Abstract
The Chesapeake Bay, a seasonally variable temperate estuary, provides a natural laboratory for examining the fluctuations and impacts of viral lysis on aquatic microorganisms. Viral abundance (VA) and viral production (VP) were monitored in the Chesapeake Bay over 4 1/2 annual cycles, producing a unique, long-term, interannual study of virioplankton production. High and dynamic VP rates, averaging 7.9 × 106 viruses per mL per h, indicate that viral lysis impacts a significant fraction of microorganisms in the Chesapeake. Viral-mediated bacterial mortality, VA, VP, and organic carbon release all displayed similar interannual and seasonal trends with higher values in 2003 and 2006 than in 2004 and 2005 and peaks in early spring and summer. Surprisingly, higher rates of viral lysis occurred in winter, resulting in a magnified effect of viral lysis on bacterioplankton during times of reduced productivity. Viral lysis directly impacted the organic carbon pool, contributing on average 76 μg of C per L per d, an amount capable of sustaining ∼55% of Chesapeake Bay bacterial production. The observed repeating interannual patterns of VP and lysis are likely interlinked with seasonal cycles of host abundance and diversity, which are in turn driven by annual cycles in environmental conditions, emphasizing the complex interplay of seasonality and microbial ecology in the Chesapeake Bay.
Keywords: viral ecology, biogeochemistry, carbon cycle, bacteriophage, tangential flow diafiltration
The Earth's ocean resources have been historically mismanaged, particularly coastal environments. Although coastal ecosystems comprise a mere 7.5% of the global ocean, they support half of all fisheries production (1) and play a disproportionately large role in oceanic biogeochemical cycles (2). The economic importance and proximity of coastal ecosystems to human populations places these environments at the forefront of human impacts on ocean health. The Chesapeake Bay, one of the world's largest and most productive estuaries, was among the first coastal ecosystems to show the dramatic ecological effects of human activity, including pressures from point and nonpoint source pollution, overfishing, shipping, agriculture, industrial activity, and seasonal hypoxia (3, 4). Ultimately, many of these issues, especially summer deep water hypoxia, are linked to the activity of microorganisms (5, 6). Thus, understanding the processes affecting microbial communities, such as viral lysis, is essential to understanding the mechanisms through which human activities impact the economic and environmental viability of the Chesapeake Bay.
Studies of the Chesapeake were among the earliest reports documenting high viral abundance (VA) in marine ecosystems (7, 8) and the seasonal and geographic variability of virioplankton assemblages (9–11). Metagenomic investigations have shown that the Chesapeake hosts a diverse virioplankton community that is endemic to the estuary (12, 13). Chesapeake virioplankton assemblages turn over in less than a day with overall viral production (VP) rates rivaling those observed in the other productive marine ecosystems (14–16), but little is known of seasonal and spatial variability in viral lysis in this ecosystem.
Estimates of the impact of viral lysis on marine ecosystems such as viral-mediated bacterial mortality (VMM) rely on VP estimates. Thus, development of reliable VP assays has been critical to understanding of the interplay of VP and marine microbiology. Only recently have protocols for estimating VP been refined for routine, high-throughput use (17). Based on the handful of studies using these new approaches, VMM varies seasonally in marine (18) and freshwater environments (19–21) and is responsible for removing 13–100% of bacterial standing stock (%BA) each day (20–23). The only previous study to examine VP interannually did not observe consistent seasonal patterns in VP or its interactions with environmental variables (24).
For a productive temperate ecosystem such as the Chesapeake, seasonal fluctuations in VP may propagate as changes in the relative impact of viral lysis on microbial diversity and productivity and the remineralization of nutrients. High rates of VP during times of microbial senescence would result in a high impact of viral lysis on bacteria. In contrast, if high rates of VP coincide with high rates of microbial production, the overall impact of viral lysis on microbial processes would be smaller. The goal of this research was to document interannual variability in VA, VP, and viral lysis and to correlate emergent patterns with microbial and environmental parameters in the Chesapeake Bay.
Results
Chesapeake Bay Environmental Parameters.
Over the study period, surface water temperatures in the Chesapeake Bay displayed significant monthly and seasonal variability, with mean values ranging from 3 °C in winter to 26 °C in summer (Fig. S1A and Table S1; month, P < 0.001; season, P < 0.001). Mean surface water salinity significantly increased (P < 0.001) from the northern Chesapeake [7 practical salinity units (psu)] to the southern Chesapeake (21 psu) but did not vary seasonally or interannually (Fig. S1B and Table S1; season, P = 0.237; year, P = 0.102). Mean nutrient values were as follows: NOx, 16 ± 1.9 μM N; NH4, 2.8 ± 0.37 μM N; and PO4, 0.42 ± 0.03 μM P. Total chlorophyll a (Chl a) concentration ranged from 1.7 to 58 μg per L with a mean of 13 ± 1.5 μg per L (Fig. S1C). Seasonal and spatial variations in nutrient (NOx, NH4, and PO4) and Chl a concentrations did not deviate from previous observations (see ref. 25 for review).
