Abstract
We summarize the genetic literature on polygamy rates and sire numbers per clutch in invertebrate animals that brood their offspring and then compare findings with analogous data previously compiled for vertebrate species displaying viviparity or other pregnancy-like syndromes. As deduced from molecular parentage analyses of several thousand broods from more than 100 “pregnant” species, invertebrate brooders had significantly higher mean incidences of multiple mating than pregnant vertebrates, a finding generally consistent with the postulate that clutch size constrains successful mate numbers in species with extended parental care. However, we uncovered no significant correlation in invertebrates between brood size and genetically deduced rates of multiple mating by the incubating sex. Instead, in embryo-gestating animals otherwise as different as mammals and mollusks, polygamy rates and histograms of successful mates per brooder proved to be strikingly similar. Most previous studies have sought to understand why gestating parents have so many mates and such high incidences of successful multiple mating; an alternative perspective based on logistical constraints turns the issue on its head by asking why mate numbers and polygamy rates are much lower than they theoretically could be, given the parentage-resolving power of molecular markers and the huge sizes of many invertebrate broods.
Keywords: genetic parentage analysis, mating systems, multiple paternity, polyandry, polygyny
Ever since Darwin (1), natural historians have understood that ecological and life-history constraints (including finite time and energy for reproductive activities coupled with limited mate availabilities) should have important impacts on mating behaviors and thereby on the evolution of mating systems and the operation of sexual selection in sexually reproducing species (2–6). Especially for species in which a parent invests heavily in offspring by incubating its young, additional constraints on successful polygamy by members of the incubating sex should arise because of the finite brood space available for rearing embryos within or attached to the incubator's body. Many invertebrate animals guard embryos on or near their bodies in ways that are quite analogous to how vertebrate animals with internal pregnancy gestate their young. Here we address the mating-system ramifications of embryo brooding by contrasting genetically deduced incidences of polygamy by invertebrate parents that tend huge clutches versus pregnant vertebrates that carry many fewer progeny per gestation.
There are several reasons to suspect that individuals who gestate large broods might have higher incidences of polygamy (more mates and/or higher frequencies of multiple mating) than parents that tend smaller broods. First, large broods provide more statistical and physical room for multiple paternity or maternity, all else being equal. Second, many direct or indirect benefits that an incubating parent might gain from multiple mating could be amplified by the additional full-sib cohorts (different patrilines in a brood) that a larger brood in principle can accommodate. Third, because of greater potential fitness payoffs, members of the nongestating sex also should have higher incentives to mate preferentially with brooder individuals that are more fecund (i.e., carry larger broods). For all these reasons, it might be expected that genetic parentage analyses would reveal that large-clutch individuals and large-clutch species average many more full-sib cohorts per litter than do their smaller-clutch counterparts. Thus, we were surprised to find that this expectation does not appear to be well met in the huge scientific literature on animal genetic mating systems.
This is the third of three papers dealing with possible relationships between clutch size (fecundity) and propensities for polygamy in species that have, in effect, various expressions of pregnancy. In the first article of this series (7), we compared incidences of multiple mating by members of the pregnant sex in fish species with three alternative gestational modes: internal female-pregnancy, internal male-pregnancy, and external male-pregnancy (nest-tending by sires). We uncovered only mild statistical trends consistent with the hypothesis that pregnancy truncates fecundity in ways that impact selective pressures on pregnant individuals' propensities to seek multiple mates. In a second article (8), we extended this type of analysis to assess polygamy rates in viviparous mammals vis-à-vis pregnant fish species. Results led us to advance a logistical-constraint hypothesis suggesting that low mate-encounter rates routinely truncate rates of multiple mating far below levels that otherwise could be accommodated, especially in species with large broods. Here we extend such appraisals of pregnancy-like phenomena by comparing published rates of polygamy by the incubating parent in invertebrate animals versus vertebrate animals that gestate their clutches of young.
In all these studies we take advantage of the fact that mate numbers and rates of multiple mating are documented much more readily for members of the gestating sex than for members of the nongestating sex (9). For any category of pregnancy, multiple successful mating by the adult caregiver is relatively straightforward to detect in nature via genetic parentage analyses because each resulting brood of embryos is physically associated with its pregnant sire or pregnant dam. By contrast, documenting the incidences of polygamy by members of the nonpregnant sex is much more problematic, because each such individual may have parented additional broods that happened not to be included in the genetic assays. Taking advantage of this inherent sex-based asymmetry, researchers in recent decades have used highly polymorphic molecular markers (notably microsatellite loci) to estimate mate numbers and rates of multiple mating by the care-giving parent in a wide diversity of viviparous and other species that gestate or otherwise tend their brooded offspring. Here we compile this scientific literature for invertebrate brooders and compare the findings with those we previously summarized for vertebrate animals that display viviparity or other pregnancy-like phenomena.
