Abstract
Combination regimens that include artemisinin derivatives are recommended as first line antimalarials in most countries where malaria is endemic. However, the mechanism of action of artemisinin is not fully understood and the usefulness of this drug class is threatened by reports of decreased parasite sensitivity. We treated Plasmodium falciparum for periods of a few hours to mimic clinical exposure to the short half-life artemisinins. We found that drug treatment retards parasite growth and inhibits uptake of hemoglobin, even at sublethal concentrations. We show that potent artemisinin activity is dependent on hemoglobin digestion by the parasite. Inhibition of hemoglobinase activity with cysteine protease inhibitors, knockout of the cysteine protease falcipain-2 by gene deletion, or direct deprivation of host cell lysate, significantly decreases artemisinin sensitivity. Hemoglobin digestion is also required for artemisinin-induced exacerbation of oxidative stress in the parasite cytoplasm. Arrest of hemoglobin digestion by early stage parasites provides a mechanism for surviving short-term artemisinin exposure. These insights will help in the design of new drugs and new treatment strategies to circumvent drug resistance.
Keywords: erythrocyte, endoperoxide
Plasmodium falciparum, the most pathogenic human malaria parasite, is estimated to have caused 781,000 deaths in 2009 (1). Current malaria treatment relies heavily on artemisinin-based combination therapies (2). Artemisinin and related endoperoxide antimalarials clear P. falciparum infections rapidly, providing prompt therapy for severe infections (3). Moreover, artemisinins are active against parasites resistant to two major classes of antimalarials, quinolines, and antifolates (3). A disadvantage is that they have very short half-lives in vivo (approximately 1–3 h), and cannot be used as monotherapies to treat uncomplicated malaria due to frequent recrudescence of infections after standard 3-day treatments (4). However, when combined with longer-acting drugs, artemisinins provide outstanding efficacy against uncomplicated malaria (5).
The antimalarial mechanism of action of artemisinins is uncertain. Artemisinin has a 1,2,4-trioxane core incorporating an endoperoxide bridge that is essential for activity (6). There is evidence that artemisinins are activated inside the parasite by ring opening, which generates radical intermediates that react with susceptible parasite targets (6, 7). However, the origin and nature of the artemisinin activator is not clear. Some studies point to hemoglobin-derived heme or ferrous iron, or other host or parasite-derived iron sources (8), suggesting that parasite uptake and hydrolysis of hemoglobin is necessary for artemisinin activity. Other studies point to metal-independent activation of artemisinins (9). An alternative hypothesis is that artemisinin exerts its activity by directly inhibiting a single target, the calcium ATPase (PfATP6), in a manner that is heme independent and analogous to the inhibition of the sarco/endoplasmic reticulum Ca2+-ATPase by thapsigargin (10, 11).
The need to understand the mechanism of action of artemisinin has become more urgent following reports of decreased clinical efficacy, manifested as delayed clearance of blood parasites in clinical trials in Cambodia (12). Artemisinin tolerant parasites have also been developed in the laboratory (13–16). In some cases, the parasites remain susceptible to artemisinin in standard growth inhibition assays, but a subpopulation of early ring stage parasites survives by arresting development when exposed to artemisinin (14). To better characterize the antimalarial mechanism of action of artemisinin, we used a flow cytometry-based assay that permitted us to simultaneously analyze the effects of drugs on parasite growth and hemoglobin uptake. We show that artemisinin activity is highly dependent on hemoglobin digestion, providing a likely explanation for the decreased artemisinin sensitivity of developmentally arrested ring stage parasites.
Results
Artemisinin Retards Parasite Growth.
Intraerythrocytic P. falciparum develops over approximately 48 h through distinct morphological stages from rings, to metabolically more active trophozoites, to multinucleated schizonts before erythrocyte rupture and release of invasive merozoites. We have characterized a cell permeant, far red fluorescent, nucleic acid-binding dye, SYTO 61, that can readily distinguish between uninfected, ring-infected and mature stage-infected red blood cells (RBCs) in a flow cytometric format (17). SYTO 61 was used to monitor parasite developmental stage (intensity) and parasitemia (fraction of parasite-infected RBCs) after treatment with artemisinin.
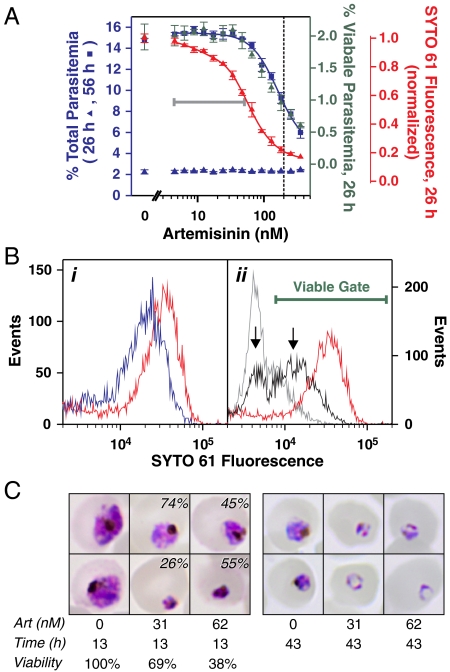
To mimic in vivo effects of short half-life artemisinins (18) we pulsed with artemisinin for 4 h and monitored the SYTO 61 profile within the same cycle (26 h later) and during the next cycle (56 h later). The parasitemia remained constant during the cycle of the drug pulse (Fig. 1A, blue triangles). In contrast, during the cycle after the pulse we observed an artemisinin-dependent decrease in the parasitemia, with an IC50 value of approximately 200 nM (Fig. 1A, blue squares). The IC50-4 h value was higher than that typically reported for artemisinin due to the short duration of the pulse (4 h) and the relatively low susceptibility of mid ring stage parasites (19). Interestingly, during the cycle of the drug pulse there was an artemisinin-dependent decrease in the intensity of the SYTO 61 signal (Fig. 1A, red triangles), reflecting the presence of different parasite populations (Fig. 1B). The decreased staining occurred even at sublethal concentrations of the drug that caused < 5% parasite death (gray bar in Fig. 1A), reflecting a moderate, but uniform decrease in staining of the entire parasite population (Fig. 1B i, blue curve) compared to the control (red curve). At the highest drug concentration examined (360 nM, Fig. 1B ii, gray curve), the major parasite population exhibited a pronounced (10-fold) decrease in staining compared to the control (red curve). This very low intensity staining during the cycle of the drug pulse is consistent with a population that is not viable (i.e., does not progress to the next cycle). Artemisinin concentrations near the IC50-4 h value (Fig. 1B ii, black curve) produced two parasite populations (arrows). These corresponded to a population that is apparently nonviable (left arrow) and another population with a moderate decrease in staining (right arrow) that likely represents a viable population. To confirm this, we used the gate indicated by the bar in Fig. 1B to exclude nonviable parasites and found that the predicted viable parasitemia in the first cycle correlated well with the observed viable parasitemia in the next cycle (Fig. 1A, green triangles and blue squares). Thus, artemisinin pulses led to a subset of nonviable parasites manifested as decreased parasitemia in the cycle after treatment.