Seasonal; and Monthly Variations.
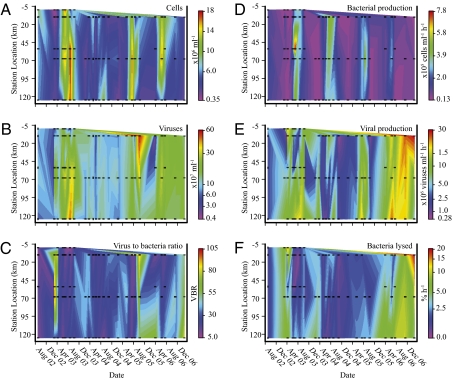
Bacterial abundance (BA) varied 50-fold from 18 × 106 cells per mL at CB818 in August 2003 to 0.35 × 106 cells per mL at CB908 in March 2003 with a grand mean of 6.6 ± 0.5 × 106 cells per mL (Fig. 1A and Table S1). Over this same period, VA averaged 1.5 ± 0.1 × 108 viruses per mL peaking at 5.9 × 108 viruses per mL at CB858 in October 2005 (Fig. 1B). The virus-to-bacteria ratio (VBR) averaged 30 ± 2.1, ranging from 7 to 95 (Fig. 1C). Significant seasonal changes occurred in BA (P < 0.001), VA (P = 0.007), and VBR (P < 0.001; Fig. 2B). Whereas BA and VA increased significantly from winter to the warmer seasons, VBR showed the opposite trend with significantly greater values in winter (Table S1).
Fig. 1.
Contour plots of BA (A), VA (B), ratio of VA to BA (C), BP (D), VP (E), and %BA (F) in the Chesapeake Bay from September 2002 through February 2007. Sampling locations are given in kilometers south from station CB908 (0 km). Black squares represent dates and locations of sample collections. In C and F, CB818, March 2003 is not plotted, as it exceeds scale. Scales are logarithmic for VA, VP, and %BA. n = 84.
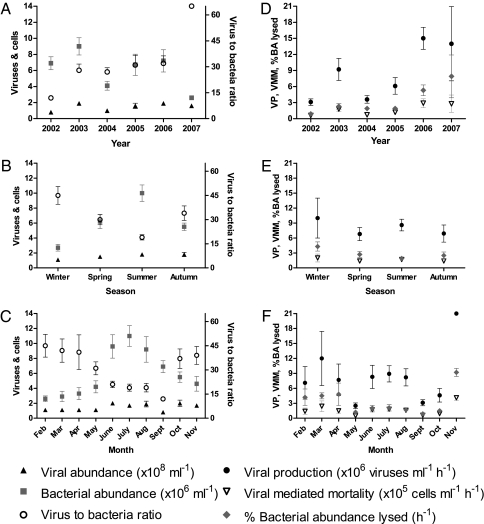
Fig. 2.
Plots of yearly (A and D), seasonal (B and E), and monthly (C and F) mean VA and BA, VBR, VP, VMM, and %BA. Error bars are ± SEM. Values for n are given in Table S1.
Over the 54-mo study, bacterial production (BP) averaged 1.5 × 105 cells per mL per h (Fig. 1D), with threefold greater values in summer than in spring and autumn and sixfold higher values in summer than in winter (P < 0.001). Mean VP was 7.9 ± 1.0 × 106 viruses per mL per h and ranged over two orders of magnitude, from 0.28 × 106 viruses per mL per h at CB858 in October 2005 to 58 × 106 viruses per mL per h at CB818 in March 2003 (Fig. 1E). Based on VP estimates, viral turnover time (VTT) averaged 1.2 ± 0.12 per d (Table S1), and an average of 76 ± 9.6 μg of C per L per d was released by viral lysis of 1.6 ± 0.20 × 105 cells per mL per h. Monthly statistical analyses of VP, VMM, organic carbon (OC) release, and VTT were obscured by samples from November 2006 and February 2007 when high VP rates were observed due to an overall increase in VP in 2006–2007 (Fig. 2D). Nevertheless, monthly trends were apparent. In particular, the magnitudes of VP, VMM, and OC release were consistently low in May between earlier peaks in March through April and later increases from June through August (Fig. 2 and Table S1). Normalizing monthly VP means to their respective yearly VP means to account for interannual variations revealed the same repeating monthly patterns in VP rates with increases in spring and summer (Fig. S2).
Viral impacts on the host bacterial community (estimated from VP rates) included the percentages of BA (%BA) and BP (%BP) lysed by viruses (Table S1 and Fig. 1F). These estimates averaged 2.7 ± 0.38% h−1 and 280 ± 59%, respectively, and varied significantly between seasons (P ≤ 0.001), with winter values twofold to threefold higher than other seasons (Fig. 2E). Across sampling years, %BA and %BP lysed by viruses peaked in February, March, April, and November (Fig. 2F; %BA, P < 0.001; %BP, P < 0.001). This trend opposes that of VA and VP but resembles that of VBR (Fig. 2C).