Results
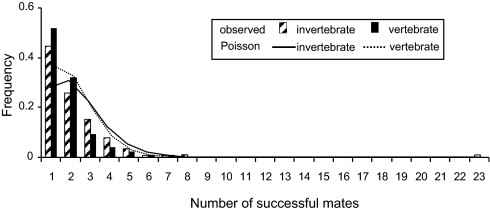
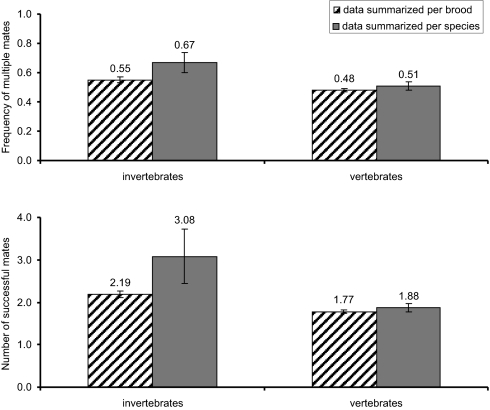
We uncovered published molecular appraisals of genetic parentage for a total of 583 broods of 29 invertebrate species (Table 1) plus 3,057 litters representing 93 vertebrate species in which either internal or external pregnancy is displayed either by females (the usual situation) or by males (7, 8). Table 2 summarizes and compares these findings for invertebrates versus vertebrates, as do Figs. 1 and 2 in graphical formats. As was true for the vertebrate litters, invertebrate broods routinely proved to be composed of several full-sib cohorts reflective of multiple successful mates for the gestating parent. Overall, slightly more than two-thirds of all surveyed invertebrate broods evidenced multiple mating, with the number of full-sib cohorts in a brood averaging 2.19 ± 0.08 (SE) as calculated per brood or 3.08 ± 0.64 as calculated per species (a calculation that, in effect, gives equal statistical weight to each species regardless of how many broods were assayed). All these summary statistics are significantly (or nearly so) higher than their vertebrate counterparts (Table 2). The record for the highest number of genetically deduced mates in any surveyed invertebrate brood was 23 [for one brood of the rough periwinkle, Littorina saxatilis (18)].
Table 1.
Summary statistics of mating parameters of invertebrates
| Species | No. of clutches examined | Clutch size | % clutches with multiple paternity (actual numbers) | Mean no. of mates (range) | No. of alleles at most polymorphic marker (no. of marker loci) | Mode of offspring housing | Reference |
| Knobbed whelk, Busycon carica | 12 | Several thousand | 92 (11/12) | 3.5 (1–7) | 36 (3) | Eggcase string | 28 |
| Slipper shell, Crepidula fornicata | 6 | Average 7,500 | 100 (6/6) | 3.3 (2–5) | 37 (4) | Give birth | 11 |
| Slipper shell, Crepidula fornicata | 18 | 25,000 | 78 (14/18) | 2.6 (1–5) | 32 (5) | Give birth | 12 |
| Slipper shell, Crepidula fornicata | 12 | Average 7,500 | 92 (11/12) | 2.8 (1–4) | 45 (5) | Give birth | 13 |
| Slipper shell, Crepidula coquimbensis | 5 | Hundreds to thousands | 100 (5/5) | 4.4 (3–5) | 18 (5) | Direct development | 14 |
| Sea hare, Aplysia californica | 33 | Hundred thousands to millions | 76 (25/33) | 2.1 (1–3) | 23 (1) | Egg clutch | 15 |
| Land snail, Anrianta arbustorum | 26 | Dozens to hundreds | 100 (26/26) | 3.7 (2–6) | 8 (4) | Egg mass, direct development | 16 |
| Flat periwinkle, Littorina obtusata | 3 | 50–150 | 100 (3/3) | 5.0 (4–6) | 14 (3) | Egg mass, direct development | 17 |
| Rough periwinkle, Littorina saxatilis | 4 | 23–302 | 100 (4/4) | 19.3 (15–23) | 23 (5) | Ovoviviparity | 18 |
| Calamary, Sepioteuthis australis | 35 | Hundred | 97 (34/35) | 2.7 (1–4) | 14 (5) | Egg strand | 19 |
| Veined squid, Loligo forbesi | 3 | Hundred | 67 (2/3) | 1.7 (1, 2) | 25 (6) | Egg string | 20 |
| Inshore squid, Loligo pealeii | 5 | Several thousand | 100 (5/5) | 3.0 (2–4) | 34 (3) | Egg strand | 21 |
| Chokka squid, Loligo vulgaris | 4 | Several thousand | 50 (2/4) | 2.8 (1–5) | 30 (4) | Egg string | 22 |
| Octopus, Graneledone boreopacifica | 1 | 57 | 100 | 2.0 (2) | 6 (18) | Egg string | 23* |
| Snapping shrimp, Alpheus angulosus | 54 | Several hundred | 22 (12/54) | 1.2 (1, 2) | 29 (5) | Extended maternal care | (24) |
| Ghost shrimp, Callichirus islagrande | 40 | Several thousand | 20 (8/40) | 1.2 (1–3) | 31 (2) | Extended maternal care | 25 |
| Freshwater shrimp, Caridina ensifera | 20 | 16 (5–30) | 100 (20/20) | 5.3 (2–11) | 25 (4) | Extended maternal care | 26 |
| Swamp crayfish, Procambarus clarkii | 30 | 276 (136–362) | 97 (29/30) | 2.6 (1–4) | 10 (4) | Extended maternal care | 27 |
| Placid crayfish, Orconectes placidus | 15 | 250 (20–514) | 60 (9/15) | 1.8 (1–4) | 22 (3) | Extended maternal care | 10 |
| Rugose squat lobster, Munida rugosa | 25 | Up to 30,000 | 84 (21/25) | 2.2 (1–3) | 14 (3) | Extended maternal care | 29 |