Fig. 1.
Sublethal concentrations of artemisinin slow parasite growth. (A, B) Flow cytometric analysis. Mid ring stage parasites (1% hematocrit, 2% parasitemia) pulsed with artemisinin for 4 h and analyzed after 26 h (in the same cycle) and after 56 h (in the next cycle). (A) Correlation of parameters measured at 26 h (triangles) with the parasitemia determined at 56 h (blue squares and curve). The dotted vertical line corresponds to the IC50 value for killing. The 26 h parameters are total parasitemia (blue triangles), median SYTO 61 signal (red triangles and curve) and viable parasitemia (green triangles). The latter was determined using the gating shown in B. The gray bar highlights the region where artemisinin affects the SYTO 61 staining at 26 h but not parasitemia at 56 h. (B) SYTO 61 profiles of parasite populations 26 h after treatment in the absence (red curves) and at sublethal (i) and lethal (ii) concentrations of artemisinin. The curves correspond to 0 (red), 47 (blue), 93 (black), and 360 (gray) nM artemisinin. The left and right arrows indicate nonviable and viable populations after the 93 nM treatment. Events are normalized to the total number of cells. Error bars represent SD of quadruplicate measurements. (C) Giemsa analysis. Early trophozoite stage parasites (0.5% hematocrit, 1.5% parasitemia) were pulsed with artemisinin (Art) for 4 h and analyzed by Giemsa staining 13 h and 43 h after treatment. Two parasite populations are evident in artemisinin-treated parasites examined at 13 h (percentage of each population is indicated on images, estimated by counting > 100 parasites in duplicate samples). The viability values correspond to the proportion of viable parasites in the cultures and were determined by flow cytometric analysis of the parasitemia in SYTO 61-stained cultures 43 h after drug treatment (performed in triplicate).
The above analyses demonstrate that SYTO 61 staining can distinguish between nonviable parasites with very low SYTO staining and viable parasites with SYTO staining that is moderately decreased. The lower staining of viable parasites compared to controls likely reflects the presence of earlier stage parasites (17). This was confirmed by monitoring the delay in entry of artemisinin-treated parasites into the next cycle by flow cytometry (Fig. S1) and by examining parasite morphology by Giemsa staining (Fig. 1C). Smears examined within the same parasite cycle as drug treatment (13 h after treatment, Fig. 1C) show two parasite populations at artemisinin concentrations near the IC50-4 h value. These populations were identified by comparing their relative numbers in the smears with the expected population of viable parasites calculated from the parasitemia measured in the cycle following treatment (viability, Fig. 1C). Thus, the population of parasites with “crisis” or pyknotic morphology (lower images) corresponds to nonviable parasites. By contrast the viable population corresponds to a population of mid trophozoites (upper images) whose growth has been retarded compared to the untreated controls (late trophozoites). The younger age of the viable artemisinin-treated parasites compared to the controls is maintained in the next parasite cycle (43 h after treatment, Fig. 1C). Thus, the data show that lower artemisinin concentrations induce a nonlethal growth retardation effect. A similar effect was observed for the clinically relevant, semisynthetic derivative dihydroartemisinin (DHA) (Fig. S1). By contrast deoxyartemisinin, an inactive derivative, showed no effect on the rate of parasite development (Fig. S2).
Artemisinin Inhibits Uptake of Host Hemoglobin.
P. falciparum takes up and degrades host hemoglobin in an acidic digestive vacuole in a process that begins in the mid ring stage and reaches a peak in the mid trophozoite stage (20, 21). Some antimalarials, including artemisinin, are reported to inhibit hemoglobin uptake (22), but it is not clear if this is a direct effect, or a consequence of parasite killing. To monitor hemoglobin uptake and parasite viability simultaneously we established a correlative flow cytometric assay employing parasites that had invaded resealed RBCs containing a fluorescent marker (21). The fluorescein-dextran was taken up into the parasite’s digestive vacuole and used to monitor the level of endocytosis (green fluorescence intensity) following selective lysis of the RBC membrane by saponin (23).