Interannual and Geographic Variations.
Only data gathered from 2003 to 2006 were used for interannual comparisons, as 2002 and 2007 were represented by single samplings. BA, but not BP (BA, P < 0.001; BP, P = 0.766), varied significantly from year to year, with low and high mean values occurring in 2004 and 2003, respectively (Fig. 2A and Table S1). VA also varied interannually (P < 0.001), with viral numbers in 2004 significantly lower than in other sampling years (Fig. 2A). VP, VMM, OC release, VTT, and %BP lysed were significantly higher in 2003 and 2006 than in 2004 and 2005 (Fig. 2D; P < 0.001). These interannual variations in VP significantly influenced the seasonal and monthly analyses, since three of five winter samplings occurred in years with significantly higher mean VP estimates. Lastly, %BA lysed by viruses varied from year to year (P < 0.001), a trend resulting from a twofold to threefold increase in 2006 (Fig. 2D).
Despite significant variations in salinity between sampling locations, BA, VA, and VBR did not change significantly with location in the Chesapeake Bay (Table S1; BA, P = 0.062; VA, P = 0.496; VBR, P = 0.071). Only the %BA lysed by viruses and VTT varied significantly between individual sampling locations (Table S1; %BA, P = 0.003; VTT, P = 0.001). The %BA lysed was significantly higher at CB707 than at station CB818, whereas VTT was higher at stations CB707 and CB908 than station CB804 (Table S1).
Correlations.
Both VA and BA were positively correlated with temperature, Synechococcus abundances, and each other (Table S2). BP was also positively correlated to temperature and BA. In contrast, VBR was negatively correlated to a number of variables, including temperature, BP, and Synechococcus abundance (Table S2). Only VA was positively correlated with VBR.
Interestingly, VP, VA, and BA were positively correlated (Table S2). Of these variables, a significant first-order linear regression only occurred between VP and BA (logVP = 8.22 + 0.42(logBA); r2 = 0.08). VP was also positively correlated with VMM, OC release, and %BA and %BP lysed because calculations of these parameters rely in part or entirely on VP estimates (Table S3).
The %BP lysed by viruses was negatively correlated with BA, BP, temperature, and Synechococcus abundances (Table S2). Only VBR was significantly positively related to %BP lysed. Like VBR, %BA lysed by viruses was negatively correlated with temperature, BA, BP, and Synechococcus abundance and positively correlated with VBR and salinity.
Discussion
In this study, VP and the impact of viral lysis on Chesapeake Bay microbial communities fluctuated in repeating annual and seasonal patterns. Increases in VA and VP occurred in early spring and summer in correlation with changes in host abundance and productivity, which are in turn related to seasonal variations in temperature and estuarine hydrology. Despite the negative impact of viral lysis on host production, the labile micronutrients and organic matter released through viral lysis can sustain a significant proportion of microbial production and may foster microbial diversity. Below, we explore some of the possible mechanisms driving the observed variations in VA, VP, and viral impacts within this estuarine ecosystem.
VA and VP Temporal Variations.
The mean VP rate observed in the Chesapeake Bay of 7.9 × 106 viruses per mL per h was similar to previous reports in other coastal or eutrophic ecosystems, where production rates varied from 5 × 106 to 1.8 × 107 viruses per mL per h (15, 26, 27). The range of VP rates observed in this study overlapped with that of earlier reports in the Chesapeake Bay (14, 16). However, the magnitude was generally higher because previous research was performed in late summer and winter, whereas this study also incorporated spring and summer values.
The temporal dynamics of VP in the Chesapeake Bay can be linked to changes in the following four interacting microbial factors: (i) host abundance, (ii) productivity, (iii) grazing pressure, and (iv) viral and host community composition. First, the positive correlations between VP, VA, and BA, and the tendency for peaks in VP to coincide with or lag slightly behind increases in BA, suggest that changes in host abundance influenced VP. Previous reports have documented positive correlations between virioplankton and bacterioplankton abundance and have linked changes in VA with increased host abundance or biomass after spring and autumn phytoplankton blooms (28, 29), which may be linked to increased VP as well. Thus, as the main resource necessary for viral replication, host organisms, increase, so too do viral abundance and production.
Second, changes in host growth rates influence VP (30–32). Although VP was not directly related to primary or secondary production in this study, these three processes can be indirectly linked through monthly and seasonal changes in temperature. Indeed, maximum rates of primary (this study and refs. 25 and 33) and secondary production (this study and ref. 4) in the Chesapeake Bay occurred in summer, when VP estimates were highest. As host productivity increases with temperature, so does the potential for viral productivity.