| Squat lobster, Munida sarsi | 5 | Up to 30,000 | 100 (5/5) | 4.0 (4) | 14 (2) | Extended maternal care | 29 |
| American lobster, Homarus americanus | 108 | Several thousand | 13 (14/108) | 1.2 (1–3) | 24 (4) | Extended maternal care | 30 |
| Norway lobster, Nephrops norvegicus | 11 | 140–4,000 | 55 (6/11) | 1.1 (1–3) | 37 (2) | Extended maternal care | 31 |
| Brown crab, Cancer pagurus | 18 | 500,000–3 million | 0 (0/18) | 1.0 (1) | 27 (3) | Extended maternal care | 32 |
| Porcelain crab, Petrolisthes cinctipes | 10 | 1,300 | 80 (8/10) | 1.9 (1–3) | 47 (2) | Extended maternal care | 33 |
| Snow crab, Chionoecetes opilio | 7 | thousands | 0 (0/7) | 1.0 (1) | 22 (2) | Extended maternal care | 34 |
| Praying mantis, Ciulfina rentzi | 8 | 12 (10–14) | 0 (0/8) | 1.0 (1) | 7 (2) | Ootheca | 35 |
| Praying mantis, Ciulfina klassi | 7 | 12 (7–23) | 57 (4/7) | 2.1 (1–4) | 9 (2) | Ootheca | 35 |
| Sea spider, Ammothea hilgendorfi | 13 | 243 (35–774) | 54 (7/13) | 1.8 (1–3) | 36 (4) | Paternal care | 36 |
| Sea spider, Ammothella biunguiculata | 45 | Dozens to hundreds | 69 (31/45) | 2.7 (1–9) | 60 (5) | Paternal care | 37 |
| Ascidian, Botryllus schlosseri | 14 | NA | 92 (13/14) | 3.2 (1–5) | 30 (4) | Viviparity | 38† |
*Not used in species analysis.
†Not used in brood analysis.
Table 2.
Comparisons of mating parameters in invertebrate brooders versus pregnant vertebrates
| Mating-system parameter | Invertebrates (mean ± SE) | Vertebrates (mean ± SE) | t-statistic | df | P* | P† |
| No. of successful mates based on per-brood data | 2.19 ± 0.08 | 1.77 ± 0.02 | 5.11 | 651 | 0.00 | 0.00 |
| No. of successful mates based on per-species data | 3.08 ± 0.64 | 1.88 ± 0.10 | 1.85 | 28 | 0.07 | 0.01 |
| Proportion of broods with multiple mates based on per-species data | 0.67 ± 0.07 | 0.51 ± 0.03 | 2.18 | 41 | 0.04 | 0.02 |
*Two-tailed t tests assuming unequal variances.
†Results of a comparison by the Mann-Whitney u test.
Fig. 1.
Frequency distributions of invertebrate and vertebrate broods in which gestators had the indicated numbers of successful mates (as deduced from genetic parentage analyses). Also shown are the relevant Poisson distributions (with same means as those that were observed) that have been truncated by apportioning the expected zero class (no mates) to the other mate classes.
Fig. 2.
Frequencies of multiple mating and the estimated mate numbers in 583 broods representing 29 invertebrate species and in 3,057 litters or broods of 93 vertebrate species that display one form or another of pregnancy.
Discussion
In many animal lineages, anisogamy (the larger size and lower motility of female gametes compared with male gametes) ultimately predisposed the evolution of female-pregnancy and maternal care of offspring, and these phenomena in turn can make females and their ova even more limiting as a reproductive resource. Thus, anisogamy set the evolutionary stage by making females more fecundity-limited than males, and gestational phenomena often amplify this sexual difference by placing further constraints on how many progeny a pregnant or brooding female can bear during her lifetime. In turn, these inherent fertility differences between the genders routinely translate into shallower sexual-selection gradients (39) for females than for males in many species, meaning that a male's reproductive success can increase greatly with higher mate counts, whereas a female's genetic fitness inherently is truncated by her limited fecundity, regardless of her particular mating behavior. Thus, conventional evolutionary wisdom holds that males often experience stronger selection pressures for multiple mating than do females, and this difference in sexual selection pressure is precisely why the relatively few species with male-pregnancy and/or sex-role reversal are of special evolutionary interest (36, 40). Such species offer mirror-image vantages on animal mating systems and sexual selection.