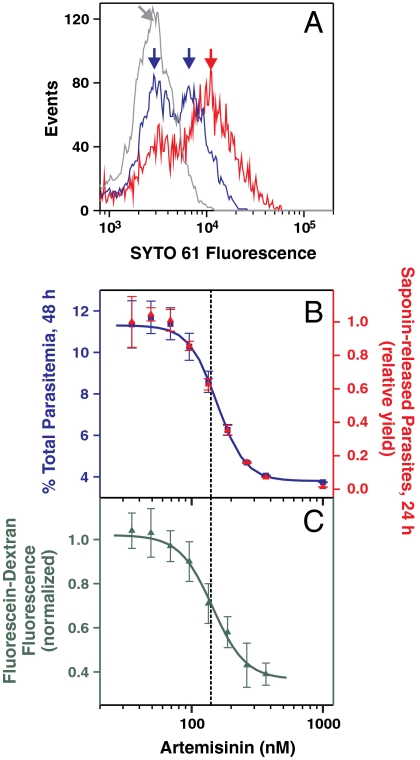
Late ring stage parasites were pulsed with artemisinin for 4 h and monitored in the same cycle (after 24 h) to assess effects on parasite development and uptake of host cytoplasm, and during the next cycle (after 48 h) to assess parasite viability. As for parasites developing in intact RBCs, no change in parasitemia was detected after 24 h, but the SYTO 61 intensity revealed different populations of infected RBCs (Fig. 2A). Again the low intensity population (1,000 nM artemisinin; Fig. 2A, gray curve) correlated with the population that did not survive into the next cycle (Fig. 2B). At an artemisinin concentration near the IC50-4 h value (190 nM) two populations were observed (blue curve), corresponding to nonviable parasites (Fig. 2A, left blue arrow) and viable parasites (right blue arrow) with delayed growth compared to the control (red curve).
Fig. 2.
Artemisinin treatment inhibits endocytosis of host cytoplasm. Fluorescein-dextran-containing resealed RBCs infected with late ring stage parasites were pulsed with artemisinin for 4 h and analyzed in the same or the next cycle. (A) SYTO 61 profiles 24 h after treatment with 35, 190, and 1,000 nM artemisinin (red, blue, and gray curves). (B) Correlation of yield of saponin-released parasites 24 h after artemisinin treatment (red triangles) with the total parasitemia 48 h after treatment (blue squares). Parasite yield was quantitated using the strict gating regime described in the text. (C) Fluorescein-dextran uptake in viable parasites 24 h after treatment. The median green fluorescence signal of the saponin-treated parasites selected in B was used as the measure of uptake. The dashed vertical line corresponds to the approximate IC50 value for killing. Error bars correspond to the range of duplicate (total parasitemia) or SD of triplicate (parasite yield and fluorescence) measurements.
We quantitated the effect of the artemisinin pulse on the uptake of RBC cytoplasm by measuring the intracellular fluorescein-dextran signal following saponin lysis. Such measurements are potentially compromised by the presence of nonviable cells; this can skew the average intensity toward low values if parasite growth had been arrested or to high intensities if the digestive vacuole is alkalinized (Fig. S3). We heavily weighted the measurements toward viable cells by using strict scattering and SYTO 61 gates based on the profile of the control cells and by only selecting cells possessing an acidic digestive vacuole using the ratio of green: yellow fluorescein fluorescence (see Fig. S3). The number of saponin-released parasites that were counted using these criteria (Fig. 2B, red triangles) closely correlated with the parasitemia measured at 48 h (blue squares), confirming that only parasites derived from viable parasites were analyzed. The median green signal of this population was used to quantitate uptake. At concentrations close to the IC50-4 h value these viable parasites exhibited up to a 60% reduction in fluorescein-dextran uptake compared to the untreated parasites (Fig. 2C), demonstrating that the concentration-response relationships for hemoglobin uptake and artemisinin toxicity are very similar.
Hemoglobin Is Required for Artemisinin-Induced Inhibition of Endocytosis.
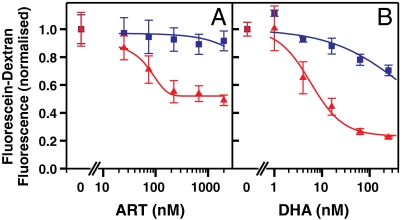
We next examined the effect of artemisinin on hemoglobin uptake by parasites that had been released from their RBCs. For these experiments, saponin-released trophozoites were incubated for 4 h in medium containing fluorescein-dextran, with or without readdition of RBC lysate. The hemoglobin content of the RBC lysate medium (3.7 mM) was approximately 20% that found in intact cells, and similar to the level in resealed RBCs (21). Control trophozoites accumulated similar levels of fluorescein-dextran from the medium into acidic compartments in the absence and presence of the RBC lysate (Fig. S4). Parasites exposed to artemisinin or DHA in the presence of RBC lysate showed up to an 80% reduction in fluorescein-dextran uptake (Fig. 3, red triangles). The IC50 for this effect was similar to the IC50-4 h value for the killing of trophozoites in intact cells in culture (Fig. 4A, red curves). By contrast, in the absence of RBC lysate no significant reduction in fluorescein-dextran uptake occurred, even at 2 μM artemisinin (> 30-fold decrease in sensitivity, Fig. 3A, blue squares). Similarly the effect of DHA on fluorescein-dextran uptake was markedly decreased in the absence of RBC lysate (> 30-fold decrease in sensitivity, Fig. 3B). These results demonstrate that a host cell component, presumably hemoglobin or a hemoglobin breakdown product, is required for potent artemisinin activity in this assay.
Fig. 3.
RBC lysate is needed for artemisinin (A) or DHA (B) -mediated inhibition of endocytosis. Mid trophozoite stage parasites were released from RBCs with 0.02% (w/v) saponin and incubated for 4 h with fluorescein-dextran in the absence (blue squares) and presence (red triangles) of RBC lysate containing 3.7 mM hemoglobin. The median green fluorescence signal of the parasite population was used as a measure of uptake. Error bars represent SD of triplicate measurements.
