molecules in plants might serve as signals to regulate cellular responses during plant pathogen invasion.
Materials and Methods
Methods for RNA extraction and cDNA synthesis, isolation, and genotyping of insertional null mutants, plasmid constructions, and plant transformations are described in SI Materials and Methods. All primers used in this study are listed in Table S1.
Plant Materials and Growth Conditions.
A. thaliana wild-type ecotypes are Col-0, Ws, and Ler. The atlpxa-1 mutation was in Ws background and was generated by the Arabidopsis Knockout Facility of the Arabidopsis Functional Genomics Consortium (17). The atlpxd1-1 (CS855902) mutation was in the Col-0 background and was obtained through the University of Wisconsin-Madison (Wisconsin DsLox lines) (15). The atlpxd2-1 (ET116191) mutation was in the Ler background and obtained from the Arabidopsis Gene Trap Collection at the Cold Spring Harbor Laboratory (16). The atlpxa-2 (SALK_092408), atlpxa-3 (SALK_101521), atlpxb-1 (SALK_087537), atlpxb-2 (SALK_087529), atlpxk-1 (SALK_063783C), atlpxk-2 (SALK_100275C), atlpxk-3 (SALK_145083), and atkdta-1 (SALK_035981) mutations were obtained from the Arabidopsis Biological Resource Center and were in Col-0 background (18).
Plants were grown in long-day conditions (16 h light/8 h dark) at 22 °C in either soil or half-strength Murashige–Skoog medium (33), solidified with 0.8% agar containing 1% sucrose. For sterile growth conditions, seeds were surface-sterilized by treatment with 50% Clorox and 0.2% Triton X-100 for 10 min, and then washed five times with sterile distilled water. All seeds suspended in sterile water were kept in the dark at 4 °C for 3 d to break seed dormancy. For lipid analysis, sterilized seeds (typically 2,000) were germinated and grown for 10 d in 250 mL full-strength Murashige–Skoog liquid medium (16 h light/8 h dark), supplemented with 0.1% Plant Preservative Mixture (Plant Cell Technology, Inc.).
Subcellular Localization Experiments.
To investigate the subcellular localization of the AtLpx-GFP fusion proteins, T2 generation plants of the transgenic lines were used to observe GFP fluorescence. Seven-day old seedlings grown on half-strength Murashige–Skoog plates were harvested in half-strength Murashige–Skoog medium containing 200 nM MitoTracker (MitoTracker Orange CMTMRos, Molecular Probes). After staining for 15 min, the seedlings were washed in half-strength Murashige–Skoog medium for 10 min. The seedlings were viewed with a Zeiss LSM 510 upright confocal microscope. To observe GFP and MitoTracker fluorescence simultaneously, GFP was excited at 488 nm and MitoTracker Orange at 543 nm with appropriate lasers. Fluorescence emissions were detected using BP505–530 (GFP) and LP560 (MitoTracker Orange) filters. Images were exported as digital files using the Zeiss LSM Image Browser. At least five T2 generation lines were viewed in this manner for each construct.
Plasma membranes, chloroplasts and mitochondria were isolated from 10-day old seedlings grown under sterile conditions (34–36). Protein concentration was determined by the bicinchoninic acid assay (37).
Lipid Extraction from Plant Seedlings.
The 10-day old seedlings grown under sterile conditions were harvested, washed with cold distilled water, and ground with a mortar and pestle at 4 °C in a buffer (4 mL/g fresh plant weight) consisting of 0.3 M sorbitol, 5 mM MgCl2, 5 mM EGTA, 5 mM EDTA, 20 mM Hepes, pH 8.0, 10 mM NaHCO3, and 1% Protease Inhibitor Cocktail (P9599-5ML, Sigma). Homogenates were filtered through two layers of Miracloth (Calbiochem Ltd.). Aliquots of plant homogenates (each containing 10 mg protein in 2–3 mL) were stored at -80 °C. Lipids were extracted by the Bligh–Dyer method (38): Homogenates from wild-type or mutant plants, containing equal amounts of protein (10 mg), were diluted into 8 mL phosphate-buffered saline (39). Each 8-mL sample was extracted for 1 h at room temperature by conversion to a single phase Bligh–Dyer system, consisting of chloroform/methanol/water (1∶2∶0.8, vol/vol). The supernatants were collected after brief centrifugation and converted to acidic two-phase Bligh–Dyer mixtures, consisting of chloroform∶methanol∶0.1 M HCl (2∶2∶1.8, vol/vol), by adding chloroform and aqueous HCl. The lower phases were collected after low-speed centrifugation and the solvent removed by rotary evaporation. The lipids were stored at -80 °C.
LC–MRM and LC–ESI/MS/MS Detection of Lipid A Precursors in Arabidopsis.