Based in part on the logic introduced by Bateman (39), we take it almost as a given that members of the nonpregnant sex (usually males) tend to evolve behavioral dispositions to seek copulations with members of the pregnant sex (usually females). Thus, at issue here is not so much the mating behaviors by members of the nongestating sex but rather the mating proclivities of members of the gestating sex (exactly the issue that molecular parentage analyses are best suited to assess). Our current interest in comparing invertebrate brooders with pregnant vertebrates relates partly to the fact that incubator parents in these two groups often have very different clutch sizes (potential fecundities or fertilities). For example, most mammalian litters contain only a small handful of young, but many invertebrate broods number in the hundreds to many thousands of embryos (Table 1). Of course, vertebrate and invertebrate animals differ in countless biotic features that might influence mating decisions by members of the pregnant sex, so any observed difference in mating proclivities between these two groups might have nothing to do with brood size per se. On the other hand, if invertebrate brooders and pregnant vertebrates prove to have similar rates and patterns of polygamy despite their many biological differences, then such a finding itself might shed some light on the evolution of mating behaviors in species with high parental-investment tactics.
From our current survey of the literature on genetic parentage in “pregnant” animals, we have confirmed that most invertebrate broods consist of multiple full-sib cohorts, meaning that the gestating parent routinely must have had several successful mates. More than 50% of all surveyed invertebrate broods consisted of two or more such cohorts of embryos, and a typical brooding parent had about two to five successful sexual partners on average. These substantial rates of polygamy may not be surprising, given the several fitness rewards that in theory can attend polygamous mating behaviors by members of the gestating sex (Table 3). Rather, what we find surprising is that the overall mating profiles of invertebrate brooders are so remarkably similar to those of their vertebrate counterparts (Table 2 and Figs. 1 and 2).
Table 3.
Selection-based hypotheses often advanced to explain unexpectedly high levels of polyandry in various species (42)
| Nature of the fitness benefit | Representative example or review |
| Direct material benefits | 43 |
| Courtship feeding (nutritional support) | 44, 45 |
| Better territories (more resources) | 46 |
| Reduced risk of sexual harassment | 48 |
| More parental care | 50 |
| Better immediate mate compatibility | 51 |
| Indirect genetic benefits | 42 |
| Fertilization insurance | 52 |
| Better genes via sperm competition | 53, 54 |
| Better genes via cryptic female choice | 47, 55 |
| Enhanced offspring viability | |
| Sons with more competitive sperm | 49 |
| Reduced risk of incompatible sperm | 56 |
| Higher genetic diversity within a brood | 57, 58 |
| Bet-hedging in unpredictable settings | 59 |
| Compatible genes for self or progeny | 60 |
Many of these hypotheses overlap and are not mutually exclusive.
When molecular markers were introduced to mating-system analyses in the latter part of the 20th century, many researchers were intrigued by what they interpreted as unexpectedly high rates of polygamy in many species otherwise suspected from field observations to be mostly monogamous (reviews in 9, 61). Thus, a research tradition arose wherein a primary goal was to explain why multiple mating by females (polyandry) was far more common than previously thought. Many hypotheses were advanced and tested in numerous taxa regarding possible direct and indirect fitness benefits that females might derive from polyandrous matings (Table 3). Of course, multiple mating was recognized to have potential downsides as well (such as the risk of contracting sexually transmitted diseases), but overall the bulk of the research effort went into understanding why females (in addition to males) often take multiple mates.
Our current overview of the genetic-parentage literature for “pregnant” species raises the diametrically opposite question: Why do invertebrate brooders have so few mates? One theoretical possibility is that the molecular assays were inadequate to document higher mate counts. This explanation is incorrect, however, because nearly all the genetic-parentage studies entailed highly polymorphic microsatellite markers that in principle could have revealed many more parents per brood than were reported. To assess the minimum number of detectable mates in each study given the genetic markers used, we searched each publication for the observed number of alleles at the most polymorphic microsatellite locus in sampled populations (Table 1). We then divided that number (minus 2) by two to assess how many sires could have contributed to each brood. In nearly all cases, the markers used could, in principle, have detected many more sires per brood (or many more dams in species with gestation by males) than actually were recorded. For example, several surveys included loci with >30 alleles in the focal populations, implying that at least 15 sires (or 15 dams in male-brooding species) could have been detected if multiple mating by the pregnant sex truly had been more rampant.