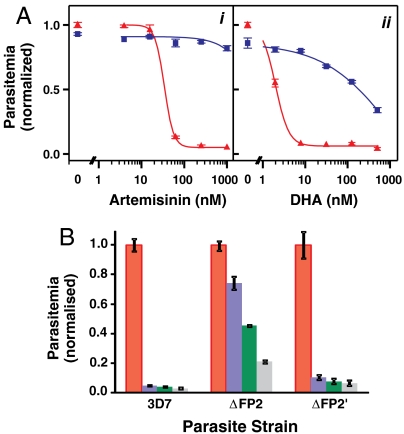
Fig. 4.
Hemoglobin digestion is required for activity of artemisinins. (A) Trophozoite stage parasites (2% hematocrit, 0.2% parasitemia) were preincubated for 1 h in the presence (blue squares) and absence (red triangles) of 5 μM ALLN before the addition of artemisinin (i) or DHA (ii). After 4 h, the cells were washed, cultured for 72 h and analyzed by flow cytometry. Parasitemias are normalized to untreated controls (no endoperoxide or inhibitor). (B) 3D7 parent and falcipain deletion mutants, 3D7_ΔFP2 and 3D7_ΔFP2′, were subjected to artemisinin for 2 h (0 nM, red; 200 nM, blue; 400 nM, green; 800 nM, gray) at mid trophozoite stage, then cultured for 48 h. The data are normalized to the sample with no artemisinin. Error bars represent SD of triplicate measurements.
Hemoglobin Digestion Is Required for Inhibition of Growth of P. falciparum by Artemisinin.
To confirm a role for a hemoglobin digestion product in artemisinin activity, we examined whether inhibition of hemoglobin degradation suppresses artemisinin activity. The cysteine protease inhibitor N-acetyl-L-leucyl-L-leucyl-L-norleucinal (ALLN), which inhibits falcipain hemoglobinases, blocks digestion leading to an accumulation of hemoglobin in the digestive vacuole (24). Mid trophozoite stage parasites treated with up to 5 μM ALLN for 5 h showed a slight retardation of growth in the next parasite cycle, but ≥90% of parasites survived the treatment (Fig. 4A). Parasites pretreated for 1 h with ALLN then for 4 h with a combination of ALLN and artemisinin were almost completely refractory to the effects of artemisinin at concentrations up to 1 μM (> 30-fold decrease in sensitivity, Fig. 4Ai). Similar results were observed for a combination of ALLN and DHA (100-fold decrease in sensitivity, Fig. 4A ii) or artesunate (> 4-fold decrease in sensitivity; Fig. S5A). By contrast deoxyartemisinin, which has no antimalarial activity, showed no interaction with ALLN (Fig. S2). Another cysteine protease inhibitor, E-64, is less able to cross membranes than ALLN and a longer incubation was required to block hemoglobin digestion, but we found that a 24 h pretreatment with this inhibitor partly protected against an artemisinin pulse (Fig. S5B i). The aspartic protease inhibitor pepstatin, which inhibits plasmepsin hemoglobinases, had less impact on artemisinin activity (Fig. S5B ii).
The cysteine proteases, falcipain-2 and falcipain-3, play major roles in hemoglobin degradation by intraerythrocytic parasites (25, 26). To further characterize the importance of hemoglobin digestion in artemisinin activity we studied falcipain deletion mutants. Falcipain-2 is expressed early in parasite development, and its deletion leads to an accumulation of undegraded hemoglobin in early to mid trophozoites (27). We found that the falcipain-2 deletion mutant 3D7_ΔFP2 was substantially protected against an artemisinin pulse at the mid trophozoite stage (Fig. 4B), showing a sixfold increase in the IC50 value. Falcipains-2, -2′ and -3 are coexpressed in mature stage parasites. Falcipain-2′ deletion parasites show no accumulation of undegraded hemoglobin, presumably due to functional redundancy with other falcipains (27). Correspondingly, the artemisinin sensitivity of a falcipain-2′ deletion mutant (3D7_ΔFP2′) was similar to that of wild type parasites (Fig. 4B). The effects of deletion of falcipain-3 could not be assessed, as erythrocytic parasites cannot tolerate the deletion (25).
Our data strongly suggest that flux of a hemoglobin degradation product, presumably heme or ferrous iron, is needed for the potent antimalarial activity of artemisinin. Once activated, artemisinin is likely to react with a number of specific targets, including heme itself, and to cause oxidative stress (28, 29). To examine the downstream consequences of artemisinin activity we measured oxidative stress levels in drug-treated P. falciparum using the reactive oxygen species reporter, 2′,7′-dichlorofluorescein (DCF). We previously showed that the DCF fluorescence signal increases as the intraerythrocytic parasite matures into the trophozoite stage when hemoglobin digestion is most active (17). We found that treatment with artemisinin or DHA for 2 h caused an increase in the DCF signal (Fig. 5, blue squares), indicating increased oxidative insult in the parasite cytoplasm. Pretreatment of control trophozoite stage parasites for 3 h with ALLN slightly decreased the DCF signal (Fig. 5, 0 nM drug), likely due to inhibited hemoglobin digestion. Interestingly pretreatment with ALLN also completely abrogated the endoperoxide-induced increase in DCF signal (Fig. 5, red triangles), indicating that hemoglobin degradation is required for an artemisinin-mediated increase in parasite oxidative stress.
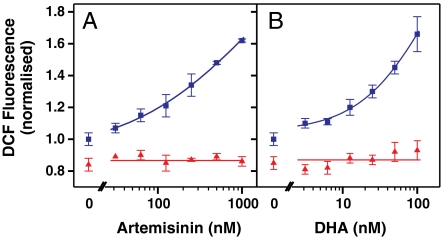
Fig. 5.