Detailed conditions for normal phase LC separation of total Arabidopsis lipids, coupled with ESI/MS/MS or MRM detection of the lipid A precursors, are provided in the SI Materials and Methods.
Supplementary Material
Acknowledgments.
The authors thank Drs. Jinshi Zhao, Hak Suk Chung, Sam Gattis, Jinhua Qian, Louis Metzger, and other lab members for helpful discussions. This research was supported by National Institutes of Health Grant GM-051310 (to C.R.H. Raetz), the Large Scale Collaborative Grant GM-069338, (to Z.G.), and the LIPID MAPS mass spectrometry facility at Duke University.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1108840108/-/DCSupplemental.
References
- 1.Raetz CRH, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raetz CRH, Reynolds CM, Trent MS, Bishop RE. Lipid A modification systems in Gram-negative bacteria. Annu Rev Biochem. 2007;76:295–329. doi: 10.1146/annurev.biochem.76.010307.145803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bos MP, Robert V, Tommassen J. Biogenesis of the Gram-negative bacterial outer membrane. Annu Rev Microbiol. 2007;61:191–214. doi: 10.1146/annurev.micro.61.080706.093245. [DOI] [PubMed] [Google Scholar]
- 4.McClerren AL, et al. A slow, tight-binding inhibitor of the zinc-dependent deacetylase LpxC of lipid A biosynthesis with antibiotic activity comparable to ciprofloxacin. Biochemistry. 2005;44:16574–16583. doi: 10.1021/bi0518186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park BS, et al. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. 2009;458:1191–1195. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- 6.Russell JA. Management of sepsis. N Engl J Med. 2006;355:1699–1713. doi: 10.1056/NEJMra043632. [DOI] [PubMed] [Google Scholar]
- 7.Liu D, Sun TP, Raetz CRH. Arabidopsis thaliana genes encoding orthologs of enzymes involved in Escherichia coli lipid A biosynthesis. FASEB J. 2003;17(Suppl S):A579. (abstr) [Google Scholar]
- 8.Metzger LE, 4th, Raetz CRH. An alternative route for UDP-diacylglucosamine hydrolysis in bacterial lipid A biosynthesis. Biochemistry. 2010;49:6715–6726. doi: 10.1021/bi1008744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeidler D, et al. Innate immunity in Arabidopsis thaliana: Lipopolysaccharides activate nitric oxide synthase (NOS) and induce defense genes. Proc Natl Acad Sci USA. 2004;101:15811–15816. doi: 10.1073/pnas.0404536101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deckert G, et al. The complete genome of the hyperthermophilic bacterium Aquifex aeolicus. Nature. 1998;392:353–358. doi: 10.1038/32831. [DOI] [PubMed] [Google Scholar]
- 11.Williams AH, Raetz CRH. Structural basis for the acyl chain selectivity and mechanism of UDP-N-acetylglucosamine acyltransferase. Proc Natl Acad Sci USA. 2007;104:13543–13550. doi: 10.1073/pnas.0705833104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackman JE, Raetz CRH, Fierke CA. Site directed mutagenesis of the bacterial metalloamidase UDP-(3-O-acyl)-N-acetylglucosamine deacetylase (LpxC). Identification of the zinc binding site. Biochemistry. 2001;40:514–523. doi: 10.1021/bi001872g. [DOI] [PubMed] [Google Scholar]
- 13.Bartling CM, Raetz CRH. Crystal structure and acyl chain selectivity of Escherichia coli LpxD, the N-acyltransferase of lipid A biosynthesis. Biochemistry. 2009;48:8672–8683. doi: 10.1021/bi901025v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armstrong MT, et al. Histochemical evidence for lipid A (endotoxin) in eukaryote chloroplasts. FASEB J. 2006;20:2145–2146. doi: 10.1096/fj.05-5484fje. [DOI] [PubMed] [Google Scholar]
- 15.Woody ST, Austin-Phillips S, Amasino RM, Krysan PJ. The WiscDsLox T-DNA collection: An arabidopsis community resource generated by using an improved high-throughput T-DNA sequencing pipeline. J Plant Res. 2007;120:157–165. doi: 10.1007/s10265-006-0048-x. [DOI] [PubMed] [Google Scholar]
- 16.Martienssen RA. Functional genomics: Probing plant gene function and expression with transposons. Proc Natl Acad Sci USA. 1998;95:2021–2026. doi: 10.1073/pnas.95.5.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krysan PJ, Young JC, Sussman MR. T-DNA as an insertional mutagen in Arabidopsis. Plant Cell. 1999;11:2283–2290. doi: 10.1105/tpc.11.12.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alonso JM, et al. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science. 2003;301:653–657. doi: 10.1126/science.1086391. [DOI] [PubMed] [Google Scholar]