Intriguingly, grazing pressure may enhance viral lysis and production (34–36). Thus, the June–August highs in VP in the Chesapeake may be associated with synergistic effects of enhanced grazing during these months (35, 36). Continued work is needed to determine the interactions, if any, between grazing and viral lysis in the Chesapeake.
Finally, monthly and seasonal variations in the composition of microbial host and virioplankton assemblages affect VA and VP. In the Chesapeake, bacterioplankton and virioplankton assemblages shift seasonally from summer and autumn through winter and spring (9, 11, 37). Thus, changes in the types of viruses present, which have inherently different rates of replication, likely contribute to the observed temporal variations in the magnitude of VP. Key features of viral biology, such as burst size, also likely respond to seasonal changes with cascading effects on viral processes such as lysis and production rates (Table S4).
However, higher levels of viral and host richness may be linked to lower VP, as increased numbers of viral–host pairs can actually reduce successful contacts between viruses and their hosts (38, 39). A previous study of bacterial diversity in the Baltimore Harbor found increased bacterioplankton richness in September 2002 compared with March 2003, and VP in this study was lower in September 2002 than March 2003 (Fig. 1E; ref. 37). Thus, the high VP rates seen in early spring, summer, and autumn may be facilitated by lower host and viral diversity, which increases successful virus–host contacts. Lower host and viral diversity at these times may result from stable hydrological conditions due to seasonal water stratification.
The same hypotheses of connections between host abundance, productivity, and diversity and VP also apply to the observed interannual variations. BA, VA, and VP were all higher in 2003 and 2006 than in 2004 (Fig. 2 A and D). BP was not higher in years of increased VP, indicating that changes in bacterial activity alone are insufficient to explain the observed interannual variations. The lower VP rates in 2004 may be tied to lower salinities throughout the Chesapeake during that year. Increased stream flows would have induced mixing that resulted in a more diverse host community and thus reduced levels of VP. By the same argument, increased VP in 2006 may be linked to higher salinities throughout the Bay in this year, which may have allowed less diverse, yet persistent, virioplankton and host communities to develop.
VA and VP Spatial Variations.
In previous studies, spatial variations in VA have been linked to changes in OC concentrations (40), BA (40–43), and phytoplankton blooms (44). Similarly, increased VP has been observed at eutrophic vs. oligotrophic sampling sites (26, 45–47). In contrast to these reports, VA and VP did not change significantly with location in the Chesapeake Bay, despite distinct gradients in salinity and nutrient concentrations. Instead, VA and VP were significantly correlated with host abundance, a parameter that varied seasonally with temperature instead of geographically with salinity in the Chesapeake. Thus, VP is indirectly linked to temperature through changes in host abundance.
Viral Lysis Impact Variations.
This study suggests that viruses play a larger role in the microbial loop, as seen by increased percentages of BA and BP lysed, during colder months in the Chesapeake Bay. Perhaps as grazing pressure declines in winter, viral lysis becomes a leading factor governing microbial mortality. Indeed, increased frequencies of infected bacteria and estimates of viral lysis have been reported in environments with few grazers such as anoxic waters and solar salterns (46, 48, 49). As predicted by contact rate theory, viral impacts on BA and BP increased with the ratio of viruses to bacteria. This correlation occurred on the scale of total VA, as VP represents a bulk estimate representing numerous viral–host pair interactions. When extended to the level of individual viral–host pairs, contact rate theory predicts that an increased ratio of viruses to hosts will result in increased viral–host contacts (50). However, increased contact does not necessarily imply increased rates of VP. Indeed, no correlation was found between VP and VBR, perhaps due to the need for specific viral–host interactions to produce infections and the influence of changing viral and host community diversity on VP rates, as discussed previously.
Many estimates of viral impacts in this study were ≥100% of BA or BP lost to viral lysis. This observation is contradictory to observations of stable or increasing BA in the Chesapeake. Four factors likely resulted in overestimation of viral impacts on bacterioplankton in this study. First, estimates of VMM and viral impacts (%BP and %BA lysed by viruses) from VP require a burst size conversion factor. Using a fixed burst size of 50 viruses per cell lysed may have contributed to the high estimates of BP consumed by viruses. Indeed, in 11 of 13 cases, recalculation using empirically determined burst sizes reduced estimates of %BP lost to viral lysis (Table S4). Second, VP estimates assume all cells lyse and does not account for the production of viruses in the absence of cell lysis, as occurs during chronic phage infection. If not all cells lyse while producing viruses, then VMM will be overestimated. Third, BP may have been underestimated because of 3H leucine isotope dilution (51), inflating values of %BP lysed. Fourth, VP estimates taken from a single time point cannot account for changes in VP that occur throughout a diel cycle. VP rates can vary hourly, and integrating VP measurements across a diel cycle results in lowered estimates of viral impacts than single measurements (23). Because of these factors, our values represent maximum estimates of viral impacts on bacterioplankton in the Chesapeake.