Artemisinin (A) and DHA (B) exert prooxidant activity that is antagonized by inhibition of hemoglobin digestion. Trophozoite stage parasites (2% hematocrit, 1% parasitemia) labeled with CM-H2DCFDA were incubated for 1 h in the absence (blue squares) or presence (red triangles) of 5 μM ALLN before an additional 2 h incubation with artemisinin or DHA. The cells were washed and the median DCF signal of the parasite population analyzed by flow cytometry. Data are normalized to untreated controls (no endoperoxide or inhibitor). Error bars represent SD of triplicate measurements.
Discussion
Artemisinin is highly potent against hemoglobin-digesting stages of malaria parasites (7, 30) and against other hemoglobin-degrading pathogens such as Schistosoma species (31), but it is much less potent against other pathogens (32). This suggests that hemoglobin digestion plays a critical role in the mechanism of action of this drug class. However, until now the dependence of artemisinin action on hemoglobin uptake and digestion has not been directly demonstrated.
In this work we provide strong evidence for a requirement for hemoglobin uptake to support artemisinin activity. We examined the effect of artemisinins on the uptake of a fluorescent marker by parasites that had been freed from their host RBCs. When saponin-released parasites were incubated in the presence of RBC lysate, artemisinin inhibited the uptake of fluorescein-dextran. In contrast, artemisinin had little effect on fluorescein-dextran uptake in the absence of RBC lysate. Thus, a hemoglobin-derived activator is needed for artemisinin activity. The studies both with freed parasites and with parasites in resealed RBCs showed that one of the effects of artemisinin is to decrease hemoglobin uptake, even in viable cells. Thus, paradoxically, one of the effects of artemisinin is to inhibit uptake of hemoglobin, the source of the artemisinin activator.
Falcipain cysteine proteases play important roles in hemoglobin degradation, both directly and as activators of plasmepsin hemoglobinases (25, 33). To confirm the importance of hemoglobin degradation in the action of artemisinin we examined the effect of pretreatment of parasites with the cysteine protease inhibitors ALLN and E-64. We designed these experiments to focus on events in the mid ring to mid trophozoite stages of infection to distinguish hemoglobinase inhibitor effects from those on schizont rupture and invasion (32). We found that cysteine protease inhibitors potently antagonized artemisinin action. These results strongly suggest that a hemoglobin degradation product is needed for the antimalarial activity of artemisinin. Previous studies using an inhibitor of a plasmepsin hemoglobinase, Ro40-4388, found no antagonistic interaction with artemisinin (11). That analysis looked for an interaction over the full intraerythrocytic cycle and so is not directly comparable with our data. In any event, in our study the aspartic protease inhibitor pepstatin had a relatively small impact on artemisinin activity compared to cysteine protease inhibitors, consistent with aspartic proteases playing a redundant role in hemoglobin digestion (26).
Falcipain-2 is the only cysteine protease hemoglobinase expressed early in erythrocytic parasite development, and knock-out parasites have markedly diminished hemoglobinase activity in the early trophozoite stage (25). These parasites exhibit a swollen vacuole phenotype, consistent with defective hemoglobin digestion, but the deletion is not lethal, apparently due to rescue by the expression of falcipain-2′ and falcipain-3 beginning at the mid trophozoite stage (25). By contrast, falcipain-2′ deletion parasites show no defect, presumably due to the expression of other falcipains in mature parasites (25). As would be expected if hemoglobin digestion is needed for artemisinin action, falcipain-2 deletion parasites showed substantial protection against a pulse of artemisinin in early to mid trophozoites, whereas falcipain-2′ deletion parasites showed an artemisinin sensitivity profile similar to that of wild type parasites.
When hemoglobin is degraded in the parasite digestive vacuole, hemoglobin is oxidized, with the production of a superoxide anion radical (O2•-) that is rapidly converted to H2O2. P. falciparum is protected against oxidative damage by thioredoxin- and glutathione-based redox systems (34). However the parasite does not encode catalase or a classical glutathione peroxidase, and it has been suggested that the detoxification processes may be barely sufficient to prevent parasite damage (34). A DCF precursor has been developed as a membrane-permeant probe to monitor intracellular oxidative stress (35). The probe is deacetylated by intracellular esterases, producing a charged, thiol-reactive product with enhanced cellular retention. Upon oxidation by reactive oxygen species (especially hydrogen peroxide in the presence of iron or hematin) the highly fluorescent DCF product is formed (35). We previously showed that the DCF signal increases as the intraerythrocytic parasite matures to the trophozoite stage, when hemoglobin digestion is most active (17). In this work, we show that treatment of parasites with artemisinin or DHA further increases the DCF signal. This result is consistent with previous studies that provided evidence of artemisinin-induced free radicals in parasite membranes (36–38). Hemoglobinase inhibitors prevented the artemisinin-induced increase in oxidative stress, indicating that hemoglobin digestion is a prerequisite for this downstream effect of artemisinin.
When studied in culture, artemisinin caused a delay in the development of a subset of ring stage parasites (13, 14). Parasite alterations that extend this period of developmental arrest may explain recent observations in Cambodia of delayed clearance of circulating parasites after treatment with artemisinins (12, 39). These observations are very concerning, as they may foretell the evolution of parasites that are fully resistant to the action of artemisinins. Thus, a better understanding of the means by which artemisinins act against malaria parasites and induce dormancy in subsets of ring stage parasites is a key research priority.