- 19.Takayama K, et al. Fatty acyl derivatives of glucosamine 1-phosphate in Escherichia coli and their relation to lipid A: Complete structure of a diacyl GlcN-1-P found in a phosphatidylglycerol-deficient mutant. J Biol Chem. 1983;258:7379–7385. [PubMed] [Google Scholar]
- 20.Bulawa CE, Raetz CRH. The biosynthesis of Gram-negative endotoxin: Identification and function of UDP-2,3-diacylglucosamine in Escherichia coli. J Biol Chem. 1984;259:4846–4851. [PubMed] [Google Scholar]
- 21.Metzger LE, 4th, Raetz CRH. Purification and characterization of the lipid A disaccharide synthase (LpxB) from Escherichia coli, a peripheral membrane protein. Biochemistry. 2009;48:11559–11571. doi: 10.1021/bi901750f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seveno M, et al. Characterization of a putative 3-deoxy-D-manno-2-octulosonic acid (Kdo) transferase gene from Arabidopsis thaliana. Glycobiology. 2010;20:617–628. doi: 10.1093/glycob/cwq011. [DOI] [PubMed] [Google Scholar]
- 23.Poot M, et al. Analysis of mitochondrial morphology and function with novel fixable fluorescent stains. J Histochem Cytochem. 1996;44:1363–1372. doi: 10.1177/44.12.8985128. [DOI] [PubMed] [Google Scholar]
- 24.Bartling CM, Raetz CRH. Steady-state kinetics and mechanism of LpxD, the N-acyltransferase of lipid A biosynthesis. Biochemistry. 2008;47:5290–5302. doi: 10.1021/bi800240r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jouhet J, et al. Phosphate deprivation induces transfer of DGDG galactolipid from chloroplast to mitochondria. J Cell Biol. 2004;167:863–874. doi: 10.1083/jcb.200407022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newman MA, Daniels MJ, Dow JM. The activity of lipid A and core components of bacterial lipopolysaccharides in the prevention of the hypersensitive response in pepper. Mol Plant Microbe Interact. 1997;10:926–928. doi: 10.1094/MPMI.1997.10.7.926. [DOI] [PubMed] [Google Scholar]
- 27.Livaja M, Zeidler D, von Rad U, Durner J. Transcriptional responses of Arabidopsis thaliana to the bacteria-derived PAMPs harpin and lipopolysaccharide. Immunobiology. 2008;213:161–171. doi: 10.1016/j.imbio.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Newman MA, Dow JM, Molinaro A, Parrilli M. Priming, induction and modulation of plant defence responses by bacterial lipopolysaccharides. J Endotoxin Res. 2007;13:69–84. doi: 10.1177/0968051907079399. [DOI] [PubMed] [Google Scholar]
- 29.Dow M, Newman MA, von Roepenack E. The induction and modulation of plant defense responses by bacterial lipopolysaccharides. Annu Rev Phytopathol. 2000;38:241–261. doi: 10.1146/annurev.phyto.38.1.241. [DOI] [PubMed] [Google Scholar]
- 30.Gerber IB, Zeidler D, Durner J, Dubery IA. Early perception responses of Nicotiana tabacum cells in response to lipopolysaccharides from Burkholderia cepacia. Planta. 2004;218:647–657. doi: 10.1007/s00425-003-1142-0. [DOI] [PubMed] [Google Scholar]
- 31.Meyer A, Puhler A, Niehaus K. The lipopolysaccharides of the phytopathogen Xanthomonas campestris pv. campestris induce an oxidative burst reaction in cell cultures of Nicotiana tabacum. Planta. 2001;213:214–222. doi: 10.1007/s004250000493. [DOI] [PubMed] [Google Scholar]
- 32.Sequeira L. Mechanisms of induced resistance in plants. Annu Rev Microbiol. 1983;37:51–79. doi: 10.1146/annurev.mi.37.100183.000411. [DOI] [PubMed] [Google Scholar]
- 33.Murashige T, Skoog F. A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. [Google Scholar]
- 34.Kubis SE, Lilley KS, Jarvis P. Isolation and preparation of chloroplasts from Arabidopsis thaliana plants. Methods Mol Biol. 2008;425:171–186. doi: 10.1007/978-1-60327-210-0_16. [DOI] [PubMed] [Google Scholar]
- 35.Millar AH, Liddell A, Leaver CJ. Isolation and subfractionation of mitochondria from plants. Methods Cell Biol. 2001;65:53–74. doi: 10.1016/s0091-679x(01)65004-0. [DOI] [PubMed] [Google Scholar]
- 36.Santoni V. Plant plasma membrane protein extraction and solubilization for proteomic analysis. Meth Mol Biol. 2007;355:93–109. doi: 10.1385/1-59745-227-0:93. [DOI] [PubMed] [Google Scholar]
- 37.Smith PK, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 38.Bligh EG, Dyer JJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 39.Dulbecco R, Vogt M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954;99:167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Altschul SF, et al. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.