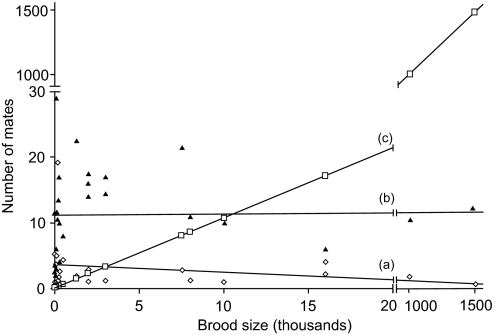
Alternatively, we suspect that the general explanation for the paucity of mates for pregnant invertebrates and vertebrates alike has to do with limited logistical opportunities for successful mating during each breeding season or episode (8). Depending on the species, such constraints on mate acquisition may include numerous ecological and natural-history factors such as low population densities, short mating seasons, low mate-encounter rates, lengthy courtships, and even postcopulatory phenomena such as sperm competition and cryptic female choice (41, 47). The net effect of such constraints is to truncate mate numbers severely, even in animal species with exceptionally large broods and high frequencies of polygamy. To illustrate the severity of such truncation, consider Fig. 3 in which lines c and b indicate how high mate counts theoretically could be in the invertebrates surveyed, given the known brood sizes and observed polymorphism levels in the genetic markers, respectively. The empirical estimates of mate numbers (line a) fall far below these theoretical maxima.
Fig. 3.
Mate numbers versus brood sizes in surveyed invertebrate species that gestate their young. Shown are the following: (a, rombi) empirical relationship between brood size and the genetically estimated number of full-sibships within a brood (r = 0.31, P = 0.23); (b, triangles) the theoretically detectable number of mates per brood based solely on the number of alleles at the most polymorphic microsatellite locus; and (c, squares) the theoretical maximum number of mates per brood based solely on brood size. For line c, the unit of the y axis is in thousands.
Again, the question becomes relevant: Why do invertebrate brooders (like pregnant vertebrates) have so few mates (i.e., why does line a fall so far below lines b and c)? This “turning the problem on its head” finds some analog in another longstanding question in population genetics: What is the adaptive significance of molecular variation? When extensive allozyme polymorphism was uncovered in the seminal protein-electrophoretic surveys of the 1960s, selectionists interpreted the data as consistent with the operation of one form or another of balancing selection, so they sought to discover the driving mechanisms in particular cases. At the same time, a dilemma facing neutralists was to account for a paucity of molecular polymorphism relative to neutrality expectations (62) given suspected mutation rates and effective population sizes. Neutrality theory eventually became molecular evolution's gigantic null hypothesis—the simplest way to view molecular variation and the overarching hypothesis that must be falsified before a selectionist interpretation can be accepted for a specific genetic polymorphism.
Mating-constraint hypotheses similarly might be viewed as null models for reproductive behaviors in nature (6, 63). If so, logistical considerations should offer a helpful alternative perspective on animal mating systems, not least as a counterbalance to standard selectionist perspectives on polygamy. For the selection–neutrality debate in molecular evolution, the final resolution proved to be a compromise: Some loci are under selection, whereas others appear neutral or nearly so. With respect to the current issue of mate numbers in “pregnant” species, the final resolution undoubtedly will prove to be some balance between fitness payoffs of polygamous mating (Table 3) and the many limitations on multiple mating imposed by what is logistically feasible within each population (64).
Materials and Methods
Our current literature review was conducted in the winter and spring of 2011. It sought to identify all substantive papers on rates of multiple paternity or multiple maternity for the assayed broods of gestating invertebrate species (most of which inhabit marine or freshwater environments), as estimated via genetic parentage analyses that used highly polymorphic molecular markers. For each species, we summarize reported information on brood size, the proportion of broods with multiple sires (or multiple dams in the case of male-brooding species), successful numbers of mates for members of the brooding sex, and the resolving power of the particular polymorphic molecular markers that were used. Different authors sometimes used slightly different statistical procedures (see ref. 65.) to quantify the incidence of multiple mating by the gestating sex, but in all cases we accepted the published estimates at face value.
For each relevant mating-system parameter (such as brood size and number of parents per brood), we calculated means and SEs and also performed regression analyses between variables. To compare the means of mating-system parameters in vertebrates versus invertebrates, we initially used t tests, but to accommodate nonnormality in the data we then verified the results with nonparametric Mann–Whitney u tests (66).
Acknowledgments
We thank Louis Bernatchez, Patty Gowaty, Steve Hubbell, and Axel Meyer for helpful comments that improved the manuscript. This work was supported by funds from the University of California, Irvine.
Footnotes
The authors declare no conflict of interest.