Carbon Cycle Implications of Viral Lysis.
In this study, estimates of OC release due to viral lysis were higher than many previous reports (24, 48, 52, 53). However, the mean OC release rate of 76 μg of C per L per d is within the range of reported viral lysis OC release values (27, 54, 55). All reports of >50 μg of C per L per d of OC released by viral lysis occur in near-shore or estuarine environments, which indicates a consistent trend of high amounts of OC released by viruses in these ecosystems. Despite these high estimates, OC from viral lysis comprises <0.1% of total OC in the Chesapeake, which ranges from 170 to 400 μM (this study; Chesapeake Bay Monitoring Program; www.chesapeakebay.net/data_waterquality.aspx).
The significant correlations between viral, bacterial, and Synechococcus abundances suggest that both heterotrophs and autotrophs are important host communities for Chesapeake Bay virioplankton. The lysis of autotrophs can have a disproportionately large impact on the C cycle by diverting photosynthate originally destined for higher trophic levels into the organic matter pool (56).
However, it is the quality, not the quantity, of OC released by viruses that is paramount. In the Chesapeake, OC from viral lysis could supply, on average, 55% of the OC needed to support BP in the Chesapeake (for calculation, see Table S3). If OC released by viral lysis is rapidly consumed, it provides an important source of carbon and micronutrients to enhance microbial production in surface waters. In contrast, if OC released by viral lysis is not rapidly consumed, the transfer of cellular material into OC would enhance the biological pump of dissolved carbon to deeper waters (56, 57) and potentially exacerbate bottom water hypoxia. Most intriguingly, only those microbes adept at using viral lysate would benefit from this release, implying that viruses influence aquatic microbial diversity directly through lysis and indirectly through OC release. Experiments directly characterizing the forms of OC released by viral lysis and how this release varies with viral–host species and season are now needed to resolve the intricate role of viruses within the aquatic microbial loop and carbon cycle.
Materials and Methods
Sampling Locations.
Surface water samples were collected from five stations: CB908, CB858, CB818, CB804, and CB707 (Fig. S3) on 24 occasions from September 2002 through February 2007. Water from ∼1-m depth was collected in 10-L Niskin bottles by using a conductivity–temperature–depth device (Sea-Bird 911 Plus, Sea-Bird Electronics). Subsamples of unfiltered water for VA and BA were collected, preserved (1% formaldehyde), and either stored at 4 °C (September 2002, March 2003) or snap-frozen in liquid nitrogen and stored at −70 °C. Water was filtered through 50-μm Spectra mesh and kept in the dark at ambient water temperature until diafiltration (1–2 h).
Microbial Abundances.
VA and BA were assessed through epifluorescence microscopy after staining with SYBR Gold (Invitrogen) as described (16). Synechococcus surface water abundances were determined by epifluorescence microscopy as reported (9). Chl a samples were size-fractionated by sequential gravity filtration through 47-mm Nucleopore filters with 20- and 3-μm pore sizes. Duplicate 100-mL samples for each fraction, including unfractionated whole water, were then vacuum-filtered onto 25-mm Whatman GF/C filters. Filters were stored at −20 °C until Chl a extraction in 90% acetone for 24 h at 4 °C in the dark (58).
Microbial Production.
The tangential flow diafiltration technique was used to measure VP (16). Three hundred milliliters of 50-μm prefiltered ambient water was filtered through a 0.22-μm Pellicon XL (Millipore) filter at a flow rate of ∼40 mL per min. Virus-free water (30 kD filtered) from the same location as the original water sample was used to replace water removed by diafiltration until four times the original sample volume had been passed through the filter. The diafiltered sample was then split into triplicate 100-mL incubations and held in the dark at ambient water temperature. Incubations were subsampled every 3 h for a total incubation time of 12 h. Primary production was measured from the assimilation of 14C-sodium bicarbonate into surface water by using deck incubators cooled with flowing surface water (59). Surface water BP was assessed in triplicate via the microcentrifuge 3H-leucine method (51). Additional details of sampling, VP experiments, and statistical analyses are presented in SI Materials and Methods. All data are reported as mean ± SEM.
Supplementary Material
Acknowledgments
We thank the crews of the R/V Cape Henlopen and R/V Hugh R. Sharp and members of the Microbial Observatory for Virioplankton Ecology, including Y. Bettarel, S. Ribblett, S. Cooney, Y. Eissler, J. Kan, and F. Chen for assistance with sampling; L. Fontana, M. Simon, S. Srinivasiah, and T. Mills for assistance with enumerations; L. Rejto, Y. Chen, and J. Yoo for help with statistical analyses. We give special thanks to D. W. Coats for Chl a data analysis and K. Wang for unrivaled support and Synechococcus counts. This work was supported by National Science Foundation (NSF) Grants MCB-0132070 and OCE-0221825 (to K.E.Wommack) and a NSF Graduate Research Fellowship and the Preston C. Townsend Biotechnology Fellowship (D.M.W.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1101907108/-/DCSupplemental.