In this work we showed that artemisinins inhibit hemoglobin uptake. This finding is in agreement with previous work showing that artemisinin treatment leads to an accumulation of undigested hemoglobin in the parasite (22). We also showed that hemoglobin uptake and subsequent hydrolysis are required for potent artemisinin activity. Thus, if parasites evolve mechanisms to delay hemoglobin uptake or hydrolysis they may circumvent artemisinin action. This effect may be particularly relevant clinically due to the short half-lives of available artemisinin antimalarials. We suggest that artemisinin-mediated inhibition of hemoglobin uptake drives some parasites toward a quiescent state. Indeed, slowed growth may be a general stress response. This artemisinin-mediated dormant state would allow the parasite to tolerate the short drug exposure afforded by treatment with artemisinin and related endoperoxides. Stalling of growth at a stage before hemoglobin uptake is initiated, or even short-term inhibition of hemoglobin uptake and degradation, may be sufficient to permit parasite survival until serum levels of artemisinins fall below inhibitory concentrations. Thus, selection of parasites with a transient developmental delay (13, 14), and thus delayed hemoglobin uptake, may explain delayed clinical clearance of parasites by artemisinins in Southeast Asia (12, 14, 39).
Our data have implications for the design of the next generation of endoperoxide antimalarials and drug combinations. Falcipain inhibitors show promise as antimalarial agents (40), however, our results suggest that inhibitors of hemoglobin hydrolysis are not suitable for use in combination with artemisinins. By contrast, unique modes of chemotherapy that facilitate hemoglobin uptake, hemoglobin hydrolysis, or intracellular oxidative stress may enhance the action of artemisinins and circumvent evolving tolerance mechanisms. Similarly, we anticipate that use of endoperoxides with longer exposure in the bloodstream will prevent parasite escape via short-term growth arrest. In this regard synthetic endoperoxides with improved in vivo half-lives that are currently under development hold great promise (32, 41, 42). A better appreciation of the interaction of artemisinins and partner drugs with the hemoglobin degradation pathway may uncover additional strategies to protect the vital resource of endoperoxide antimalarials.
Materials and Methods
Materials.
Deoxyartemisinin was from Santa Cruz Biotechnology. SYTO 61, 5-(and-6)-chloromethyl-2′,7′-dichlorodihydro-fluorescein diacetate acetyl ester (CM-H2DCFDA) and fluorescein-dextran (anionic, 10,000 MW) were from Invitrogen. Artemisinin, dihydroartemisinin (DHA), L-transepoxysuccinyl-leucylamido-[4-guanidino] butane (E-64), pepstatin A, ALLN and carbonyl cyanide 3-chlorophenylhydrazone (CCCP) were purchased from Sigma Aldrich.
Culturing of Parasites and Assessment of Growth and Viability.
D10, 3D7, and falcipain-2 (3D7_ΔFP2) and -2′ (3D7_ΔFP2′) deletion mutants (25) were cultured as described previously (17). For assessment of growth and viability, cultures were stained with SYTO 61 and analyzed by flow cytometry. For analyses of oxidative stress, trophozoite stage parasites (2% hematocrit, 1% parasitemia) were labeled with 5 μM CM-H2DCFDA for 15 min at room temperature, washed, incubated for 1 h with or without 5 μM ALLN, then 2 h with artemisinin or DHA. The cells were washed, stained with SYTO 61, and the DCF signal was analyzed by flow cytometry.
Drug Interactions and Effects of Protease Inhibitors on the Antimalarial Activity of Artemisinins.
Synchronized mid trophozoite stage 3D7 or falcipain mutant parasites were pretreated for 1 h with ALLN and cultured with artemisinin or DHA for an additional 4 h, or with E-64 or pepstatin A for 24 h, and artemisinin for 2 h, before analysis by flow cytometry.
Fluorescein-Dextran Uptake in Parasite-Infected Resealed RBCs.
Resealed RBCs were prepared (21) in the presence of 50 μM fluorescein-dextran. Harvested mature stage-infected RBCs (D10 strain) were added to generate parasite-infected resealed RBCs (21). Late ring stage parasites were incubated with artemisinin for 4 h, washed and incubated for a further 24 h before being split into three aliquots. One of these was stained with SYTO 61 and analyzed by flow cytometry to assess growth within the same parasite cycle. Saponin (0.025% w/v) was added to another aliquot to prepare saponin-released parasites for measurement of fluorescein-dextran uptake. These parasites were washed 3 times in endocytosis buffer (EB; RPMI supplemented with 11 mM glucose, 0.125% AlbuMAX, 0.02 mg/mL gentamycin, 0.4 mM hypoxanthine, 25 mM Hepes, pH 7.1) prior to flow cytometry analysis. The remaining aliquot was cultured for an additional 24 h prior to flow cytometry analysis.
Fluorescein-Dextran Uptake in Saponin-Released Parasites.
RBC lysate was prepared (22) and the hemoglobin content determined (43). Packed mid-trophozoite-infected RBCs (1–3% parasitemia, 3D7) were lysed in 10 volumes of EB containing 0.02% saponin, washed and resuspended at 10–50,000 parasites/μL in EB or RBC lysate, containing 50 μM fluorescein-dextran and dilutions of artemisinin or DHA. After a 4 h incubation, washed cells were stained with SYTO 61 and analyzed by fluorescence microscopy (21) or flow cytometry in the absence or presence of 20 μM CCCP.
SYTO 61 Staining and Flow Cytometry.
Saponin-lysed samples were stained with 0.5 μM SYTO 61 and analyzed within 1 h of staining to minimize time-dependent loss of the signal. Intact cells (1–3% hematocrit) were stained with an equal volume of SYTO 61 (0.5 or 5 μM in PBS), incubated for 15 min, diluted to approximately 0.1% hematocrit in PBS and incubated for at least 30 min before being measured. The higher SYTO 61 concentration was used for samples containing higher parasitemias to ensure that staining was not SYTO 61-limited.