References
- 1.Darwin CD. The Descent of Man and Selection in Relation to Sex. London: John Murray; 1871. [Google Scholar]
- 2.Arnold SJ, Duvall D. Animal mating systems: A synthesis based on selection theory. Am Nat. 1994;143:317–348. [Google Scholar]
- 3.Parker GA, Simmons LW. Parental investment and the control of sexual selection: Predicting the direction of sexual competition. Proc R Soc Lond B Biol Sci. 1996;263:315–321. [Google Scholar]
- 4.Shuster SM, Wade MJ. Mating Systems and Strategies. Princeton, NJ: Princeton Univ. Press; 2003. [Google Scholar]
- 5.Oliveira RF, Taborsky M, Brockmann HJ, editors. Alternative Reproductive Tactics: An Integrative Approach. New York: Cambridge Univ Press; 2008. [Google Scholar]
- 6.Gowaty PA, Hubbell SP. Reproductive decisions under ecological constraints: It's about time. Proc Natl Acad Sci USA. 2009;106(Suppl 1):10017–10024. doi: 10.1073/pnas.0901130106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avise JC, Liu J-X. Multiple mating and its relationship to alternative modes of gestation in male-pregnant versus female-pregnant fish species. Proc Natl Acad Sci USA. 2010;107:18915–18920. doi: 10.1073/pnas.1013786107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avise JC, Liu J-X. Multiple mating and its relationship to brood size in pregnant fishes versus pregnant mammals and other viviparous vertebrates. Proc Natl Acad Sci USA. 2011;108:7091–7095. doi: 10.1073/pnas.1103329108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Avise JC. Molecular Markers, Natural History, and Evolution. Sunderland, MA: Sinauer; 2004. [Google Scholar]
- 10.Walker D, Porter BA, Avise JC. Genetic parentage assessment in the crayfish Orconectes placidus, a high-fecundity invertebrate with extended maternal brood care. Mol Ecol. 2002;11:2115–2122. doi: 10.1046/j.1365-294x.2002.01609.x. [DOI] [PubMed] [Google Scholar]
- 11.Le Cam S, Pechenik JA, Cagnon M, Viard F. Fast versus slow larval growth in an invasive marine mollusc: Does paternity matter? J Hered. 2009;100:455–464. doi: 10.1093/jhered/esp007. [DOI] [PubMed] [Google Scholar]
- 12.Dupont L, Richard J, Paulet YM, Thouzeau G, Viard F. Gregariousness and protandry promote reproductive insurance in the invasive gastropod Crepidula fornicata: Evidence from assignment of larval paternity. Mol Ecol. 2006;15:3009–3021. doi: 10.1111/j.1365-294X.2006.02988.x. [DOI] [PubMed] [Google Scholar]
- 13.Proestou DA, Goldsmith MR, Twombly S. Patterns of male reproductive success in Crepidula fornicata provide new insight for sex allocation and optimal sex change. Biol Bull. 2008;214:194–202. doi: 10.2307/25066676. [DOI] [PubMed] [Google Scholar]
- 14.Brante A, Fernandez M, Viard F. Microsatellite evidence for sperm storage and multiple paternity in the marine gastropod Crepiduda coquimbensis. J Exp Mar Biol Ecol. 2011;396:83–88. [Google Scholar]
- 15.Angeloni L, Bradbury JW, Burton RS. Multiple mating, paternity, and brood size in a simultaneous hermaphrodite, Aplysia californica. Behav Ecol. 2002;14:544–560. [Google Scholar]
- 16.Kupfernagel S, Rusterholz HP, Baur B. Variation in multiple paternity and sperm utilization patterns in natural populations of a simultaneous hermaphrodite land snail. Biol J Linn Soc Lond. 2010;99:350–361. [Google Scholar]
- 17.Paterson IG, Partridge V, Buckland-Nicks J. Multiple paternity in Littorina obtusata (Gastropoda, Littorinidae) revealed by microsatellite analyses. Biol Bull. 2001;200:261–267. doi: 10.2307/1543508. [DOI] [PubMed] [Google Scholar]
- 18.Panova M, et al. Extreme female promiscuity in a non-social invertebrate species. PLoS ONE. 2010;5:e9640. doi: 10.1371/journal.pone.0009640. 10.1371/journal.pone.0009640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Camp LM, Donnellan SC, Dyer AR, Fairweather PG. Multiple paternity in field and captive-laid egg strands of Sepioteuthis australis (Cephalopoda: Loliginidae) Mar Freshw Res. 2004;55:819–823. [Google Scholar]
- 20.Shaw PW, Boyle PR. Multiple paternity within the brood of single females of Loligo forbesi (Cephalopoda: Loliginidae), demonstrated with microsatellite DNA markers. Mar Ecol Prog Ser. 1997;160:279–282. [Google Scholar]
- 21.Buresch KM, Hanlon RT, Maxwell MR, Ring S. Microsatellite DNA markers indicate a high frequency of multiple paternity within individual field collected egg capsules of the squid Loligo pealeii. Mar Ecol Prog Ser. 2001;210:161–165. [Google Scholar]