References
- 1.Ryther JH. Photosynthesis and fish production in the sea. Science. 1969;166:72–76. doi: 10.1126/science.166.3901.72. [DOI] [PubMed] [Google Scholar]
- 2.Siefert R, Plattner G. The role of coastal zones in global biogeochemical cycles. Eos Trans AGU. 2004;85:469. [Google Scholar]
- 3.Lomas MW, Glibert PM, Shiah FK, Smith EM. Microbial processes and temperature in Chesapeake Bay: Current relationships and potential impacts of regional warming. Glob Change Biol. 2002;8:51–70. [Google Scholar]
- 4.Shiah FK, Ducklow HW. Temperature regulation of heterotrophic bacterioplankton abundance, production, and specific growth-rate in Chesapeake Bay. Limnol Oceanogr. 1994;39:1243–1258. [Google Scholar]
- 5.Crump BC, Peranteau C, Beckingham B, Cornwell JC. Respiratory succession and community succession of bacterioplankton in seasonally anoxic estuarine waters. Appl Environ Microbiol. 2007;73:6802–6810. doi: 10.1128/AEM.00648-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Officer CB, et al. Chesapeake bay anoxia: origin, development, and significance. Science. 1984;223:22–27. doi: 10.1126/science.223.4631.22. [DOI] [PubMed] [Google Scholar]
- 7.Bergh O, Børsheim KY, Bratbak G, Heldal M. High abundance of viruses found in aquatic environments. Nature. 1989;340:467–468. doi: 10.1038/340467a0. [DOI] [PubMed] [Google Scholar]
- 8.Wommack KE, Hill RT, Kessel M, Russek-Cohen E, Colwell RR. Distribution of viruses in the Chesapeake Bay. Appl Environ Microbiol. 1992;58:2965–2970. doi: 10.1128/aem.58.9.2965-2970.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang K, Chen F. Genetic diversity and population dynamics of cyanophage communities in the Chesapeake Bay. Aquat Microb Ecol. 2004;34:105–116. [Google Scholar]
- 10.Wommack KE, Ravel J, Hill RT, Colwell RR. Hybridization analysis of Chesapeake bay virioplankton. Appl Environ Microbiol. 1999;65:241–250. doi: 10.1128/aem.65.1.241-250.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wommack KE, Ravel J, Hill RT, Chun J, Colwell RR. Population dynamics of Chesapeake bay virioplankton: Total-community analysis by pulsed-field gel electrophoresis. Appl Environ Microbiol. 1999;65:231–240. doi: 10.1128/aem.65.1.231-240.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bench SR, et al. Metagenomic characterization of Chesapeake Bay virioplankton. Appl Environ Microbiol. 2007;73:7629–7641. doi: 10.1128/AEM.00938-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winget DM, Wommack KE. Randomly amplified polymorphic DNA PCR as a tool for assessment of marine viral richness. Appl Environ Microbiol. 2008;74:2612–2618. doi: 10.1128/AEM.02829-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Helton RR, Liu L, Wommack KE. Assessment of factors influencing direct enumeration of viruses within estuarine sediments. Appl Environ Microbiol. 2006;72:4767–4774. doi: 10.1128/AEM.00297-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poorvin L, Rinta-Kanto JM, Hutchins DA, Wilhelm SW. Viral release of iron and its bioavailability to marine plankton. Limnol Oceanogr. 2004;49:1734–1741. [Google Scholar]
- 16.Winget DM, Williamson KE, Helton RR, Wommack KE. Tangential flow diafiltration: An improved technique for estimation of virioplankton production. Aquat Microb Ecol. 2005;41:221–232. [Google Scholar]
- 17.Weinbauer MG, Rowe JM, Wilhelm SW. In: Manual of Aquatic Viral Ecology. Wilhelm SW, Weinbauer MG, Suttle CA, editors. Waco: ASLO; 2010. pp. 1–8. [Google Scholar]
- 18.Weinbauer MG, Fuks D, Puskaric S, Peduzzi P. Diel, seasonal, and depth-related variability of viruses and dissolved DNA in the Northern Adriatic Sea. Microb Ecol. 1995;30:25–41. doi: 10.1007/BF00184511. [DOI] [PubMed] [Google Scholar]
- 19.Bettarel Y, Sime-Ngando T, Amblard C, Dolan J. Viral activity in two contrasting lake ecosystems. Appl Environ Microbiol. 2004;70:2941–2951. doi: 10.1128/AEM.70.5.2941-2951.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peduzzi P, Schiemer F. Bacteria and viruses in the water column of tropical freshwater reservoirs. Environ Microbiol. 2004;6:707–715. doi: 10.1111/j.1462-2920.2004.00602.x. [DOI] [PubMed] [Google Scholar]