Flow cytometric measurements (17) were performed on a six-color FACSCanto™ II cytometer (Becton Dickinson) equipped with a high throughput sampler. The channels and probes were green (DCF and fluorescein-dextran; 488 nm excitation, emission 530/30 nm), yellow (fluorescein-dextran; 488 nm, 585/42 nm) and red (SYTO 61; 633 nm, 660/20 nm). Analysis was performed using FCS Express Version 3 (De Novo Software) or FlowJo (Tree Star, Inc.). For intact cells, total cells (uninfected and infected) were gated on their forward and side scatter profiles and the parasite-infected RBCs selected on their SYTO staining. Saponin-released parasites were selected based on their scatter and SYTO staining profiles. Fluorescein-dextran uptake from parasites in resealed RBCs was monitored using an additional gate based on the green/yellow fluorescence ratio of fluorescein (see Fig. S3). Fluorescein-dextran uptake in saponin-released parasites was monitored in the presence of 20 μM CCCP.
Supplementary Material
Acknowledgments.
We thank Sam Deed and Jiri Gut for technical support and Puran S. Sijwali for advice regarding falcipain knockout parasites. We acknowledge support from the Australian Research Council, the Australian National Health and Medical Research Council, and the United States National Institutes of Health. P.J.R. is a Doris Duke Charitable Foundation Distinguished Clinical Scientist. I.B. receives a Fellowship from the Swiss National Science Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1104063108/-/DCSupplemental.
References
- 1.World Heath Organization. World Malaria Report 2010. 2010. http://www.who.int/malaria/world_malaria_report_2010/world_malaria_report_2010.pdf.
- 2.Enserink M. Malaria’s drug miracle in danger. Science. 2010;328:844–846. doi: 10.1126/science.328.5980.844. [DOI] [PubMed] [Google Scholar]
- 3.Dondorp AM, et al. Artemisinin resistance: Current status and scenarios for containment. Nat Rev Microbiol. 2010;8:272–280. doi: 10.1038/nrmicro2331. [DOI] [PubMed] [Google Scholar]
- 4.Eastman RT, Fidock DA. Artemisinin-based combination therapies: A vital tool in efforts to eliminate malaria. Nat Rev Microbiol. 2009;7:864–874. doi: 10.1038/nrmicro2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nosten F, White NJ. Artemisinin-based combination treatment of falciparum malaria. Am J Trop Med Hyg. 2007;77:181–192. [PubMed] [Google Scholar]
- 6.Meshnick SR. Artemisinin: Mechanisms of action, resistance and toxicity. Int J Parasitol. 2002;32:1655–1660. doi: 10.1016/s0020-7519(02)00194-7. [DOI] [PubMed] [Google Scholar]
- 7.O’Neill PM, Barton VE, Ward SA. The molecular mechanism of action of artemisinin. The debate continues. Molecules. 2010;15:1705–1721. doi: 10.3390/molecules15031705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Neill PM, Posner GH. A medicinal chemistry perspective on artemisinin and related endoperoxides. J Med Chem. 2004;47:2945–2964. doi: 10.1021/jm030571c. [DOI] [PubMed] [Google Scholar]
- 9.Haynes RK, et al. Facile oxidation of leucomethylene blue and dihydroflavins by artemisinins: Relationship with flavoenzyme function and antimalarial mechanism of action. ChemMedChem. 2010;5:1282–1299. doi: 10.1002/cmdc.201000225. [DOI] [PubMed] [Google Scholar]
- 10.Krishna S, Pulcini S, Fatih F, Staines H. Artemisinins and the biological basis for the PfATP6/SERCA hypothesis. Trends Parasitol. 2010;26:517–523. doi: 10.1016/j.pt.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 11.Eckstein-Ludwig U, et al. Artemisinins target the SERCA of Plasmodium falciparum. Nature. 2003;424:957–961. doi: 10.1038/nature01813. [DOI] [PubMed] [Google Scholar]
- 12.Dondorp AM, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teuscher F, et al. Artemisinin-induced dormancy in Plasmodium falciparum: Duration, recovery rates, and implications in treatment failure. J Infect Dis. 2010;202:1362–1368. doi: 10.1086/656476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Witkowski B, et al. Increased tolerance to artemisinin in Plasmodium falciparum is mediated by a quiescence mechanism. Antimicrob Agents Chemother. 2010;54:1872–1877. doi: 10.1128/AAC.01636-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imwong M, et al. Exploring the contribution of candidate genes to artemisinin resistance in Plasmodium falciparum. Antimicrob Agents Chemother. 2010;54:2886–2892. doi: 10.1128/AAC.00032-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chavchich M, et al. Role of pfmdr1 amplification and expression in induction of resistance to artemisinin derivatives in Plasmodium falciparum. Antimicrob Agents Chemother. 2010;54:2455–2464. doi: 10.1128/AAC.00947-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu Y, Tilley L, Kenny S, Klonis N. Dual labeling with a far red probe permits analysis of growth and oxidative stress in P. falciparum-infected erythrocytes. Cytometry. 2010;77:253–263. doi: 10.1002/cyto.a.20856. [DOI] [PubMed] [Google Scholar]
- 18.German PI, Aweeka FT. Clinical pharmacology of artemisinin-based combination therapies. Clin Pharmacokinet. 2008;47:91–102. doi: 10.2165/00003088-200847020-00002. [DOI] [PubMed] [Google Scholar]
- 19.ter Kuile F, White NJ, Holloway P, Pasvol G, Krishna S. Plasmodium falciparum: In vitro studies of the pharmacodynamic properties of drugs used for the treatment of severe malaria. Exp Parasitol. 1993;76:85–95. doi: 10.1006/expr.1993.1010. [DOI] [PubMed] [Google Scholar]