- 22.Shaw PW, Sauer WHH. Multiple paternity and complex fertilization dynamics in the squid Loligo vulgaris reynaudii. Mar Ecol Prog Ser. 2004;270:173–179. [Google Scholar]
- 23.Voight JR, Feldheim KA. Microsatellite inheritance and multiple paternity in the deep sea octopus Graneledone boreopacifica (Mullusca: Cephalopoda) Invertebr Biol. 2009;128:26–30. [Google Scholar]
- 24.Mathews LM. Evidence for high rates of in-pair paternity in the socially monogamous snapping shrimp Alpheus angulosus. Aquat Biol. 2007;1:55–62. [Google Scholar]
- 25.Bilodeau AL, Felder DL, Neigel JE. Multiple paternity in the thalassinidean ghost shrimp, Callichirus islagrande, (Crustacea: Decapoda, Callianassidae) Mar Biol. 2005;146:381–385. [Google Scholar]
- 26.Yue GH, Chang A. Molecular evidence for high frequency of multiple paternity in a freshwater shrimp species Caridina ensifera. PLoS ONE. 2010;5:e12721. doi: 10.1371/journal.pone.0012721. 10.1371/journal.pone.0012721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yue GH, et al. High prevalence of multiple paternity in the invasive crayfish species, Procambarus clarkii. Int J Biol Sci. 2010;6:107–115. doi: 10.7150/ijbs.6.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walker D, Power AJ, Sweeney-Reeves M, Avise JC. Multiple paternity and female sperm storage along egg-case strings of the knobbed whelk, Busycon carica (Mollusca; Melongenidae) Mar Biol. 2007;151:53–61. [Google Scholar]
- 29.Bailie DA, Hynes R, Prodöhl PA. Genetic parentage in the squat lobsters Munida rugosa and M. sarsi (Crustacea, Anomura, Galatheidae) Mar Ecol Prog Ser. 2011;421:173–182. [Google Scholar]
- 30.Gosselin T, Sainte-Marie B, Bernatchez L. Geographic variation of multiple paternity in the American lobster, Homarus americanus. Mol Ecol. 2005;14:1517–1525. doi: 10.1111/j.1365-294X.2005.02498.x. [DOI] [PubMed] [Google Scholar]
- 31.Streiff R, Mira S, Castro M, Cancela ML. Multiple paternity in Norway lobster (Nephrops norvegicus L.) assessed with microsatellite markers. Mar Biotechnol (NY) 2004;6:60–66. doi: 10.1007/s10126-003-0015-7. [DOI] [PubMed] [Google Scholar]
- 32.McKeown NJ, Shaw PW. Single paternity within broods of the brown crab Cancer pagurus: A highly fecund species with long-term sperm storage. Mar Ecol Prog Ser. 2008;368:209–215. [Google Scholar]
- 33.Toonen RJ. Genetic evidence of multiple paternity of brood in the intertidal crab Petrolisthes cinctipes. Mar Ecol Prog Ser. 2004;270:259–263. [Google Scholar]
- 34.Urbani N, Sainte-Marie B, Sevigny JM, Zadworny D, Kuhnlein U. Sperm competition and paternity assurance during the first breeding period of female snow crab (Chionoecetes opilio) (Brachyura: Majidae) Can J Fish Aquat Sci. 1998;55:1104–1113. [Google Scholar]
- 35.Umbers KDL, Holwell GI, Stow AJ, Herberstein ME. Molecular evidence for variation in polyandry among praying mantids (Mantodea: Ciulfina) J Zool (Lond) 2011;284:40–45. [Google Scholar]
- 36.Barreto FS, Avise JC. Polygynandry and sexual size dimorphism in the sea spider Ammothea hilgendorfi (Pycnogonida: Ammotheidae), a marine arthropod with brood-carrying males. Mol Ecol. 2008;17:4164–4175. doi: 10.1111/j.1365-294X.2008.03895.x. [DOI] [PubMed] [Google Scholar]
- 37.Barreto FS, Avise JC. The genetic mating system of a sea spider with male-biased sex size dimorphism: Evidence for paternity skew despite random mating success. Behav Ecol Sociobiol. 2011 doi: 10.1007/s00265-011-1170-x. in press 10.1007/s00265-011-1170-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson SL, Yund PO. Variation in multiple paternity in natural populations of a free-spawning marine invertebrate. Mol Ecol. 2007;16:3253–3262. doi: 10.1111/j.1365-294X.2007.03366.x. [DOI] [PubMed] [Google Scholar]
- 39.Bateman AJ. Intra-sexual selection in Drosophila. Heredity. 1948;2:349–368. doi: 10.1038/hdy.1948.21. [DOI] [PubMed] [Google Scholar]
- 40.Jones AG, Avise JC. Mating systems and sexual selection in male-pregnant pipefishes and seahorses: Insights from microsatellite-based studies of maternity. J Hered. 2001;92:150–158. doi: 10.1093/jhered/92.2.150. [DOI] [PubMed] [Google Scholar]