- 21.Säwström C, Anesio MA, Granéli W, Laybourn-Parry J. Seasonal viral loop dynamics in two large ultraoligotrophic Antarctic freshwater lakes. Microb Ecol. 2007;53:1–11. doi: 10.1007/s00248-006-9146-5. [DOI] [PubMed] [Google Scholar]
- 22.Winter C, Smit A, Herndl GJ, Weinbauer MG. Linking bacterial richness with viral abundance and prokaryotic activity. Limnol Oceanogr. 2005;50:968–977. [Google Scholar]
- 23.Winget DM, Wommack KE. Diel and daily fluctuations in virioplankton production in coastal ecosystems. Environ Microbiol. 2009;11:2904–2914. doi: 10.1111/j.1462-2920.2009.02038.x. [DOI] [PubMed] [Google Scholar]
- 24.Boras JA, Sala MM, Vázquez-Domínguez E, Weinbauer MG, Vaqué D. Annual changes of bacterial mortality due to viruses and protists in an oligotrophic coastal environment (NW Mediterranean) Environ Microbiol. 2009;11:1181–1193. doi: 10.1111/j.1462-2920.2008.01849.x. [DOI] [PubMed] [Google Scholar]
- 25.Malone TC, et al. Scales of nutrient-limited phytoplankton productivity in Chesapeake Bay. Estuaries. 1996;19:371–385. [Google Scholar]
- 26.Bongiorni L, Magagnini M, Armeni M, Noble R, Danovaro R. Viral production, decay rates, and life strategies along a trophic gradient in the North Adriatic Sea. Appl Environ Microbiol. 2005;71:6644–6650. doi: 10.1128/AEM.71.11.6644-6650.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilhelm SW, Brigden SM, Suttle CA. A dilution technique for the direct measurement of viral production: A comparison in stratified and tidally mixed coastal waters. Microb Ecol. 2002;43:168–173. doi: 10.1007/s00248-001-1021-9. [DOI] [PubMed] [Google Scholar]
- 28.Bettarel Y, Sime-Ngando T, Amblard C, Carrias J-F, Portelli C. Virioplankton and microbial communities in aquatic systems: A seasonal study in two lakes of differing trophy. Freshw Biol. 2003;48:810–822. [Google Scholar]
- 29.Middelboe M, Riemann L, Steward GF, Hansen V, Nybroe O. Virus-induced transfer of organic carbon between marine bacteria in a model community. Aquat Microb Ecol. 2003;33:1–10. [Google Scholar]
- 30.Guixa-Boixereu N, Lysnes K, Pedros-Alio C. Viral lysis and bacterivory during a phytoplankton bloom in a coastal water microcosm. Appl Environ Microbiol. 1999;65:1949–1958. doi: 10.1128/aem.65.5.1949-1958.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tuomi P, Kuuppo P. Viral lysis and grazing loss of bacteria in nutrient- and carbon-manipulated brackish water enclosures. J Plankton Res. 1999;21:923–937. [Google Scholar]
- 32.Williamson SJ, Paul JH. Nutrient stimulation of lytic phage production in bacterial populations of the Gulf of Mexico. Aquat Microb Ecol. 2004;36:9–17. [Google Scholar]
- 33.Malone TC, Ducklow HW, Peele ER, Pike SE. Picoplankton carbon flux in Chesapeake Bay. Mar Ecol Prog Ser. 1991;78:11–22. [Google Scholar]
- 34.Simek K, et al. Changes in bacterial community composition and dynamics and viral mortality rates associated with enhanced flagellate grazing in a mesoeutrophic reservoir. Appl Environ Microbiol. 2001;67:2723–2733. doi: 10.1128/AEM.67.6.2723-2733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weinbauer MG, Christaki U, Nedoma A, Simek K. Comparing the effects of resource enrichment and grazing on viral production in a meso-eutrophic reservoir. Aquat Microb Ecol. 2003;31:137–144. [Google Scholar]
- 36.Weinbauer MG, et al. Synergistic and antagonistic effects of viral lysis and protistan grazing on bacterial biomass, production and diversity. Environ Microbiol. 2007;9:777–788. doi: 10.1111/j.1462-2920.2006.01200.x. [DOI] [PubMed] [Google Scholar]
- 37.Kan JJ, Wang K, Chen F. Temporal variation and detection limit of an estuarine bacterioplankton community analyzed by denaturing gradient gel electrophoresis (DGGE) Aquat Microb Ecol. 2006;42:7–18. [Google Scholar]
- 38.Bratbak G, Thingstad F, Heldal M. Viruses and the microbial loop. Microb Ecol. 1994;28:209–221. doi: 10.1007/BF00166811. [DOI] [PubMed] [Google Scholar]