- 20.Slomianny C. Three-dimensional reconstruction of the feeding process of the malaria parasite. Blood Cells. 1990;16:369–378. [PubMed] [Google Scholar]
- 21.Abu Bakar NA, Klonis N, Hanssen E, Chan C, Tilley L. Digestive-vacuole genesis and endocytic processes in the early intraerythrocytic stages of Plasmodium falciparum. J Cell Sci. 2010;123:441–450. doi: 10.1242/jcs.061499. [DOI] [PubMed] [Google Scholar]
- 22.Hoppe HC, et al. Antimalarial quinolines and artemisinin inhibit endocytosis in Plasmodium falciparum. Antimicrob Agents Chemother. 2004;48:2370–2378. doi: 10.1128/AAC.48.7.2370-2378.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayward R, Saliba KJ, Kirk K. The pH of the digestive vacuole of Plasmodium falciparum is not associated with chloroquine resistance. J Cell Sci. 2006;119:1016–1025. doi: 10.1242/jcs.02795. [DOI] [PubMed] [Google Scholar]
- 24.Rosenthal PJ. Cysteine proteases of malaria parasites. Int J Parasitol. 2004;34:1489–1499. doi: 10.1016/j.ijpara.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Sijwali PS, Koo J, Singh N, Rosenthal PJ. Gene disruptions demonstrate independent roles for the four falcipain cysteine proteases of Plasmodium falciparum. Mol Biochem Parasitol. 2006;150:96–106. doi: 10.1016/j.molbiopara.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 26.Liu J, Istvan ES, Gluzman IY, Gross J, Goldberg DE. Plasmodium falciparum ensures its amino acid supply with multiple acquisition pathways and redundant proteolytic enzyme systems. Proc Natl Acad Sci USA. 2006;103:8840–8845. doi: 10.1073/pnas.0601876103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sijwali PS, Rosenthal PJ. Gene disruption confirms a critical role for the cysteine protease falcipain-2 in hemoglobin hydrolysis by Plasmodium falciparum. Proc Natl Acad Sci USA. 2004;101:4384–4389. doi: 10.1073/pnas.0307720101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mercer AE, Copple IM, Maggs JL, O’Neill PM, Park BK. The role of heme and the mitochondrion in the chemical and molecular mechanisms of mammalian cell death induced by the artemisinin antimalarials. J Biol Chem. 2011;286:987–996. doi: 10.1074/jbc.M110.144188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meunier B, Robert A. Heme as trigger and target for trioxane-containing antimalarial drugs. Acc Chem Res. 2010;43:1444–1451. doi: 10.1021/ar100070k. [DOI] [PubMed] [Google Scholar]
- 30.Peatey CL, et al. Effect of antimalarial drugs on Plasmodium falciparum gametocytes. J Infect Dis. 2009;200:1518–1521. doi: 10.1086/644645. [DOI] [PubMed] [Google Scholar]
- 31.Klayman DL. Qinghaosu (artemisinin): An antimalarial drug from China. Science. 1985;228:1049–1055. doi: 10.1126/science.3887571. [DOI] [PubMed] [Google Scholar]
- 32.Kaiser M, et al. Peroxide bond-dependent antiplasmodial specificity of artemisinin and OZ277 (RBx11160) Antimicrob Agents Chemother. 2007;51:2991–2993. doi: 10.1128/AAC.00225-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drew ME, et al. Plasmodium food vacuole plasmepsins are activated by falcipains. J Biol Chem. 2008;283:12870–12876. doi: 10.1074/jbc.M708949200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Becker K, et al. Oxidative stress in malaria parasite-infected erythrocytes: host-parasite interactions. Int J Parasitol. 2004;34:163–189. doi: 10.1016/j.ijpara.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 35.LeBel CP, Ischiropoulos H, Bondy SC. Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol. 1992;5:227–231. doi: 10.1021/tx00026a012. [DOI] [PubMed] [Google Scholar]
- 36.Hartwig CL, et al. Accumulation of artemisinin trioxane derivatives within neutral lipids of Plasmodium falciparum malaria parasites is endoperoxide-dependent. Biochem Pharmacol. 2009;77:322–336. doi: 10.1016/j.bcp.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krungkrai SR, Yuthavong Y. The antimalarial action on Plasmodium falciparum of qinghaosu and artesunate in combination with agents which modulate oxidant stress. Trans R Soc Trop Med Hyg. 1987;81:710–714. doi: 10.1016/0035-9203(87)90003-4. [DOI] [PubMed] [Google Scholar]
- 38.Scott MD, et al. Qinghaosu-mediated oxidation in normal and abnormal erythrocytes. J Lab Clin Med. 1989;114:401–406. [PubMed] [Google Scholar]
- 39.Noedl H, et al. Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med. 2008;359:2619–2620. doi: 10.1056/NEJMc0805011. [DOI] [PubMed] [Google Scholar]
- 40.Coteron JM, et al. Falcipain inhibitors: Optimization studies of the 2-pyrimidinecarbonitrile lead series. J Med Chem. 2010;53:6129–6152. doi: 10.1021/jm100556b. [DOI] [PubMed] [Google Scholar]
- 41.Charman SA, et al. Synthetic ozonide drug candidate OZ439 offers new hope for a single-dose cure of uncomplicated malaria. Proc Natl Acad Sci USA. 2011;108:4400–4405. doi: 10.1073/pnas.1015762108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vennerstrom JL, et al. Identification of an antimalarial synthetic trioxolane drug development candidate. Nature. 2004;430:900–904. doi: 10.1038/nature02779. [DOI] [PubMed] [Google Scholar]
- 43.Di Iorio EE. Preparation of derivatives of ferrous and ferric hemoglobin. Methods Enzymol. 1981;76:57–72. doi: 10.1016/0076-6879(81)76114-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.