- 41.Jones AG, Ratterman NL. Mate choice and sexual selection: What have we learned since Darwin? Proc Natl Acad Sci USA. 2009;106(Suppl 1):10001–10008. doi: 10.1073/pnas.0901129106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jennions MD, Petrie M. Why do females mate multiply? A review of the genetic benefits. Biol Rev Camb Philos Soc. 2000;75:21–64. doi: 10.1017/s0006323199005423. [DOI] [PubMed] [Google Scholar]
- 43.Møller AP, Jennions MD. How important are direct fitness benefits of sexual selection? Naturwissenschaften. 2001;88:401–415. doi: 10.1007/s001140100255. [DOI] [PubMed] [Google Scholar]
- 44.Gwynne DT. Courtship feeding increases female reproductive success in bush crickets. Nature. 1984;307:361–363. [Google Scholar]
- 45.Griffith SC, Owens IP, Thuman KA. Extra pair paternity in birds: A review of interspecific variation and adaptive function. Mol Ecol. 2002;11:2195–2212. doi: 10.1046/j.1365-294x.2002.01613.x. [DOI] [PubMed] [Google Scholar]
- 46.Andersson M, Simmons LW. Sexual selection and mate choice. Trends Ecol Evol. 2006;21:296–302. doi: 10.1016/j.tree.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 47.Birkhead TR, Pizzari T. Postcopulatory sexual selection. Nat Rev Genet. 2002;3:262–273. doi: 10.1038/nrg774. [DOI] [PubMed] [Google Scholar]
- 48.Lee PLM, Hays GC. Polyandry in a marine turtle: Females make the best of a bad job. Proc Natl Acad Sci USA. 2004;101:6530–6535. doi: 10.1073/pnas.0307982101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Keller L, Reeve HK. Why do females mate with multiple males? The sexually selected sperm hypothesis. Adv Stud Behav. 1995;24:291–315. [Google Scholar]
- 50.Östlund S, Ahnesjö I., I Female fifteen-spined sticklebacks prefer better fathers. Anim Behav. 1998;56:1177–1183. doi: 10.1006/anbe.1998.0878. [DOI] [PubMed] [Google Scholar]
- 51.Ryan KK, Altmann J. Selection for male choice based primarily on mate compatibility in the Oldfield mouse, Peromyscus polionotus rhoadsi. Behav Ecol Sociobiol. 2001;50:436–440. [Google Scholar]
- 52.Baker HG. Self-compatibility and establishment after “long-distance” dispersal. Evolution. 1955;9:347–349. [Google Scholar]
- 53.Parker GA. Sperm competition games—raffles and role. Proc R Soc Lond B Biol Sci. 1990;242:120–126. [Google Scholar]
- 54.Birkhead TR, Møller AP. Sperm Competition and Sexual Selection. London: Academic; 1998. [Google Scholar]
- 55.Eberhard WG. Postcopulatory sexual selection: Darwin's omission and its consequences. Proc Natl Acad Sci USA. 2009;106(Suppl 1):10025–10032. doi: 10.1073/pnas.0901217106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zeh JA, Zeh DW. The evolution of polyandry II: Post-copulatory defenses against genetic incompatibility. Proc R Soc Lond B Biol Sci. 1997;264:69–75. [Google Scholar]
- 57.Yasui Y. The ‘genetic benefits' of female multiple mating reconsidered. Trends Ecol Evol. 1998;13:246–250. doi: 10.1016/s0169-5347(98)01383-4. [DOI] [PubMed] [Google Scholar]
- 58.Stockley P. Female multiple mating behaviour, early reproductive failure and litter size variation in mammals. Proc Biol Sci. 2003;270:271–278. doi: 10.1098/rspb.2002.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yasui Y. Female multiple mating as a genetic bet-hedging strategy when mate choice criteria are unreliable. Ecol Res. 2001;16:605–616. [Google Scholar]
- 60.Pryke SR, Rollins LA, Griffith SC. Females use multiple mating and genetically loaded sperm competition to target compatible genes. Science. 2010;329:964–967. doi: 10.1126/science.1192407. [DOI] [PubMed] [Google Scholar]
- 61.Burke T. DNA fingerprinting and other methods for the study of mating success. Trends Ecol Evol. 1989;4:139–144. doi: 10.1016/0169-5347(89)90213-9. [DOI] [PubMed] [Google Scholar]
- 62.Nei M, Graur D. Extent of protein polymorphism and the neutral mutation theory. Evol Biol. 1984;17:73–118. [Google Scholar]
- 63.Hubbell SP, Johnson LK. Environmental variance in lifetime mating success, mate choice, and sexual selection. Am Nat. 1987;130:91–112. [Google Scholar]
- 64.Uller T, Olsson M. Multiple paternity in reptiles: Patterns and processes. Mol Ecol. 2008;17:2566–2580. doi: 10.1111/j.1365-294X.2008.03772.x. [DOI] [PubMed] [Google Scholar]
- 65.Jones AG, Ardren WR. Methods of parentage analysis in natural populations. Mol Ecol. 2003;12:2511–2523. doi: 10.1046/j.1365-294x.2003.01928.x. [DOI] [PubMed] [Google Scholar]
- 66.Sokal RR, Rohlf FJ. Biometry. 3rd Ed. New York: Freeman & Co.; 1995. [Google Scholar]