- 39.Weinbauer MG, Höfle MG. Significance of viral lysis and flagellate grazing as factors controlling bacterioplankton production in a eutrophic lake. Appl Environ Microbiol. 1998;64:431–438. doi: 10.1128/aem.64.2.431-438.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farnell-Jackson EA, Ward AK. Seasonal patterns of viruses, bacteria, and dissolved organic carbon in a riverine wetland. Freshw Biol. 2003;48:841–851. [Google Scholar]
- 41.Corinaldesi C, et al. Large-scale spatial distribution of virioplankton in the Adriatic Sea: Testing the trophic state control hypothesis. Appl Environ Microbiol. 2003;69:2664–2673. doi: 10.1128/AEM.69.5.2664-2673.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steward GF, Smith DC, Azam F. Abundance and production of bacteria and viruses in the Bering and Chukchi Seas. Mar Ecol Prog Ser. 1996;131:287–300. [Google Scholar]
- 43.Jiang SC, Paul JH. Seasonal and diel abundance of viruses and occurrence of lysogeny/bacteriocinogeny in the marine environment. Mar Ecol Prog Ser. 1994;104:163–172. [Google Scholar]
- 44.Wilson WH, Tarran G, Zubkov MV. Virus dynamics in a coccolithophore-dominated bloom in the North Sea. Deep Sea Res Part II Top Stud Oceanogr. 2002;49:2951–2963. [Google Scholar]
- 45.Guixa-Boixereu N, Vaque D, Gasol JM, Sanchez-Camara J, Pedros-Alio C. Viral distribution and activity in Antarctic waters. Deep Sea Res Part II Top Stud Oceanogr. 2002;49:827–845. [Google Scholar]
- 46.Weinbauer MG, Brettar I, Hofle MG. Lysogeny and virus-induced mortality of bacterioplankton in surface, deep, and anoxic marine waters. Limnol Oceanogr. 2003;48:1457–1465. [Google Scholar]
- 47.Weinbauer MG, Fuks D, Peduzzi P. Distribution of viruses and dissolved DNA along a coastal trophic gradient in the northern Adriatic Sea. Appl Environ Microbiol. 1993;59:4074–4082. doi: 10.1128/aem.59.12.4074-4082.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weinbauer MG, Hofle MG. Size-specific mortality of lake bacterioplankton by natural virus communities. Aquat Microb Ecol. 1998;15:103–113. [Google Scholar]
- 49.Guixa-Boixareu N, Calderon-Paz JI, Heldal M, Bratbak G, Pedros-Alio C. Viral lysis and bacterivory as prokaryotic loss factors along a salinity gradient. Aquat Microb Ecol. 1996;11:215–227. [Google Scholar]
- 50.Murray AG, Jackson GA. Viral dynamics: A model of the effects of size, shape, motion, and abundance of single-celled planktonic organisms and other particles. Mar Ecol Prog Ser. 1992;89:103–116. [Google Scholar]
- 51.Kirchman D. In: Methods in Microbiology: Marine Microbiology. Paul JH, editor. New York: Academic; 2001. pp. 227–237. [Google Scholar]
- 52.Noble RT, Fuhrman JA. Rapid virus production and removal as measured with fluorescently labeled viruses as tracers. Appl Environ Microbiol. 2000;66:3790–3797. doi: 10.1128/aem.66.9.3790-3797.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilhelm SW, Weinbauer MG, Suttle CA, Jeffrey WH. The role of sunlight in the removal and repair of viruses in the sea. Limnol Oceanogr. 1998;43:586–592. [Google Scholar]
- 54.Bratbak G, Heldal M, Thingstad TF, Riemann B, Haslund OH. Incorporation of viruses into the budget of microbial C-transfer: A first approach. Mar Ecol Prog Ser. 1992;83:273–280. [Google Scholar]
- 55.Noble RT, Middelboe M, Fuhrman JA. Effects of viral enrichment on the mortality and growth of heterotrophic bacterioplankton. Aquat Microb Ecol. 1999;18:1–13. [Google Scholar]
- 56.Suttle CA. Marine viruses—major players in the global ecosystem. Nat Rev Microbiol. 2007;5:801–812. doi: 10.1038/nrmicro1750. [DOI] [PubMed] [Google Scholar]
- 57.Motegi C, et al. Viral control of bacterial growth efficiency in marine pelagic environments. Limnol Oceanogr. 2009;54:1901–1910. [Google Scholar]
- 58.Kan J, Suzuki MT, Wang K, Evans SE, Chen F. High temporal but low spatial heterogeneity of bacterioplankton in the Chesapeake Bay. Appl Environ Microbiol. 2007;73:6776–6789. doi: 10.1128/AEM.00541-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harding LW, Mallonee ME, Perry ES. Toward a predictive understanding of primary productivity in a temperate, partially stratified estuary. Estuar Coast Shelf Sci. 2002;55:437–463. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.